Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990–2019 Findings from Global Burden of Disease Study 2019
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Case Definitions
2.2. Socio-Demographic Index (SDI)
2.3. Estimation of Prevalence, Incidence, and Disability-Adjusted Life-Years
2.4. Risk Factors for DALYs
2.5. Statistical Analysis
3. Results
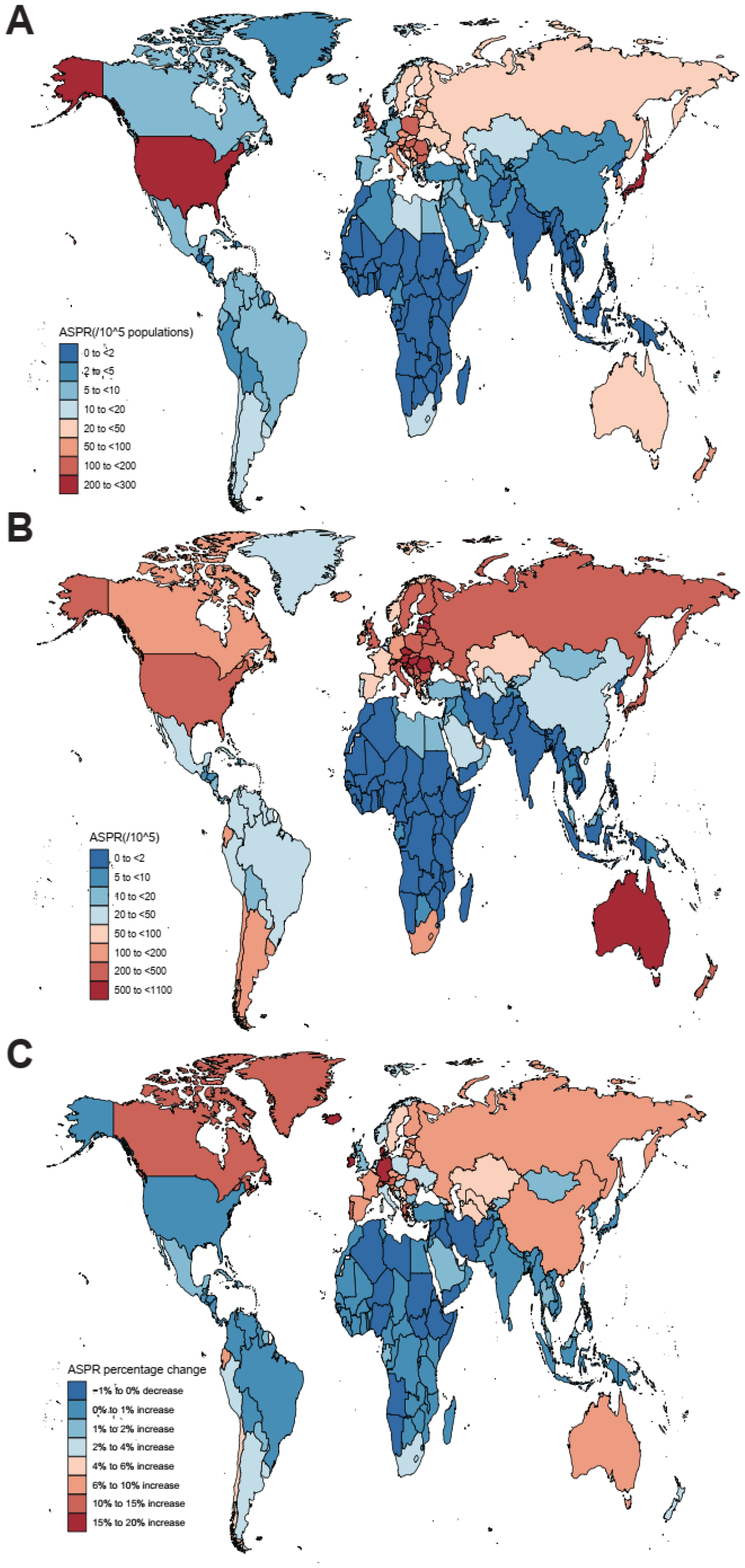
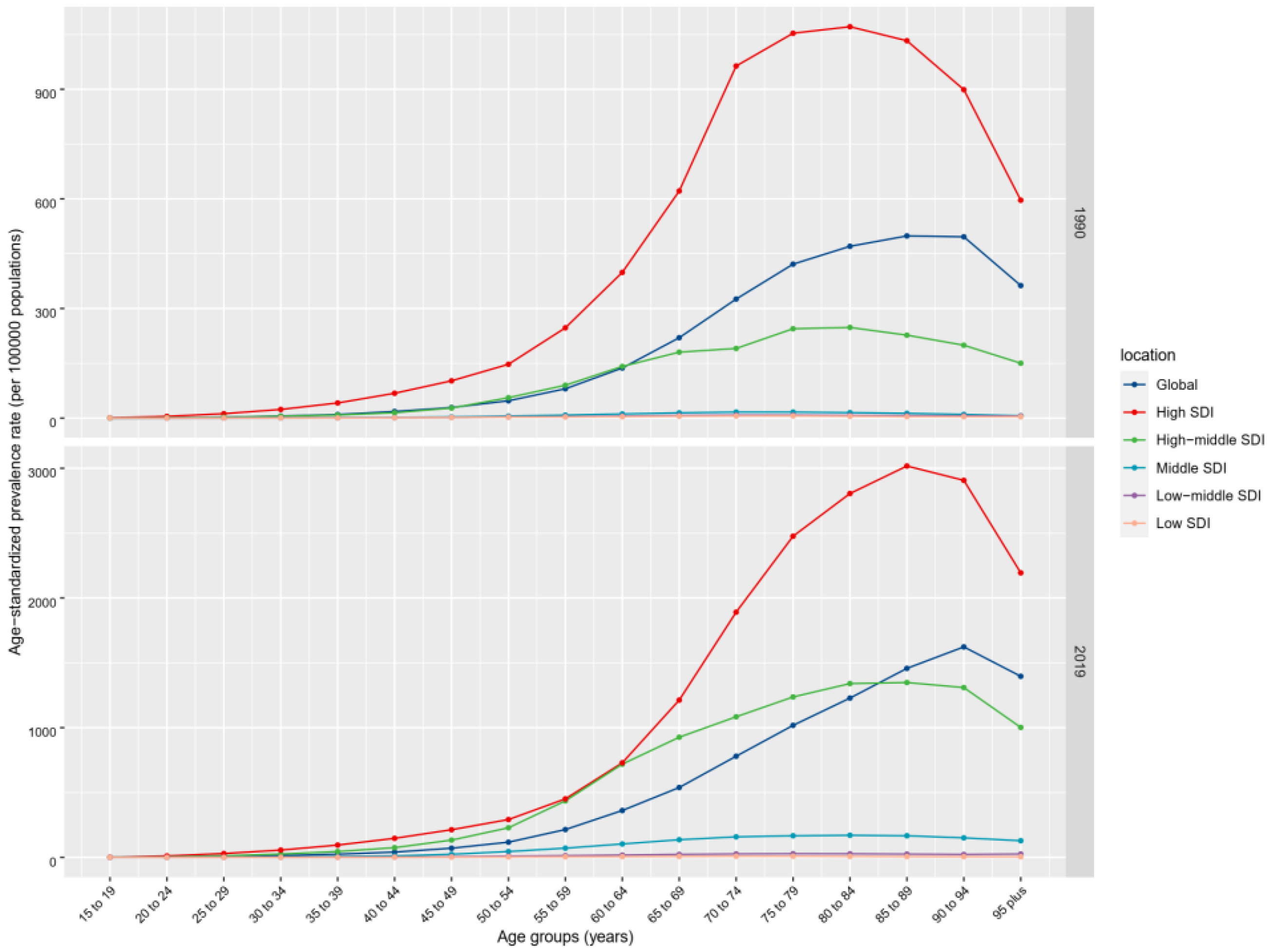
3.1. Prevalence, Incidence, DALYs, and Mortality of nrCAVD
3.2. Risk Factors for nrCAVD
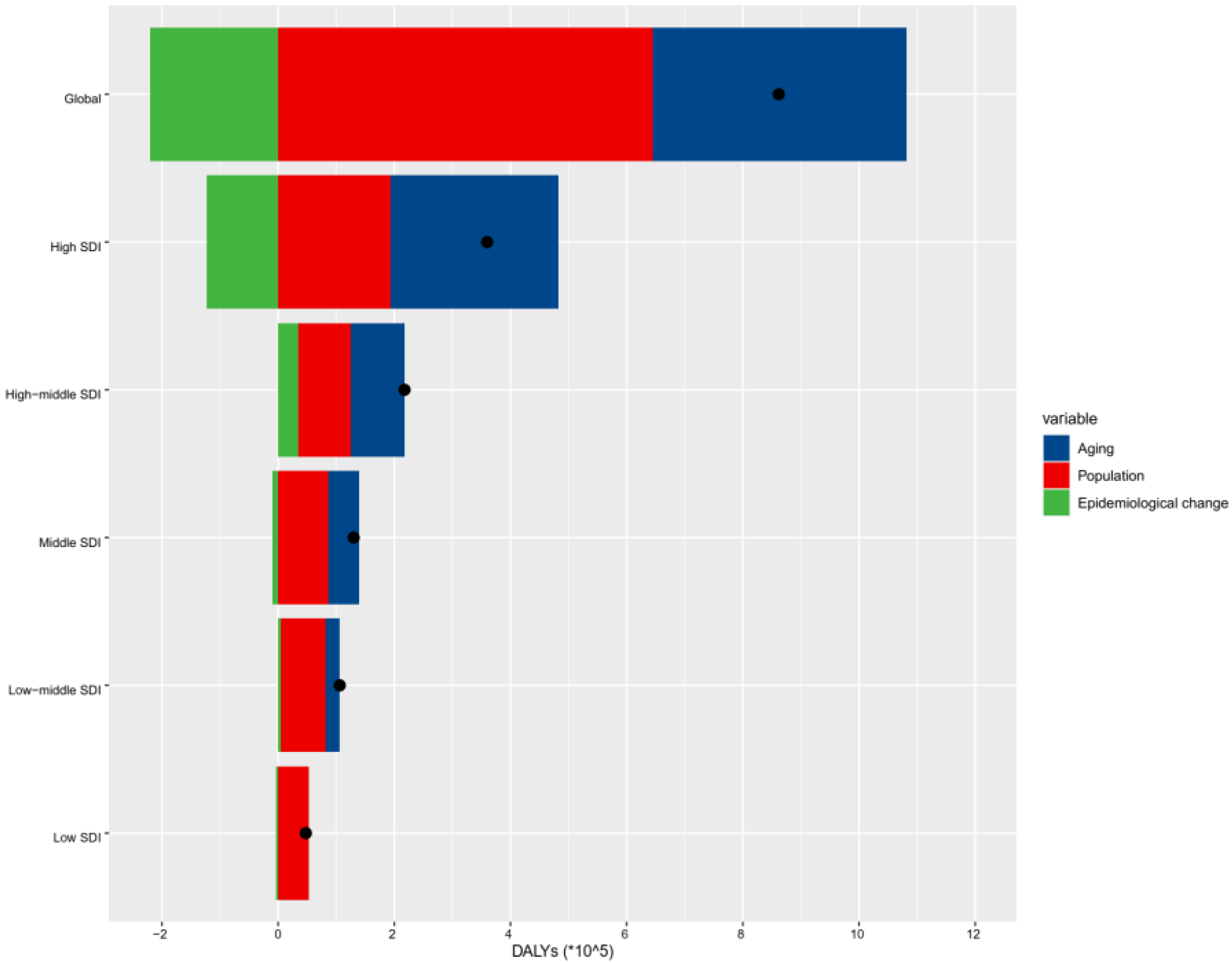
3.3. Decomposition of nrCAVD
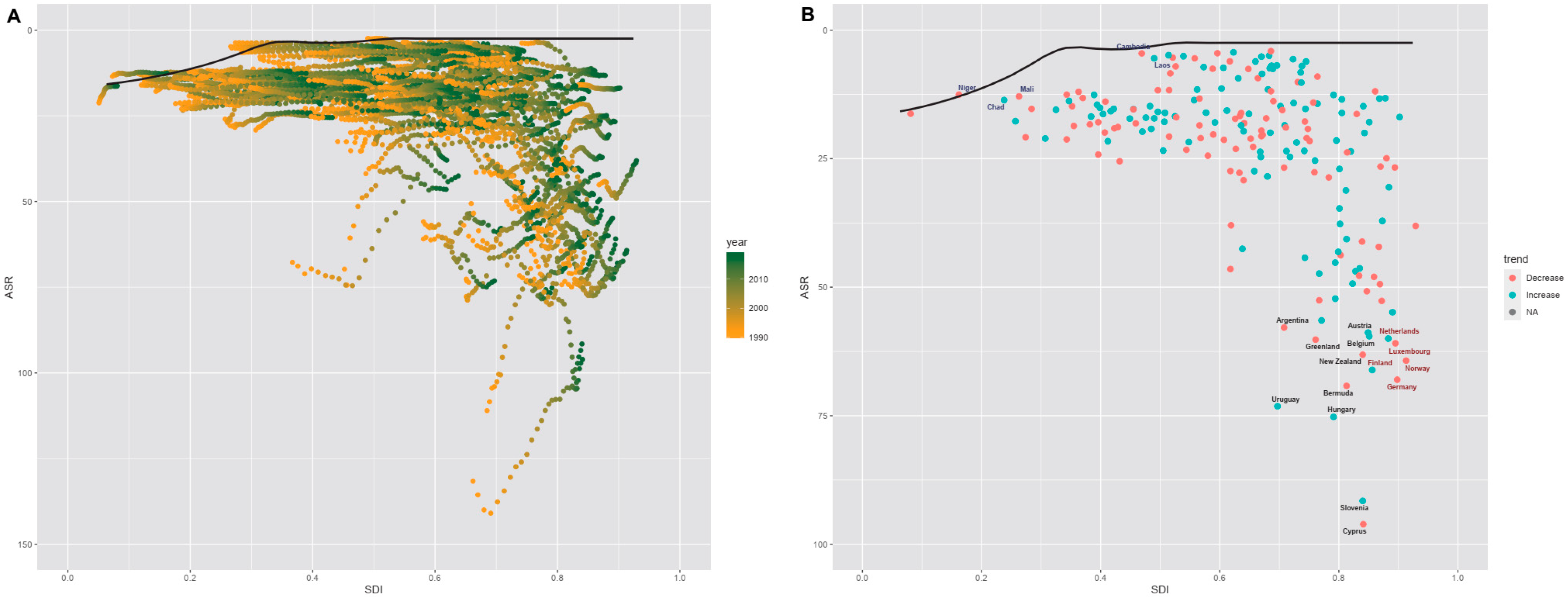
3.4. Frontier Analysis of nrCAVD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Carbone, R.G. Advancements in calcific aortic valve disease. Int. J. Cardiol. 2022, 358, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Buttner, P.; Feistner, L.; Lurz, P.; Thiele, H.; Hutcheson, J.D.; Schlotter, F. Dissecting Calcific Aortic Valve Disease-The Role, Etiology, and Drivers of Valvular Fibrosis. Front. Cardiovasc. Med. 2021, 8, 660797. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Bhutta, Z.A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Arabloo, J.; Omidi, N.; Rezapour, A.; Sarabi, A.A.; Mojtaba, G.S.; Azari, S. The burden of nonrheumatic valvular heart diseases in Iran between 1990 and 2017: Results from the global burden of disease study 2017. Int. J. Cardiol. Heart Vasc. 2022, 39, 100956. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.; Ahmadi, N.; Mohammadi, E.; Shabani, M.; Sherafati, A.; Aryannejad, A.; Rezaei, N.; Ghanbari, A.; Yoosefi, M.; Aminorroaya, A.; et al. Global and regional burden and quality of care of non-rheumatic valvular heart diseases: A systematic analysis of Global Burden of Disease 1990–2017. Int. J. Qual. Health Care 2022, 34, mzac026. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Massera, D.; Moss, A.J.; Bing, R.; Bularga, A.; Adamson, P.D.; Hunter, A.; Alam, S.; Shah, A.; Pawade, T.; et al. Prevalence and clinical implications of valvular calcification on coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 262–270. [Google Scholar] [CrossRef]
- Coffey, S.; Cox, B.; Williams, M.J. The prevalence, incidence, progression, and risks of aortic valve sclerosis: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2014, 63, 2852–2861. [Google Scholar] [CrossRef]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Das, G.P. Standardization and decomposition of rates from cross-classified data. Genus 1994, 50, 171–196. [Google Scholar]
- Chevan, A.; Sutherland, M. Revisiting Das Gupta: Refinement and extension of standardization and decomposition. Demography 2009, 46, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Rate of Kidney Function Decline and Risk of Hospitalizations in Stage 3A CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.M.; Fullman, N.; Sorensen, R.J.; Bollyky, T.; McKee, M.; Nolte, E.; Abajobir, A.A.; Avate, K.H.; Abbafati, C.; Davey, G.; et al. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: A novel analysis from the Global Burden of Disease Study 2015. Lancet 2017, 390, 231–266. [Google Scholar] [CrossRef]
- Ferreira-Gonzalez, I.; Pinar-Sopena, J.; Ribera, A.; Marsal, J.R.; Cascant, P.; Gonzalez-Alujas, T.; Evangelista, A.; Brotons, C.; Moral, I.; Permanyer-Miralda, G.; et al. Prevalence of calcific aortic valve disease in the elderly and associated risk factors: A population-based study in a Mediterranean area. Eur. J. Prev. Cardiol. 2013, 20, 1022–1030. [Google Scholar] [CrossRef]
- Lindman, B.R.; Sukul, D.; Dweck, M.R.; Madhavan, M.V.; Arsenault, B.J.; Coylewright, M.; Merryman, W.D.; Newby, D.E.; Lewis, J.; Harrell, F.J.; et al. Evaluating Medical Therapy for Calcific Aortic Stenosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2354–2376. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Ovcharenko, E.A.; Kutikhin, A.G. Development of calcific aortic valve disease: Do we know enough for new clinical trials? J. Mol. Cell. Cardiol. 2019, 132, 189–209. [Google Scholar] [CrossRef]
- Kraler, S.; Blaser, M.C.; Aikawa, E.; Camici, G.G.; Luscher, T.F. Calcific aortic valve disease: From molecular and cellular mechanisms to medical therapy. Eur. Heart J. 2022, 43, 683–697. [Google Scholar] [CrossRef]
- Ye, T.; Ma, T.; Wang, Q.; Zhang, C.M.; Cao, L.; Xu, B.D.; Zong, G.J. Prevalence and risk factors of aortic valve calcification among the elderly residents of Wuxi city, Jiangsu province. Zhonghua Xin Xue Guan Bing Za Zhi 2019, 47, 544–548. [Google Scholar]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Lindroos, M.; Kupari, M.; Valvanne, J.; Strandberg, T.; Heikkila, J.; Tilvis, R. Factors associated with calcific aortic valve degeneration in the elderly. Eur. Heart J. 1994, 15, 865–870. [Google Scholar] [CrossRef]
- Chen, H.Y.; Engert, J.C.; Thanassoulis, G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tastet, L.; Capoulade, R.; Clavel, M.A.; Larose, E.; Shen, M.; Dahou, A.; Arsenault, M.; Mathieu, P.; Bedard, E.; Dumesnil, J.G.; et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: Results from the PROGRESSA study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Mohseni, H.; Kiran, A.; Tran, J.; Nazarzadeh, M.; Rahimian, F.; Woodward, M.; Dwyer, T.; MacMahon, S.; Otto, C.M. Elevated blood pressure and risk of aortic valve disease: A cohort analysis of 5.4 million UK adults. Eur. Heart J. 2018, 39, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Bevan, G.H.; Zidar, D.A.; Josephson, R.A.; Al-Kindi, S.G. Mortality Due to Aortic Stenosis in the United States, 2008–2017. JAMA 2019, 321, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Borzouei, S.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Walsh, C.R.; Larson, M.G.; Kupka, M.J.; Levy, D.; Vasan, R.S.; Benjamin, E.J.; Manning, W.J.; Clouse, M.E.; O’Donnell, C.J. Association of aortic valve calcium detected by electron beam computed tomography with echocardiographic aortic valve disease and with calcium deposits in the coronary arteries and thoracic aorta. Am. J. Cardiol. 2004, 93, 421–425. [Google Scholar] [CrossRef]
- Shah, R.G.; Novaro, G.M.; Blandon, R.J.; Whiteman, M.S.; Asher, C.R.; Kirsch, J. Aortic valve area: Meta-analysis of diagnostic performance of multi-detector computed tomography for aortic valve area measurements as compared to transthoracic echocardiography. Int. J. Cardiovasc. Imaging 2009, 25, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-N.; Lee, H.-S.; Zhu, J. Handbook of Operations Analytics Using Data Envelopment Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
| 1990 | 2019 | 1990–2019 | 1990–2019 | |||
|---|---|---|---|---|---|---|
| Cases No. (95% UI) | ASPR (per 100,000) No. (95% UI) | Cases No. (95% UI) | ASPR (per 100,000) No. (95% UI) | ASPR Percentage Change (95% UI) | AAPC No. (95% CI) | |
| Global | 1,732,989 (1,431,469 to 2,074,809) | 45.54 (37.61 to 54.67) | 9,404,078 (8,079,604 to 10,889,727) | 116.34 (100.39 to 134.5) | 155.47 (141.66 to 171.7) | 3.36 (2.77 to 3.95) |
| Female | 838,493 (690,259 to 1,016,936) | 40.28 (32.99 to 48.82) | 4,376,817 (3,771,235 to 5,082,805) | 99.86 (86.1 to 115.88) | 147.89 (134.1 to 165.7) | 3.31 (2.86 to 3.75) |
| Male | 894,496 (741,593 to 1,067,498) | 51.19 (42.68 to 60.91) | 5,027,261 (4,276,877 to 5,861,586) | 133.38 (113.79 to 154.58) | 160.54 (146.7 to 176.16) | 3.38 (2.68 to 4.08) |
| High SDI | 1,324,934 (1,090,000 to 1,602,157) | 126.83 (104.61 to 152.72) | 5,095,444 (4,402,067 to 5,933,379) | 273.52 (237.08 to 315.22) | 115.66 (102.05 to 130.27) | 2.79 (2.18 to 3.41) |
| High-middle SDI | 364,934 (300,689 to 436,285) | 33.9 (27.98 to 40.44) | 3,569,820 (3,002,415 to 4,203,730) | 174.53 (147.33 to 204.54) | 414.83 (391.56 to 443.21) | 5.89 (5.55 to 6.23) |
| Middle SDI | 30,505 (24,085 to 37,764) | 2.82 (2.23 to 3.49) | 658,545 (529,926 to 800,781) | 25.93 (20.92 to 31.47) | 821.11 (709.87 to 944.23) | 8.04 (7.89 to 8.19) |
| Low-middle SDI | 9361 (7345 to 11,713) | 1.45 (1.15 to 1.79) | 67,258 (54,355 to 81,974) | 4.78 (3.86 to 5.82) | 229.82 (201.99 to 259.51) | 4.16 (4.08 to 4.24) |
| Low SDI | 2930 (2295 to 3719) | 1.12 (0.88 to 1.38) | 9675 (7605 to 12,135) | 1.67 (1.34 to 2.07) | 49.94 (44.14 to 56.39) | 1.39 (1.34 to 1.44) |
| High-income Asia Pacific | 469,556 (383,483 to 566,437) | 233.42 (191.41 to 280.25) | 1,715,700 (1,450,883 to 2,042,127) | 408.4 (348.93 to 479.89) | 74.96 (63.35 to 86.36) | 1.94 (1.8 to 2.08) |
| High-income North America | 687,664 (557,975 to 840,921) | 191.35 (155.87 to 232.93) | 1,492,891 (1,305,203 to 1,727,501) | 244.39 (214.46 to 279.6) | 27.72 (13.83 to 44.93) | 1.86 (0.81 to 2.92) |
| Western Europe | 218,212 (177,736 to 268,116) | 37.65 (30.75 to 45.97) | 1,862,787 (1,577,560 to 2,209,085) | 204.84 (174.62 to 240.59) | 444 (398.78 to 494.86) | 6.38 (5.73 to 7.03) |
| Australasia | 9877 (8177 to 11,826) | 41.77 (34.78 to 49.88) | 320,825 (272,234 to 381,057) | 649.5 (552 to 772.74) | 1454.86 (1236.35 to 1697.07) | 9.97 (9.85 to 10.08) |
| Andean Latin America | 831 (661 to 1026) | 3.77 (3.02 to 4.63) | 33,352 (28,251 to 39,016) | 59.04 (50.07 to 69.05) | 1465.67 (1241.46 to 1733.05) | 10.1 (9.49 to 10.72) |
| Tropical Latin America | 8034 (6435 to 9814) | 7.79 (6.23 to 9.54) | 58,601 (47,232 to 72,054) | 23.73 (19.16 to 29.01) | 204.51 (180.25 to 234) | 3.81 (3.7 to 3.92) |
| Central Latin America | 6996 (5589 to 8531) | 7.98 (6.44 to 9.71) | 76,957 (63,860 to 91,384) | 31.82 (26.45 to 37.75) | 298.86 (261.69 to 344.2) | 5.06 (4.14 to 6) |
| Southern Latin America | 5497 (4391 to 6936) | 11.87 (9.53 to 14.91) | 101,893 (87,124 to 120,797) | 122.72 (104.78 to 145.67) | 933.61 (766.84 to 1118.16) | 8.22 (8.07 to 8.38) |
| Caribbean | 3359 (2751 to 4009) | 12.61 (10.3 to 15.1) | 45,467 (37,694 to 54,849) | 87.51 (72.62 to 105.56) | 594.1 (504.42 to 696.1) | 7.07 (6.73 to 7.41) |
| Central Europe | 154,665 (128,343 to 184,574) | 104.18 (86.62 to 123.47) | 1,260,558 (1,067,648 to 1,479,616) | 608.31 (517.94 to 713.51) | 483.91 (434.98 to 537.45) | 6.63 (6.24 to 7.02) |
| Eastern Europe | 121,662 (97,120 to 148,877) | 43.32 (34.79 to 52.76) | 1,328,687 (1,065,696 to 1,605,206) | 395.8 (319.64 to 477) | 813.6 (752.33 to 886.02) | 8.12 (7.89 to 8.34) |
| Central Asia | 3767 (2956 to 4713) | 7.92 (6.22 to 9.83) | 41,060 (33,495 to 48,810) | 53.22 (43.89 to 62.92) | 572.13 (489.66 to 669.91) | 6.82 (6.7 to 6.93) |
| North Africa and Middle East | 9696 (7638 to 11,898) | 4.92 (3.9 to 6.04) | 54,300 (43,226 to 66,785) | 10.83 (8.67 to 13.35) | 120.05 (104.74 to 135.33) | 3.01 (2.55 to 3.47) |
| South Asia | 8332 (6398 to 10,638) | 1.34 (1.04 to 1.67) | 30,188 (23,678 to 37,633) | 2.03 (1.6 to 2.52) | 51.55 (45.4 to 58.54) | 1.44 (1.4 to 1.48) |
| Southeast Asia | 1785 (1337 to 2357) | 0.7 (0.53 to 0.91) | 23,986 (18,739 to 30,273) | 4.06 (3.21 to 5.09) | 480.45 (408.41 to 566.35) | 6.17 (6.05 to 6.29) |
| East Asia | 18,272 (13,682 to 24,325) | 2.1 (1.57 to 2.74) | 891,018 (707,255 to 1,093,313) | 42.41 (33.87 to 51.68) | 1920.64 (1545.86 to 2360.13) | 11.07 (10.58 to 11.57) |
| Oceania | 110 (89 to 136) | 4.3 (3.49 to 5.36) | 1130 (904 to 1374) | 18.61 (14.95 to 22.75) | 332.86 (271.32 to 396.31) | 4.7 (4.17 to 5.24) |
| Western Sub-Saharan Africa | 1300 (1004 to 1627) | 1.27 (0.99 to 1.58) | 3870 (3002 to 4876) | 1.65 (1.3 to 2.04) | 29.76 (25.16 to 34.98) | 0.85 (0.76 to 0.94) |
| Eastern Sub-Saharan Africa | 763 (589 to 963) | 0.93 (0.73 to 1.15) | 2602 (2004 to 3271) | 1.4 (1.1 to 1.74) | 51.64 (43.67 to 61.1) | 1.47 (1.41 to 1.53) |
| Central Sub-Saharan Africa | 245 (189 to 315) | 1 (0.79 to 1.26) | 796 (621 to 1012) | 1.36 (1.08 to 1.69) | 36.9 (28.71 to 46.73) | 1.12 (1.06 to 1.18) |
| Southern Sub-Saharan Africa | 2365 (1845 to 2936) | 8.15 (6.36 to 10.13) | 57409 (44,278 to 73,386) | 94.86 (72.97 to 120.7) | 1064.03 (877.48 to 1280.35) | 9.02 (8.62 to 9.41) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xu, H.; Jia, R.; Jin, Z.; Zhang, C.; Yuan, J. Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990–2019 Findings from Global Burden of Disease Study 2019. J. Clin. Med. 2022, 11, 6733. https://doi.org/10.3390/jcm11226733
Yang C, Xu H, Jia R, Jin Z, Zhang C, Yuan J. Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990–2019 Findings from Global Burden of Disease Study 2019. Journal of Clinical Medicine. 2022; 11(22):6733. https://doi.org/10.3390/jcm11226733
Chicago/Turabian StyleYang, Chengzhi, Haobo Xu, Ruofei Jia, Zening Jin, Changlin Zhang, and Jiansong Yuan. 2022. "Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990–2019 Findings from Global Burden of Disease Study 2019" Journal of Clinical Medicine 11, no. 22: 6733. https://doi.org/10.3390/jcm11226733
APA StyleYang, C., Xu, H., Jia, R., Jin, Z., Zhang, C., & Yuan, J. (2022). Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990–2019 Findings from Global Burden of Disease Study 2019. Journal of Clinical Medicine, 11(22), 6733. https://doi.org/10.3390/jcm11226733




