Robotics in Total Hip Arthroplasty: Current Concepts
Abstract
1. Introduction
2. History
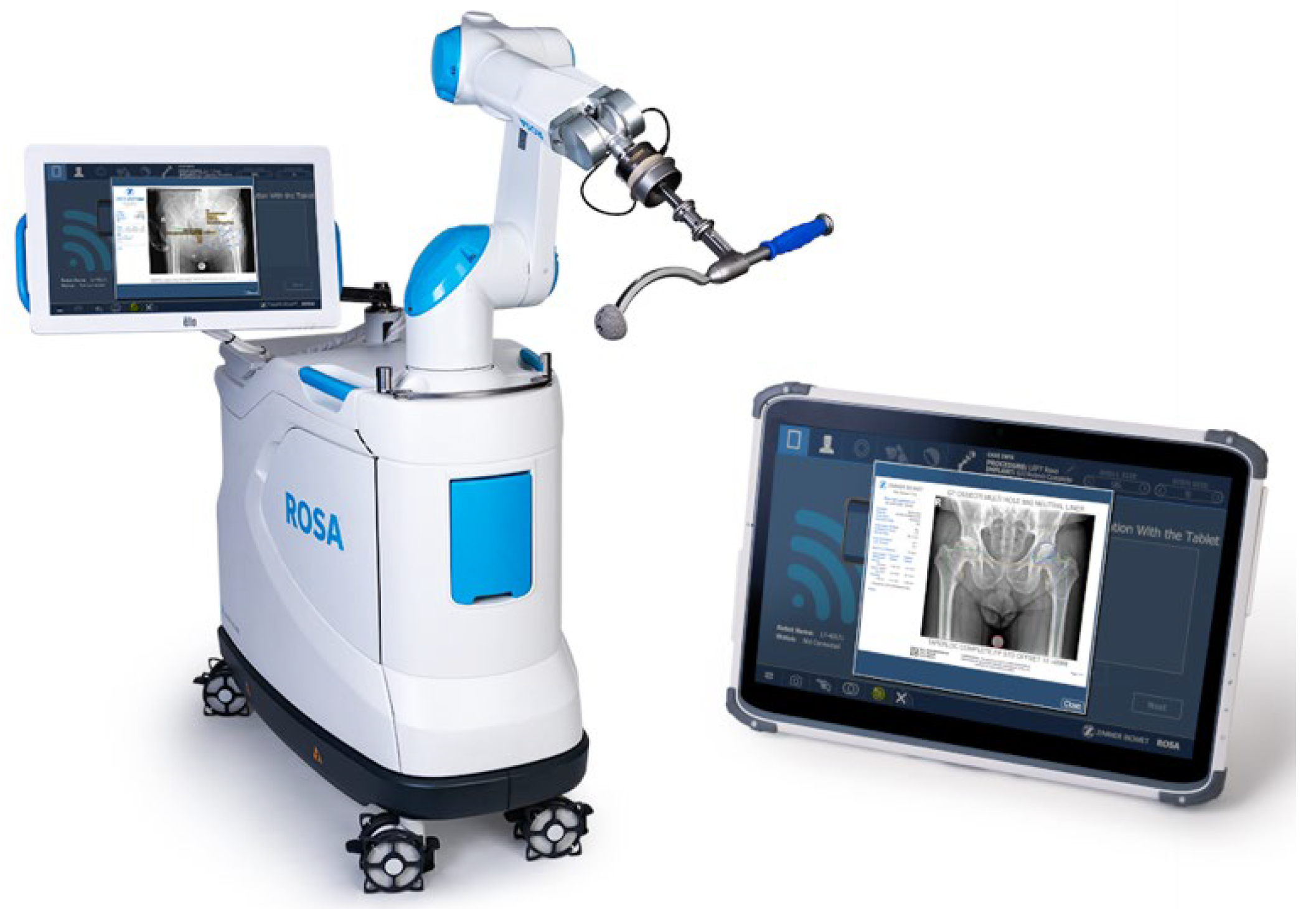
3. Current Role
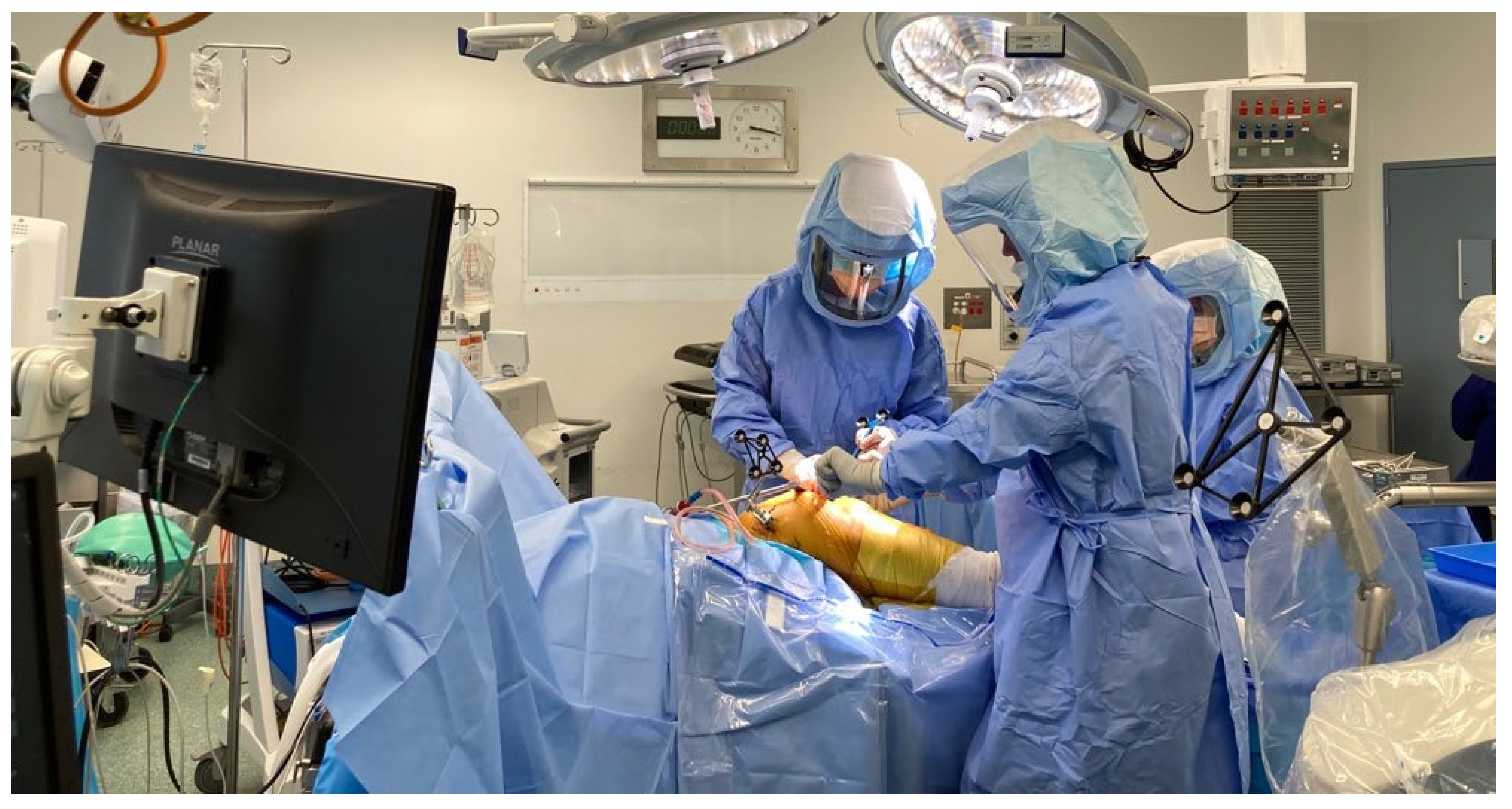
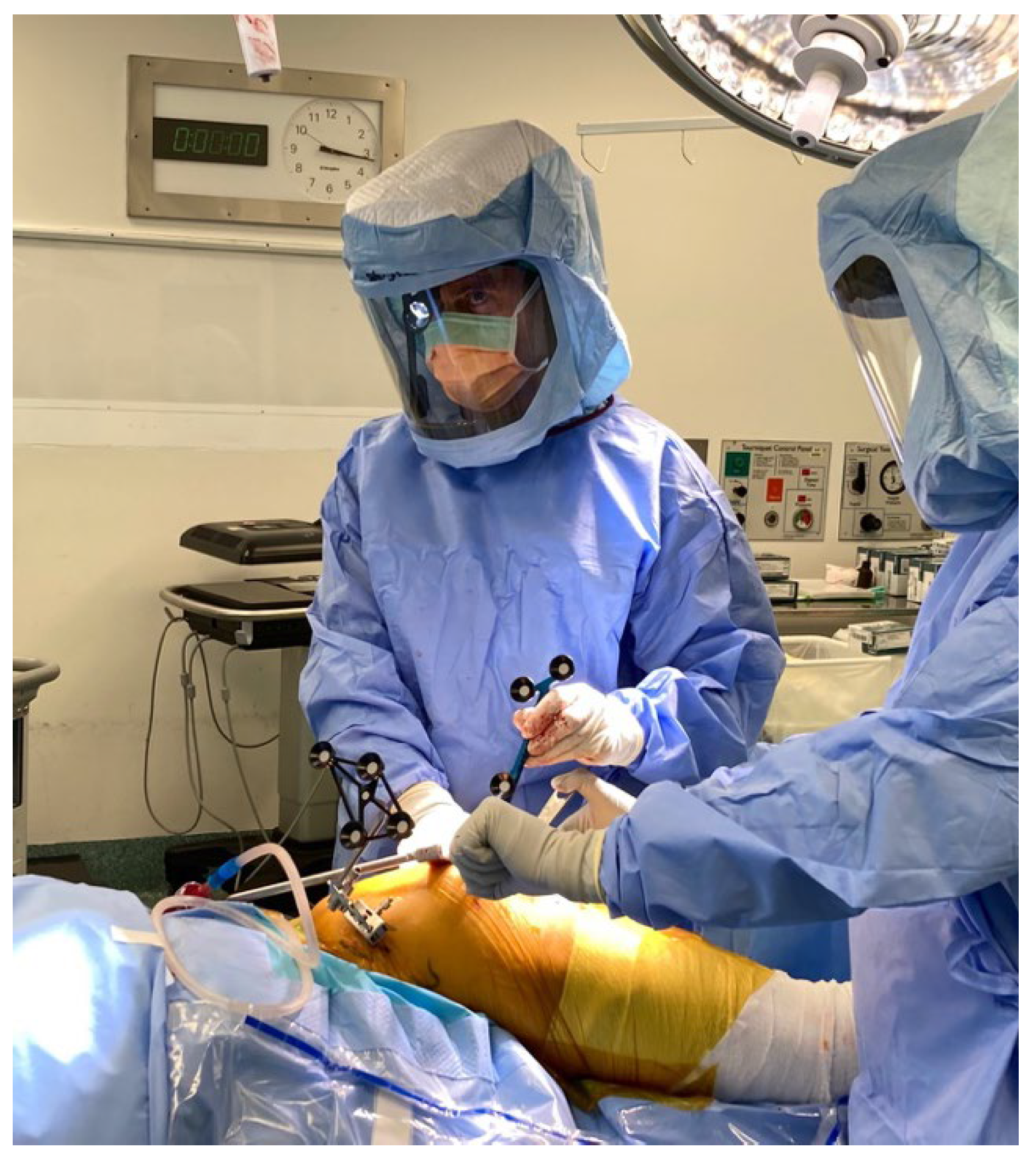
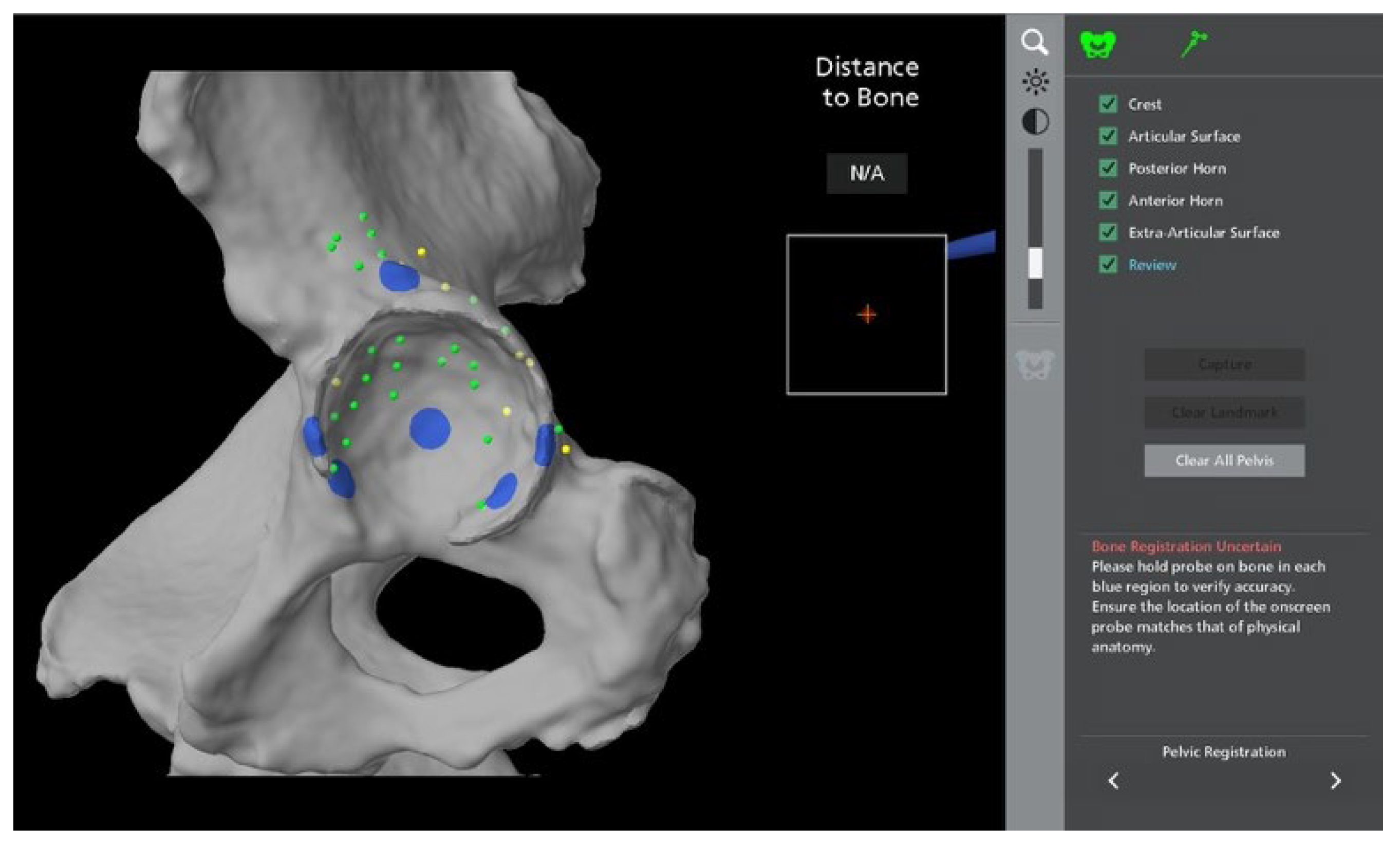
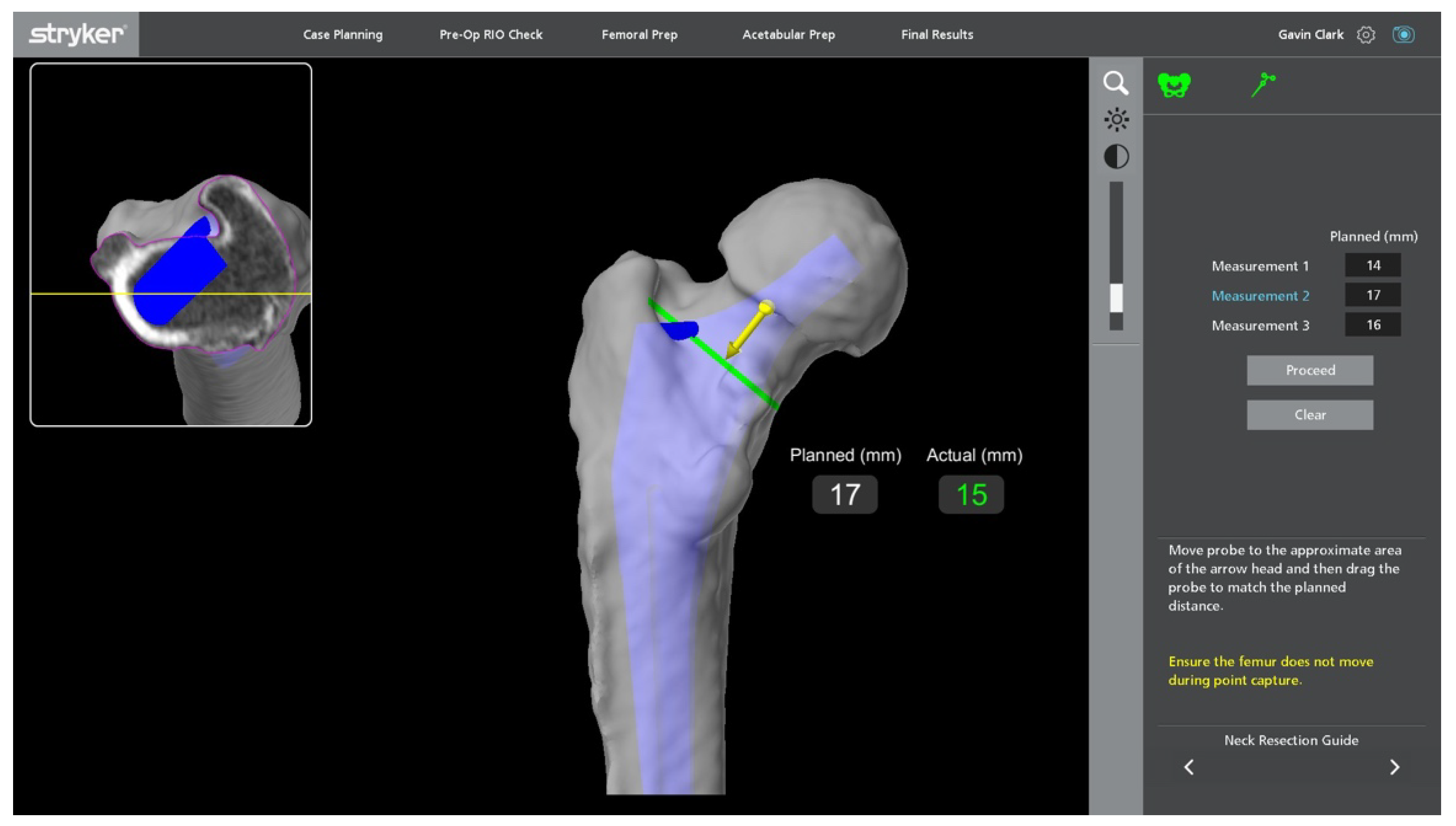
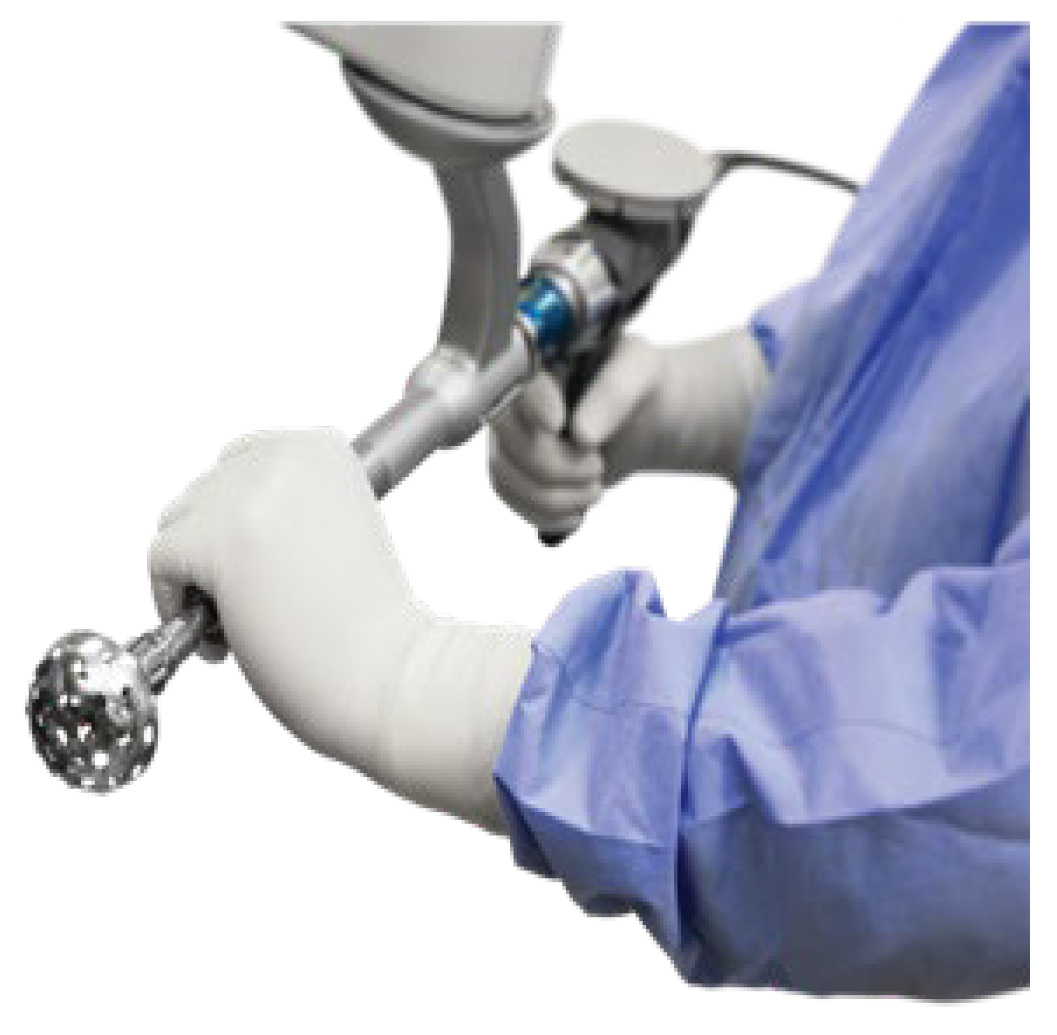
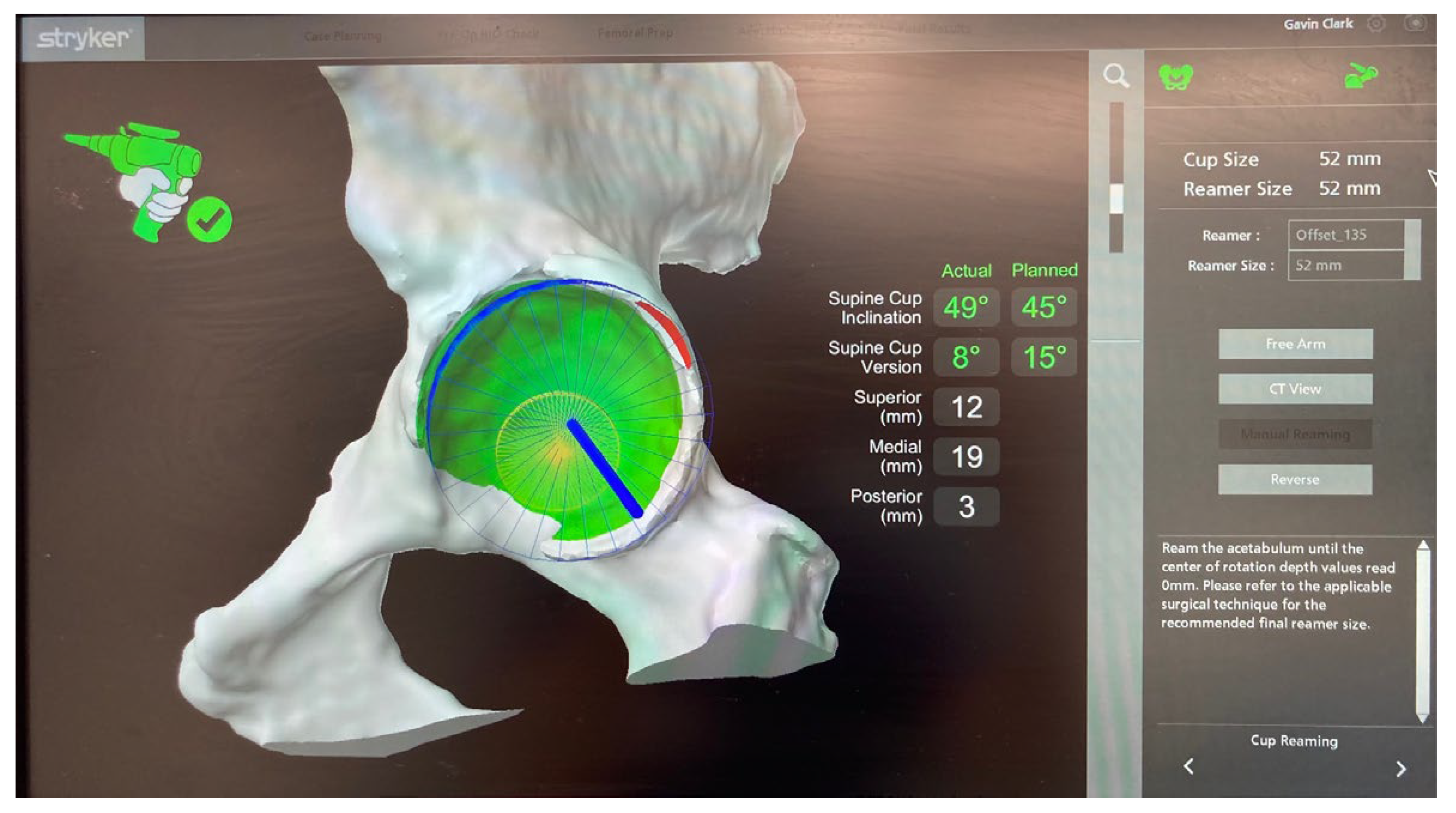
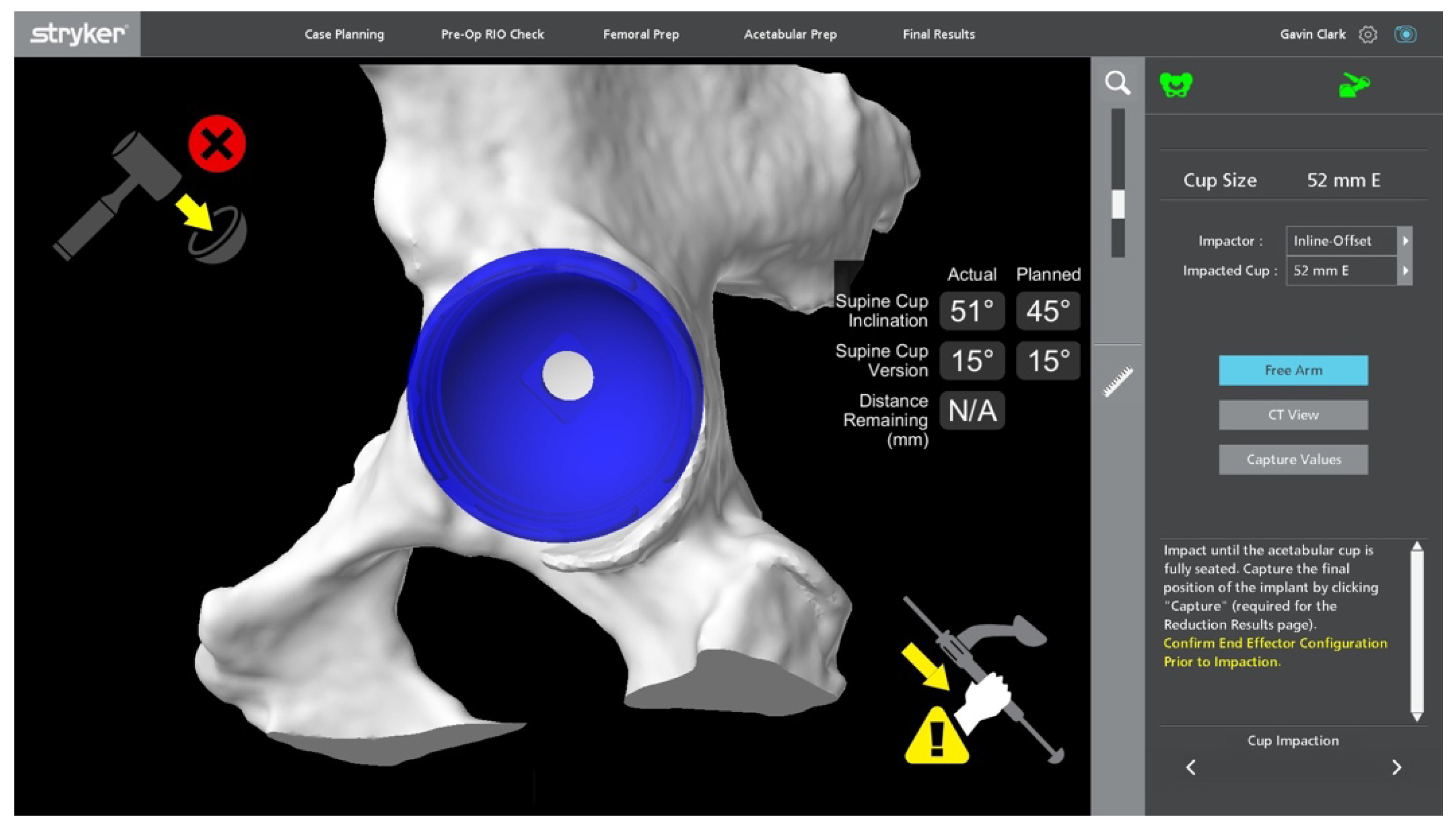
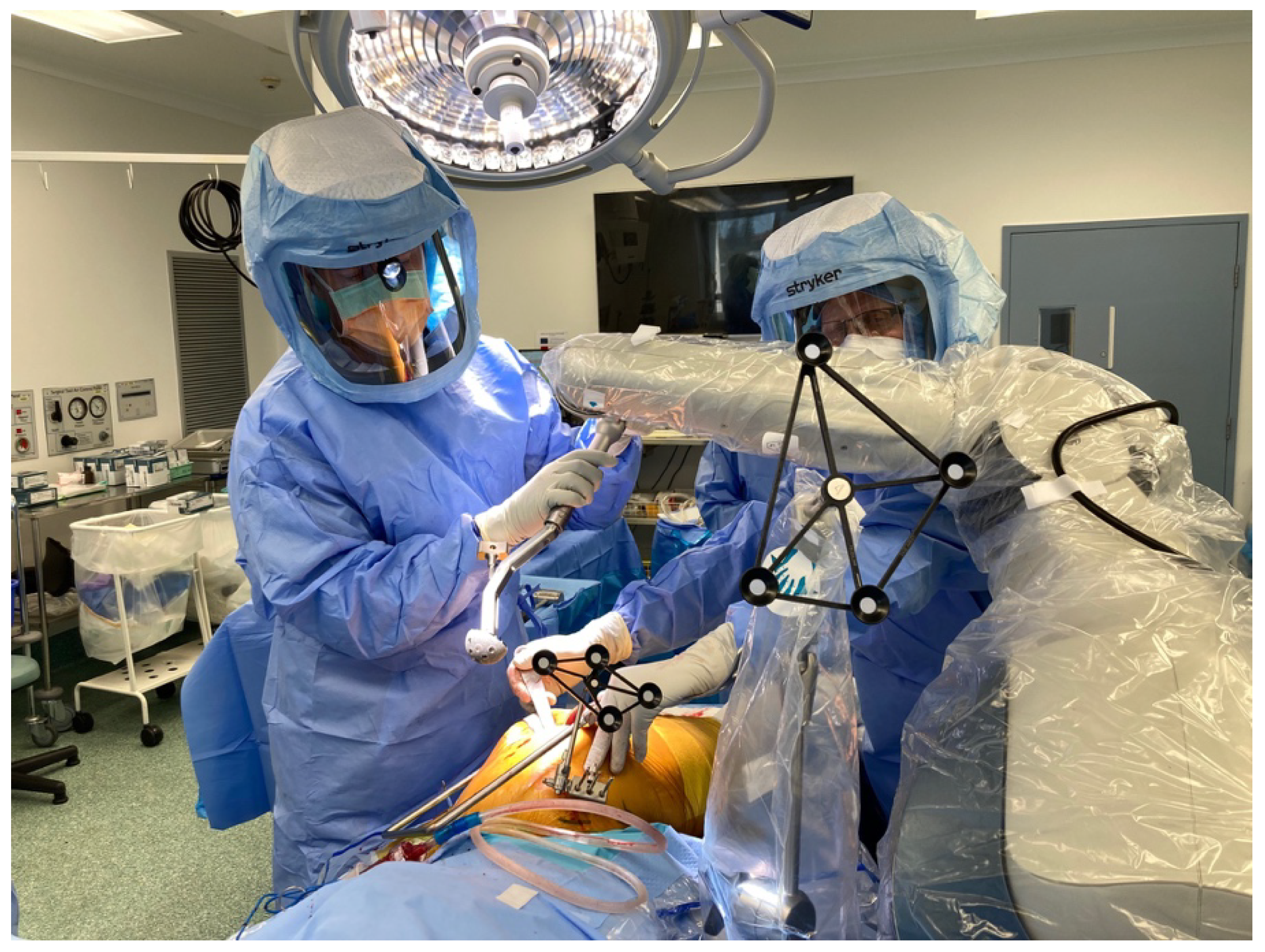
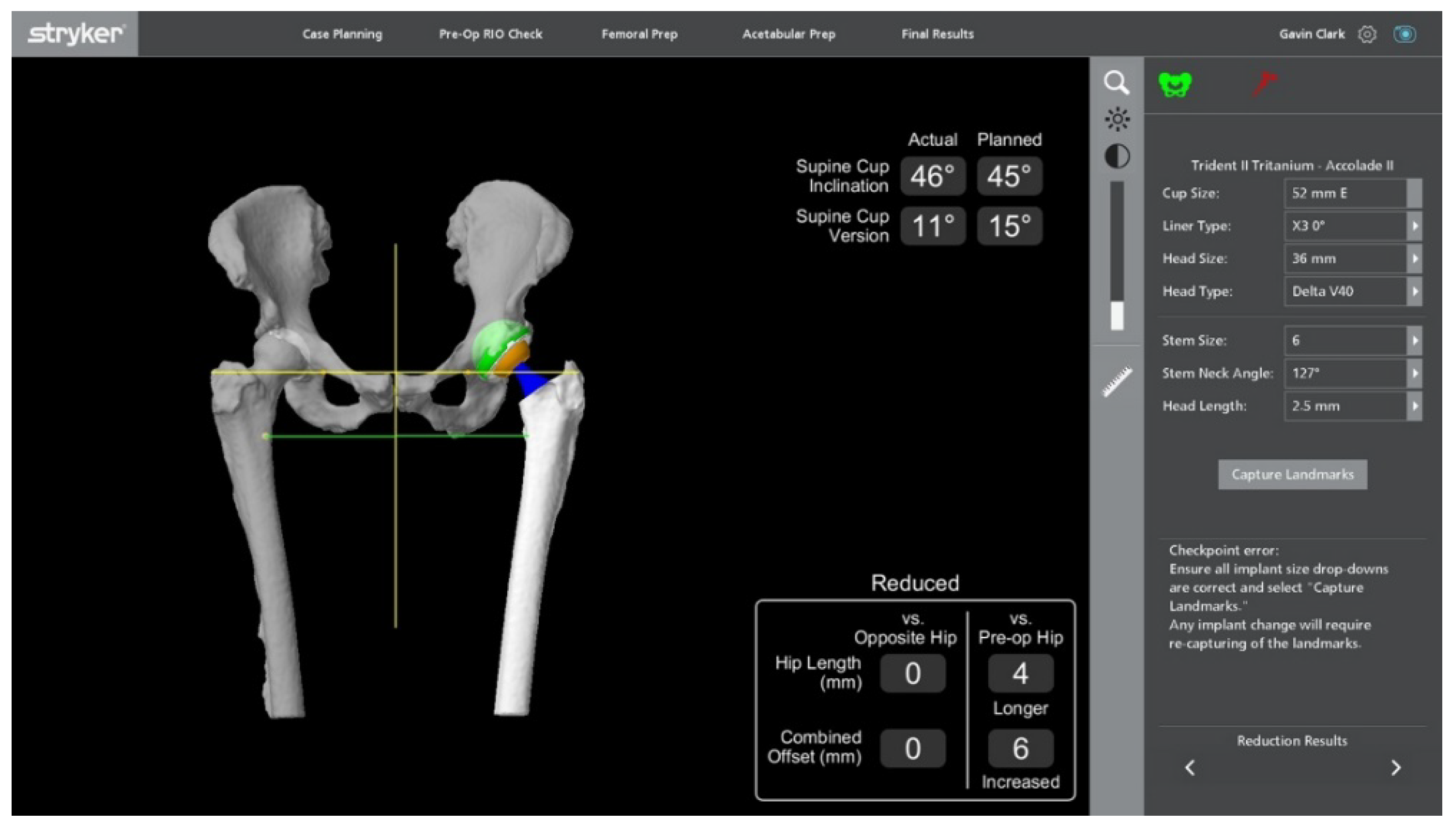
Stages of Robotic Total Hip Arthroplasty
4. Outcomes and Current Controversies
4.1. Radiological Outcomes
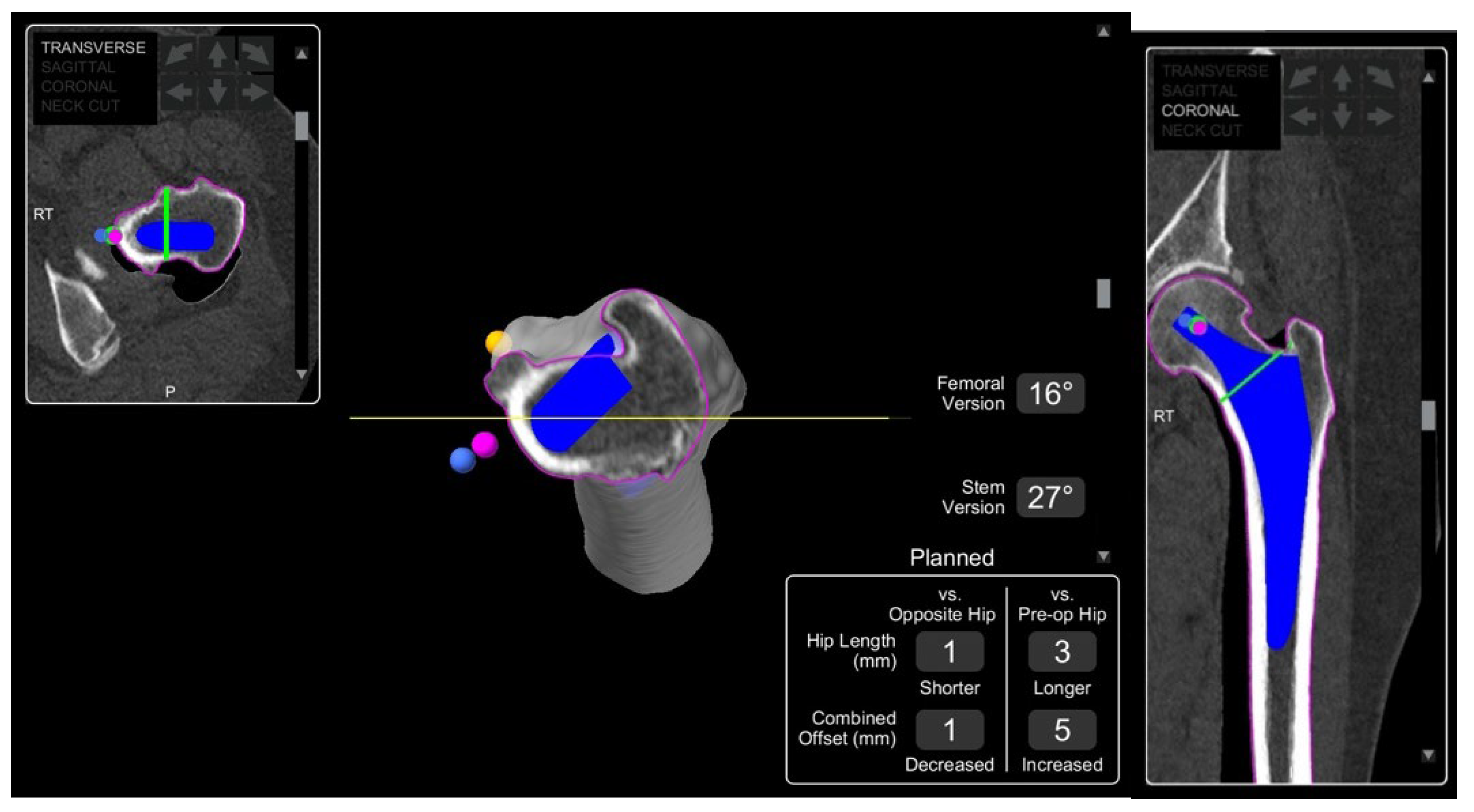
4.1.1. Accuracy of Implant Placement
4.1.2. Heterotopic Ossification (HO)
4.1.3. Leg Length Discrepancy (LLD)
4.2. Functional Outcomes
4.3. Complications
4.3.1. Infection
4.3.2. Blood Loss
4.3.3. Operative Time
4.3.4. Learning Curve
4.3.5. Dislocation and Revision Rates
4.3.6. Radiation Exposure
4.3.7. Cost
5. A Look to the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKee, G.K.; Watson-Farrar, J. Replacement of Arthritic Hips by the McKee-Farrar Prosthesis. J. Bone Joint Surg. Br. 1966, 48, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Charnley, J. The Long Term Results of Low-Friction Arthroplasty of the Hip Performed as a Primary Intervention. J. Bone Joint Surg. 1974, 54, 61–76. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report, Adelaide. Available online: https://aoanjrr.sahmri.com/annual-reports-2021 (accessed on 26 February 2022).
- Wan, Z.; Boutary, M. The Influence of Acetabular Component Position on Wear in Total Hip Arthroplasty. J. Arthroplast. 2008, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Maheshwari, A. Impingement with Total Hip Replacement. J. Bone Joint Surg. 2007, 89, 1832–1842. [Google Scholar] [CrossRef]
- Pransky, J. ROBODOC—Surgical robot success story. Ind. Robot. 1997, 24, 231–233. [Google Scholar] [CrossRef]
- Schulz, A.P.; Seide, K. Results of total hip replacement using the Robodoc surgical assistant system: Clinical outcome and evaluation of complications for 97 procedures: Evaluation of total hip replacement using the Robodoc system. Int. J. Med. Robot. Comput. Assist. Surg. 2007, 43, 301–306. [Google Scholar] [CrossRef]
- Paul, H.; Bargar, W.L. Development of a Surgical Robot for Cementless Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1992, 285, 57–66. [Google Scholar] [CrossRef]
- Bargar, W.L.; Bauer, A. Primary and Revision Total Hip Replacement Using the Robodoc System. Clin. Orthop. Relat. Res. 1998, 354, 82–91. [Google Scholar] [CrossRef]
- Bargar, W.L.; Parise, C.A. Fourteen Year Follow-Up of Randomized Clinical Trials of Active Robotic-Assisted Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 810–814. [Google Scholar] [CrossRef]
- Fontalis, A.; Kayani, B. Robotic total hip arthroplasty: Past, present and future. Orthop. Trauma 2022, 36, 6–13. [Google Scholar] [CrossRef]
- Siebel, T.; Käfer, W. Clinical outcome after robot-assisted versus conventionally implanted hip arthroplasty: Prospective, controlled study of 71 patients. Z. Orthop. Ihre Grenzgeb. 2005, 143, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Wainwright, T.W. A review of the evolution of robotic-assisted total hip arthroplasty. Hip Int. 2019, 29, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, J. Robotic-assisted compared with conventional total hip arthroplasty: Systematic review and meta-analysis. Postgrad. Med. J. 2018, 94, 335–341. [Google Scholar] [CrossRef]
- Mako® Stryker Robotic-Arm Assisted Surgery. Available online: https://www.boa.ac.uk/static/e823e0b7-278c-4666-9da48e0d65b630b8/Mako-System-design-rationale.pdf (accessed on 26 February 2022).
- Kamath, A.F.; Durbhakula, S.M. Improved accuracy and fewer outliers with a novel CT-free robotic THA system in matched-pair analysis with manual THA. J. Robot. Surg. 2022, 16, 905–913. [Google Scholar] [CrossRef]
- Li, C.; Wang, L. Clinical application of robotic orthopedic surgery: A bibliometric study. BMC Musculoskelet. Disord. 2021, 22, 968. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S. The current role of robotics in total hip arthroplasty. EFORT Open Rev. 2019, 4, 618–625. [Google Scholar] [CrossRef]
- ROSA® Hip System: User Manual and Surgical Technique V1.0. Available online: https://www.zimmerbiomet.com/content/dam/zb-corporate/en/products/specialties/robotics/3500.2-GLBL-en-ROSA-Hip-System-User-Manual-and-Surgical-Technique.pdf (accessed on 27 June 2022).
- Haffer, H.; Adl Amini, D. The Impact of Spinopelvic Mobility on Arthroplasty: Implications for Hip and Spine Surgeons. JCM 2020, 9, 2569. [Google Scholar] [CrossRef]
- Stefl, M.; Lundergan, W. Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone Jt. J. 2017, 99-B, 37–45. [Google Scholar] [CrossRef]
- Sicat, C.S.; Buchalter, D.B. Intraoperative Technology Use Improves Accuracy of Functional Safe Zone Targeting in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, S540–S545. [Google Scholar] [CrossRef]
- Tarwala, R.; Dorr, L.D. Robotic assisted total hip arthroplasty using the MAKO platform. Curr. Rev. Musculoskelet. Med. 2011, 4, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, P.; Mansour, J. Current concepts in robotic total hip arthroplasty. SICOT-J. 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Perazzini, P.; Trevisan, M. The Mako™ robotic arm-assisted total hip arthroplasty using direct anterior approach: Surgical technique, skills and pitfalls. Acta Biomed. 2020, 91, 21–30. [Google Scholar] [PubMed]
- MakoTM Total Hip Posterolateral Approach Surgical Reference Guide. Available online: https://www.strykermeded.com/media/2041/mako-tha-posterolateral-approach-surgical-technique.pdf (accessed on 29 June 2022).
- Callanan, M.C.; Jarrett, B. The John Charnley Award: Risk Factors for Cup Malpositioning: Quality Improvement Through a Joint Registry at a Tertiary Hospital. Clin. Orthop. Relat. Res. 2011, 469, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lewinnek, G.E.; Lewis, J.L. Dislocations after total hip-replacement arthroplasties. J. Bone Joint Surg. Am. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Jolles, B.M.; Zangger, P. Factors predisposing to dislocation after primary total hip arthroplasty. J. Arthroplast. 2002, 17, 282–288. [Google Scholar] [CrossRef]
- Emara, A.K.; Samuel, L.T. Robotic-arm assisted versus manual total hip arthroplasty: Systematic review and meta-analysis of radiographic accuracy. Int. J. Med. Robot. 2021, 17, e2332. [Google Scholar] [CrossRef]
- Chen, X.; Deng, S. Robotic arm-assisted arthroplasty: The latest developments. Chin. J. Traumatol. 2021, 25, 125–131. [Google Scholar] [CrossRef]
- Han, P.-F.; Chen, C.-L. Robotics-assisted versus conventional manual approaches for total hip arthroplasty: A systematic review and meta-analysis of comparative studies. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e1990. [Google Scholar] [CrossRef]
- Honl, M.; Dierk, O. Comparison of robotic-assisted and manual implantation of a primary total hip replacement: A prospective study. J. Bone Jt. Surg 2003, 85, 1470–1478. [Google Scholar] [CrossRef]
- Hofmann, A.A.; Skrzynski, M.C. Hip Arthroplasty: Headaches & Migraines: Leg-Length Inequality and Nerve Palsy in Total Hip Arthroplasty: A Lawyer Awaits! Orthopedics 2000, 23, 943–944. [Google Scholar] [PubMed]
- Sarangi, P.P.; Bannister, G.C. Leg Length Discrepancy after Total Hip Replacement. HIP Int. 1997, 3, 121–124. [Google Scholar] [CrossRef]
- Clement, N.D.; Gaston, P. Robotic arm-assisted versus manual total hip arthroplasty: A propensity score matched cohort study. Bone Jt. Res. 2021, 10, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S. Does robotic-assisted surgery improve outcomes of total hip arthroplasty compared to manual technique? A systematic review and meta-analysis. Postgrad. Med. J. 2021. [Google Scholar] [CrossRef]
- Domb, B.G.; Redmond, J.M. Accuracy of Component Positioning in 1980 Total Hip Arthroplasties: A Comparative Analysis by Surgical Technique and Mode of Guidance. J. Arthroplast. 2015, 30, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Domb, B.G.; Chen, J.W. Minimum 5-Year Outcomes of Robotic-assisted Primary Total Hip Arthroplasty With a Nested Comparison Against Manual Primary Total Hip Arthroplasty: A Propensity Score–Matched Study. J. Am. Acad. Orthop. Surg. 2020, 28, 847–856. [Google Scholar] [CrossRef]
- Nishihara, S.; Sugano, N.; Nishii, T.; Miki, H.; Nakamura, N.; Yoshikawa, H. Comparison Between Hand Rasping and Robotic Milling for Stem Implantation in Cementless Total Hip Arthroplasty. J. Arthroplast. 2006, 21, 957–966. [Google Scholar] [CrossRef]
- Samuel, L.T.; Acuna, A.J. Comparing early and mid-term outcomes between robotic-arm assisted and manual total hip arthroplasty: A systematic review. J. Robot. Surg. 2022, 16, 735–748. [Google Scholar] [CrossRef]
- Illgen, R.L.; Bukowski, B.R. Robotic-Assisted Total Hip Arthroplasty: Outcomes at Minimum Two-Year Follow-Up. Surg. Technol. Int. 2017, 30, 365–372. [Google Scholar]
- Bukowski, B.R.; Anderson, P. Improved Functional Outcomes with Robotic Compared with Manual Total Hip Arthroplasty. Surg. Technol. Int. 2016, 29, 303–308. [Google Scholar]
- Lim, S.-J.; Ko, K.-R. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: A prospective randomized short-term outcome study. Comput. Aided. Surg. 2015, 20, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Gaston, P. Robotic arm-assisted versus manual total hip arthroplasty: A systematic review and meta-analysis. Bone Jt. J. 2021, 103-B, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.M.; Gupta, A. The Learning Curve Associated With Robotic-Assisted Total Hip Arthroplasty. J. Arthroplast. 2015, 30, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kamara, E.; Robinson, J. Adoption of Robotic vs Fluoroscopic Guidance in Total Hip Arthroplasty: Is Acetabular Positioning Improved in the Learning Curve? J. Arthroplast. 2017, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S. The learning curve of robotic-arm assisted acetabular cup positioning during total hip arthroplasty. HIP Int. 2021, 31, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kolodychuk, N.; Su, E. Can robotic technology mitigate the learning curve of total hip arthroplasty? Bone Jt. Open 2021, 2, 365–370. [Google Scholar] [CrossRef]
- Booij, R.; Budde, R.P.J. Technological developments of X-ray computed tomography over half a century: User’s influence on protocol optimization. Eur. J. Radiol. 2020, 131, 109261. [Google Scholar] [CrossRef]
- Perets, I.; Mu, B.H. Current topics in robotic-assisted total hip arthroplasty: A review. HIP Int. 2020, 30, 118–124. [Google Scholar] [CrossRef]
- Pierce, J.; Needham, K. Robotic-assisted total hip arthroplasty: An economic analysis. J. Comp. Eff. Res. 2022, 10, 1225–1234. [Google Scholar] [CrossRef]
- Maldonado, D.R.; Go, C.C. Robotic Arm-assisted Total Hip Arthroplasty is More Cost-Effective Than Manual Total Hip Arthroplasty: A Markov Model Analysis. J. Am. Acad. Orthop. Surg. 2021, 29, 4. [Google Scholar] [CrossRef]
- Kirchner, G.J.; Lieber, A.M. The Cost of Robot-assisted Total Hip Arthroplasty: Comparing Safety and Hospital Charges to Conventional Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2021, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Joint Surg. Am 2022, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, I.N.; Bohensky, M.A. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Nawabi, D.H.; Conditt, M.A. Haptically guided robotic technology in total hip arthroplasty: A cadaveric investigation. Proc. Inst. Mech. Eng. Part H 2013, 227, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Redmond, J.M. Does Robotic-Assisted Computer Navigation Affect Acetabular Cup Positioning in Total Hip Arthroplasty in the Obese Patient? A Comparison Study. J. Arthroplast. 2015, 30, 2204–2207. [Google Scholar] [CrossRef]
- Haddad, F.S.; Horriat, S. Robotic and other enhanced technologies: Are we prepared for such innovation? J. Bone Joint Surg. Br. 2019, 101-B, 1469–1471. [Google Scholar] [CrossRef]

| Author | Year | Design | Robot Type | Evidence |
|---|---|---|---|---|
| Bukowski et al. [44] | 2016 | Retrospective | MAKO | rTHA reduced blood loss vs. cTHA (374 +/− 133 mL vs. 423 +/− 186 mL, p = 0.035) |
| Chen et al. [15] | 2018 | Systematic review and meta-analysis | No significant difference in surgical time rTHA vs. cTHA (however, favoured cTHA) Higher HO rate post rTHA (32/142 rTHA vs. 18/133 cTHA, p = 0.04) | |
| Chen et al. [32] | 2021 | Review | rTHA more accurate acetabular cup placement Equivocal functional scores rTHA vs. cTHA at 5 years Equivocal blood loss rTHA vs. cTHA | |
| Clement et al. [37] | 2021 | Propensity score-matched prospective | MAKO | rTHA significantly superior restoration of leg length (2.3 mm greater rTHA vs. 3.6 mm cTHA) rTHA–significantly higher OHS (2.5 points) and FJS (21.1 points) with FJS having clinical significance |
| Domb et al. [39] | 2015 | Retrospective | MAKO | Comparable LLD rates rTHA vs. cTHA (97% <10 mm, no significant difference rTHA vs. cTHA) |
| Domb et al. [40] | 2020 | Propensity score-matched retrospective | MAKO | rTHA higher HHS, FJS and VR-12 Physical (all significant) No significant difference in revision rates over 5 years |
| Emara et al. [31] | 2021 | Systematic review and meta-analysis | rTHA superior acetabular cup positioning (significant) rTHA significantly lower LLD vs. cTHA (−0.33 mm vs. −1.24 mm) | |
| Han et al. [33] | 2019 | Systematic review and meta-analysis | No significant difference in development of HO rTHA vs. cTHA No difference rTHA vs. cTHA in HHS, WOMAC, Merle D’Aubigne scores post-operatively (none reaching significance) | |
| Illgen et al. [43] | 2017 | Retrospective | MAKO | rTHA vs. cTHA no difference in infection rates |
| Kamara et al. [48] | 2017 | Retrospective | MAKO | rTHA competency achieved after 10 procedures |
| Kayani et al. [49] | 2021 | Prospective | MAKO | rTHA competency achieved after 12 procedures |
| Kirchner et al. [55] | 2021 | Retrospective | Not specified | rTHA higher cost (USD $20,046 vs. cTHA USD $18,258), despite shorter hospital LOS |
| Kolodychuk et al. [50] | 2021 | Prospective | Not specified | rTHA mitigated learning curve, with no significant difference in radiological outcomes and operative time between new and experienced surgeons |
| Kumar et al. [38] | 2021 | Systematic review and meta-analysis | rTHA reduced LLD vs. cTHA (mean difference 1.44 mm, p = 0.01) rTHA longer operative time (mean difference 19.48 min, p = 0.02) No significant difference in dislocation and revision rates rTHA vs. cTHA | |
| Lim et al. [45] | 2015 | Prospective | ROBODOC | No significant difference in blood loss (rTHA 1010cc vs. cTHA 895cc) |
| Maldonado et al. [54] | 2021 | Computer simulation | rTHA significant cost reduction, saving USD 945 per public patient, and USD 1810 for private patients | |
| Ng et al. [46] | 2021 | Systematic review and meta-analysis | Learning curve to rTHA competency 12–35 patients | |
| Nishihara et al. [41] | 2006 | Prospective | ORTHODOC ISS | Equivocal time to walking 500 m, rTHA > cTHA number of patients walking 6 blocks in 13 days (significant) Equivocal Merle d’Aubigne hip score 3 months post-operatively (rTHA 15.8 vs. cTHA 15.3, insignificant), rTHA significantly improved scores on the same scale at 2 years (rTHA 17.4 vs. 17.1) |
| Pierce et al. [53] | 2022 | Propensity score-matched retrospective | Not specified | rTHA overall lower 90-day cost (assessing index procedure, hospital LOS and rehabilitation) averaging USD 785 less per patient. |
| Redmond et al. [47] | 2015 | Retrospective | MAKO | Significantly lower risk of malpositioned acetabular cup (103/105 in Lewinnek’s safe zone, and 99/105 in Callanan’s safe zone) and a shorter operating time with the final 70 rTHA cases, which reached significance. |
| Samuel et al. [42] | 2021 | Systematic review | No significant difference in functional outcomes, and between MAKO and ROBODOC No significant difference in infection rates Equivocal dislocation and revision rates rTHA vs. cTHA | |
| Schulz et al. [8] | 2007 | Prospective | ROBODOC | rTHA higher intraoperative blood loss and transfusion requirement |
| Tarwala et al. [24] | 2011 | Review | rTHA 3x increase in radiation exposure due to pre-operative planning CT scan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullock, E.K.C.; Brown, M.J.; Clark, G.; Plant, J.G.A.; Blakeney, W.G. Robotics in Total Hip Arthroplasty: Current Concepts. J. Clin. Med. 2022, 11, 6674. https://doi.org/10.3390/jcm11226674
Bullock EKC, Brown MJ, Clark G, Plant JGA, Blakeney WG. Robotics in Total Hip Arthroplasty: Current Concepts. Journal of Clinical Medicine. 2022; 11(22):6674. https://doi.org/10.3390/jcm11226674
Chicago/Turabian StyleBullock, Emily K. C., Michael J. Brown, Gavin Clark, James G. A. Plant, and William G. Blakeney. 2022. "Robotics in Total Hip Arthroplasty: Current Concepts" Journal of Clinical Medicine 11, no. 22: 6674. https://doi.org/10.3390/jcm11226674
APA StyleBullock, E. K. C., Brown, M. J., Clark, G., Plant, J. G. A., & Blakeney, W. G. (2022). Robotics in Total Hip Arthroplasty: Current Concepts. Journal of Clinical Medicine, 11(22), 6674. https://doi.org/10.3390/jcm11226674