Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice
Abstract
1. Introduction
2. Vulnerable Plaque and Its Visualization
2.1. Pathology of Vulnerable Plaque
2.2. Intravascular Ultrasound (GS-IVUS, VH-IVUS, HD-IVUS)
2.2.1. Grey Scale Intravascular Ultrasound (GS-IVUS)
2.2.2. Virtual Histology Intravascular Ultrasound (VH-IVUS)
2.3. Near-Infrared Spectroscopy (NIRS)
2.4. Optical Coherence Tomography (OCT)
The Impact of OCT Finding on Patients’ Risk
2.5. OCT vs. VH-IVUS and NIRS
2.6. Fused Imaging
2.6.1. Concept of Vulnerable Plaque in Fusion Imaging
2.6.2. NIRS-IVUS Imaging
2.6.3. IVUS-OCT Imaging
2.6.4. NIRS-OCT IMAGING/NIR(A)F-OCT Imaging
2.6.5. NIRF-OCT-IVUS Imaging
2.7. Fusion of Coronary Angiography and IVUS/OCT in 3D Reconstructions
2.8. High-Frequency and Dual-Frequency IVUS
3. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrick, J.B. Landmark Article (JAMA 1912). Clinical Features of Sudden Obstruction of the Coronary Arteries. By James B. Herrick. JAMA 1983, 250, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Mintz, G.S.; Virmani, R. Vulnerable Plaques, Vulnerable Patients, and Intravascular Imaging. J. Am. Coll. Cardiol. 2018, 72, 2022–2026. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Russo, M.; Fracassi, F.; Kurihara, O.; Kim, H.O.; Thondapu, V.; Araki, M.; Shinohara, H.; Sugiyama, T.; Yamamoto, E.; Lee, H.; et al. Healed Plaques in Patients with Stable Angina Pectoris. Arter. Thromb. Vasc. Biol. 2020, 40, 1587–1597. [Google Scholar] [CrossRef]
- Narula, J.; Nakano, M.; Virmani, R.; Kolodgie, F.D.; Petersen, R.; Newcomb, R.; Malik, S.; Fuster, V.; Finn, A.V. Histopathologic Characteristics of Atherosclerotic Coronary Disease and Implications of the Findings for the Invasive and Noninvasive Detection of Vulnerable Plaques. J. Am. Coll. Cardiol. 2013, 61, 1041–1051. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients with ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [CrossRef]
- Fahed, A.C.; Jang, I.-K. Plaque erosion and acute coronary syndromes: Phenotype, molecular characteristics and future directions. Nat. Rev. Cardiol. 2021, 18, 724–734. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M.; et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus): A report of the american college of cardiology task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Tuzcu, E.M.; Sipahi, I.; Schoenhagen, P.; Nissen, S.E. Intravascular Ultrasound in Cardiovascular Medicine. Circulation 2006, 114, e55–e59. [Google Scholar] [CrossRef]
- Mintz, G.S.; Painter, J.A.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Popma, J.J.; Chuang, Y.C.; Bucher, T.A.; Sokolowicz, L.E.; Leon, M.B. Atherosclerosis in angiographically “normal” coronary artery reference segments: An intravascular ultrasound study with clinical correlations. J. Am. Coll. Cardiol. 1995, 25, 1479–1485. [Google Scholar] [CrossRef]
- Schoenhagen, P.; Ziada, K.M.; Kapadia, S.R.; Crowe, T.D.; Nissen, S.E.; Tuzcu, E.M. Extent and Direction of Arterial Remodeling in Stable Versus Unstable Coronary Syndromes: An Intravascular Ultrasound Study. Circulation 2000, 101, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Batty, J.A.; Subba, S.; Luke, P.; Gigi, L.W.C.; Sinclair, H.; Kunadian, V. Intracoronary Imaging in the Detection of Vulnerable Plaques. Curr. Cardiol. Rep. 2016, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Yonetsu, T.; Jang, I.-K. Advances in Intravascular Imaging: New Insights into the Vulnerable Plaque from Imaging Studies. Korean Circ. J. 2018, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Hao, H.; Shibuya, M.; Imanaka, T.; Fukunaga, M.; Miki, K.; Tamaru, H.; Sawada, H.; Naito, Y.; Ohyanagi, M.; et al. Accuracy of OCT, Grayscale IVUS, and Their Combination for the Diagnosis of Coronary TCFA: An Ex Vivo Validation Study. JACC Cardiovasc. Imaging 2015, 8, 451–460. [Google Scholar] [CrossRef]
- Puri, R.; Worthley, M.I.; Nicholls, S.J. Intravascular imaging of vulnerable coronary plaque: Current and future concepts. Nat. Rev. Cardiol. 2011, 8, 131–139. [Google Scholar] [CrossRef]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary Plaque Classification With Intravascular Ultrasound Radiofrequency Data Analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef]
- Kitahara, S.; Kataoka, Y.; Sugane, H.; Otsuka, F.; Asaumi, Y.; Noguchi, T.; Yasuda, S. In vivo imaging of vulnerable plaque with intravascular modalities: Its advantages and limitations. Cardiovasc. Diagn. Ther. 2020, 10, 1461–1479. [Google Scholar] [CrossRef]
- Nasu, K.; Tsuchikane, E.; Katoh, O.; Vince, D.G.; Virmani, R.; Surmely, J.-F.; Murata, A.; Takeda, Y.; Ito, T.; Ehara, M.; et al. Accuracy of In Vivo Coronary Plaque Morphology Assessment: A Validation Study of In Vivo Virtual Histology Compared With In Vitro Histopathology. J. Am. Coll. Cardiol. 2006, 47, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Obaid, D.R.; Costopoulos, C.; Parker, R.A.; Calvert, P.A.; Teng, Z.; Hoole, S.P.; West, N.E.J.; Goddard, M.; Bennett, M.R. Direct Comparison of Virtual-Histology Intravascular Ultrasound and Optical Coherence Tomography Imaging for Identification of Thin-Cap Fibroatheroma. Circ. Cardiovasc. Imaging 2015, 8, e003487. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.A.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.; Densem, C.G.; Schofield, P.M.; Braganza, D.; Clarke, S.C.; Ray, K.K.; et al. Association Between IVUS Findings and Adverse Outcomes in Patients With Coronary Artery Disease: The VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc. Imaging 2011, 4, 894–901. [Google Scholar] [CrossRef]
- Cheng, J.M.; Garcia-Garcia, H.M.; De Boer, S.P.M.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; Van Domburg, R.T.; Ligthart, J.; Witberg, K.T.; et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur. Heart J. 2013, 35, 639–647. [Google Scholar] [CrossRef]
- Schuurman, A.-S.; Vroegindewey, M.M.; Kardys, I.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; van Geuns, R.-J.; Regar, E.; Van Mieghem, N.M.; Ligthart, J.; Serruys, P.W.; et al. Prognostic Value of Intravascular Ultrasound in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 2003–2011. [Google Scholar] [CrossRef]
- Kubo, T.; Maehara, A.; Mintz, G.S.; Doi, H.; Tsujita, K.; Choi, S.-Y.; Katoh, O.; Nasu, K.; Koenig, A.; Pieper, M.; et al. The Dynamic Nature of Coronary Artery Lesion Morphology Assessed by Serial Virtual Histology Intravascular Ultrasound Tissue Characterization. J. Am. Coll. Cardiol. 2010, 55, 1590–1597. [Google Scholar] [CrossRef]
- Puri, R.; Libby, P.; Nissen, S.E.; Wolski, K.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.; Raichlen, J.S.; Uno, K.; et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: Insights from SATURN. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 380–388. [Google Scholar] [CrossRef]
- Tian, J.; Gu, X.; Sun, Y.; Ban, X.; Xiao, Y.; Hu, S.; Yu, B. Effect of statin therapy on the progression of coronary atherosclerosis. BMC Cardiovasc. Disord. 2012, 12, 70. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Legutko, J.; Jakala, J.; Mintz, G.S.; Wizimirski, M.; Rzeszutko, L.; Partyka, L.; Mrevlje, B.; Richter, A.; Margolis, P.; Kaluza, G.L.; et al. Virtual Histology-Intravascular Ultrasound Assessment of Lesion Coverage After Angiographically-Guided Stent Implantation in Patients With ST Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2012, 109, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Legutko, J.; Jakala, J.; Mintz, G.S.; Kaluza, G.L.; Mrevlje, B.; Partyka, L.; Wizimirski, M.; Rzeszutko, L.; Richter, A.; Margolis, P.; et al. Radiofrequency–Intravascular Ultrasound Assessment of Lesion Coverage After Angiography-Guided Emergent Percutaneous Coronary Intervention in Patients With Non–ST Elevation Myocardial Infarction. Am. J. Cardiol. 2013, 112, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Mrevlje, B.; Kleczyński, P.; Kranjec, I.; Jąkała, J.; Noc, M.; Rzeszutko, Ł.; Dziewierz, A.; Wizimirski, M.; Dudek, D.; Legutko, J. Optical coherence tomography versus intravascular ultrasound for culprit lesion assessment in patients with acute myocardial infarction. Postep. Kardiol Interwencyjnej 2020, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Ikeda, T.; Toda, M.; Okubo, R.; Yabe, T.; Koike, M.; Saito, D.; Yamazaki, J. Assessment of Angiographic Coronary Calcification and Plaque Composition in Virtual Histology Intravascular Ultrasound. J. Interv. Cardiol. 2015, 28, 205–214. [Google Scholar] [CrossRef]
- Stone, P.H.; Saito, S.; Takahashi, S.; Makita, Y.; Nakamura, S.; Kawasaki, T.; Takahashi, A.; Katsuki, T.; Nakamura, S.; Namiki, A.; et al. Prediction of Progression of Coronary Artery Disease and Clinical Outcomes Using Vascular Profiling of Endothelial Shear Stress and Arterial Plaque Characteristics: The PREDICTION Study. Circulation 2012, 126, 172–181. [Google Scholar] [CrossRef]
- Rodriguez-Granillo, G.A.; García-García, H.M.; Mc Fadden, E.P.; Valgimigli, M.; Aoki, J.; de Feyter, P.; Serruys, P.W. In Vivo Intravascular Ultrasound-Derived Thin-Cap Fibroatheroma Detection Using Ultrasound Radiofrequency Data Analysis. J. Am. Coll. Cardiol. 2005, 46, 2038–2042. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2019, 41, 383–391. [Google Scholar] [CrossRef]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; Ijsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT–FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; Di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; Hernandez, J.M.D.L.T.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions: Endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter Endocardiovascular Therapeutics (HKSTENT) and the Cardiac Society of Australia and New Zealand. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef]
- Pu, J.; Mintz, G.S.; Brilakis, E.S.; Banerjee, S.; Abdel-Karim, A.-R.R.; Maini, B.; Biro, S.; Lee, J.-B.; Stone, G.W.; Weisz, G.; et al. In vivo characterization of coronary plaques: Novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur. Heart J. 2011, 33, 372–383. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; van Geuns, R.-J.; de Boer, S.P.M.; Simsek, C.; Kardys, I.; Lenzen, M.J.; van Domburg, R.T.; Regar, E.; et al. Near-Infrared Spectroscopy Predicts Cardiovascular Outcome in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.-S.; Vroegindewey, M.; Kardys, I.; Oemrawsingh, R.M.; Cheng, J.M.; de Boer, S.; Garcia-Garcia, H.M.; van Geuns, R.-J.; Regar, E.S.; Daemen, J.; et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur. Heart J. 2017, 39, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef]
- Kini, A.S.; Baber, U.; Kovacic, J.C.; Limaye, A.; Ali, Z.A.; Sweeny, J.; Maehara, A.; Mehran, R.; Dangas, G.; Mintz, G.S.; et al. Changes in Plaque Lipid Content After Short-Term Intensive Versus Standard Statin Therapy: The YELLOW Trial (Reduction in Yellow Plaque by Aggressive Lipid-Lowering Therapy). J. Am. Coll. Cardiol. 2013, 62, 21–29. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2020, 14, 1440–1450. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Muller, J.E.; Rizik, D.G.; Shunk, K.A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Madden, S.; et al. Plaque Characterization to Inform the Prediction and Prevention of Periprocedural Myocardial Infarction During Percutaneous Coronary Intervention: The CANARY Trial (Coronary Assessment by Near-Infrared of Atherosclerotic Rupture-Prone Yellow). JACC Cardiovasc. Interv. 2015, 8, 927–936. [Google Scholar] [CrossRef]
- Prati, F.; Jenkins, M.W.; Di Giorgio, A.; Rollins, A.M. Intracoronary optical coherence tomography, basic theory and image acquisition techniques. Int. J. Cardiovasc. Imaging 2011, 27, 251–258. [Google Scholar] [CrossRef]
- Roleder, T.; Jąkała, J.; Kałuża, G.L.; Partyka, Ł.; Proniewska, K.; Pociask, E.; Zasada, W.; Wojakowski, W.; Gasior, Z.; Dudek, D. The basics of intravascular optical coherence tomography. Postep. Kardiol Interwencyjnej 2015, 11, 74–83. [Google Scholar] [CrossRef]
- Sinclair, H.; Bourantas, C.; Bagnall, A.; Mintz, G.S.; Kunadian, V. OCT for the Identification of Vulnerable Plaque in Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2015, 8, 198–209. [Google Scholar] [CrossRef]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.-H.; Halpern, E.F.; et al. Characterization of Human Atherosclerosis by Optical Coherence Tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Jaworski, C.; Corrigan, J.P.; de Silva, R.; Bennett, M.R.; Mahmoudi, M.; Hoole, S.P.; West, N.E.J. Optical coherence tomography imaging of coronary atherosclerosis is affected by intraobserver and interobserver variability. J. Cardiovasc. Med. 2016, 17, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, L.; Agozzino, M.; Marco, V.; Ricciardi, A.; Concardi, M.; Romagnoli, E.; Gatto, L.; Calogero, G.; Tavazzi, L.; Arbustini, E.; et al. Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kume, T.; Okura, H.; Yamada, R.; Koyama, T.; Fukuhara, K.; Kawamura, A.; Imai, K.; Neishi, Y.; Uemura, S. Detection of Plaque Neovascularization by Optical Coherence Tomography: Ex Vivo Feasibility Study and In Vivo Observation in Patients With Angina Pectoris. J. Invasive Cardiol. 2016, 28, 17–22. [Google Scholar]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Y.; Yang, J.; Zhai, G.; Zhou, Y.; Guo, Q. Prevalence of Healed Plaque and Factors Influencing Its Characteristics Under Optical Coherence Tomography in Patients With Coronary Artery Disease: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Cardiovasc. Med. 2021, 8, 761208. [Google Scholar] [CrossRef]
- Fracassi, F.; Crea, F.; Sugiyama, T.; Yamamoto, E.; Uemura, S.; Vergallo, R.; Porto, I.; Lee, H.; Fujimoto, J.; Fuster, V.; et al. Healed Culprit Plaques in Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 73, 2253–2263. [Google Scholar] [CrossRef]
- Usui, E.; Mintz, G.S.; Lee, T.; Matsumura, M.; Zhang, Y.; Hada, M.; Yamaguchi, M.; Hoshino, M.; Kanaji, Y.; Sugiyama, T.; et al. Prognostic impact of healed coronary plaque in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis 2020, 309, 1–7. [Google Scholar] [CrossRef]
- Roleder, T.; Galougahi, K.K.; Chin, C.Y.; Bhatti, N.K.; Brilakis, E.; Nazif, T.M.; Kirtane, A.J.; Karmpaliotis, D.; Wojakowski, W.; Leon, M.B.; et al. Utility of near-infrared spectroscopy for detection of thin-cap neoatherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 663–669. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, J.-H.; Shin, D.-H.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; Jang, Y.; Hong, M.-K. Long-Term Outcomes of Neointimal Hyperplasia without Neoatherosclerosis after Drug-Eluting Stent Implantation. JACC Cardiovasc. Imaging 2014, 7, 788–795. [Google Scholar] [CrossRef]
- Xing, L.; Higuma, T.; Wang, Z.; Aguirre, A.D.; Mizuno, K.; Takano, M.; Dauerman, H.L.; Park, S.-J.; Jang, Y.; Kim, C.-J.; et al. Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study. J. Am. Coll. Cardiol. 2017, 69, 2502–2513. [Google Scholar] [CrossRef]
- Kubo, T.; Ino, Y.; Mintz, G.S.; Shiono, Y.; Shimamura, K.; Takahata, M.; Terada, K.; Higashioka, D.; Emori, H.; Wada, T.; et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1376–1384. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nissen, S.E.; Prati, F.; Windecker, S.; Kataoka, Y.; Puri, R.; Hucko, T.; Kassahun, H.; Liao, J.; Somaratne, R.; et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: Rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 2021, 11, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.D.; Fabris, E.; Kedhi, E.; Ghilencea, L.-N.; Caiazzo, G.; Sherif, S.A.; Di Mario, C. Intra-coronary Imaging for the Evaluation of Plaque Modifications Induced by Drug Therapies for Secondary Prevention. Curr. Atheroscler. Rep. 2020, 22, 76. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Ali, Z.A.; Held, C.; Matsumura, M.; Kjøller-Hansen, L.; Bøtker, H.E.; Maeng, M.; Engstrøm, T.; Wiseth, R.; et al. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J. Am. Coll. Cardiol. 2020, 76, 2289–2301. [Google Scholar] [CrossRef]
- Jang, I.-K. Pursuit for the detection of vulnerable plaque. Eur. Heart J. 2019, 41, 392–393. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2016, 38, 792–800. [Google Scholar] [CrossRef]
- Bryniarski, K.; Gasior, P.; Legutko, J.; Makowicz, D.; Kedziora, A.; Szolc, P.; Bryniarski, L.; Kleczynski, P.; Jang, I.-K. OCT Findings in MINOCA. J. Clin. Med. 2021, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Kim, H.O.; Kurihara, O.; Araki, M.; Shinohara, H.; Thondapu, V.; Yonetsu, T.; Soeda, T.; Minami, Y.; Higuma, T.; et al. Characteristics of non-culprit plaques in acute coronary syndrome patients with layered culprit plaque. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Fovino, L.N.; Varbella, F.; Trabattoni, D.; Caramanno, G.; Trani, C.; De Cesare, N.; Esposito, G.; Montorfano, M.; Musto, C.; et al. A Large, Prospective, Multicentre Study of Left Main PCI Using a Latest-Generation Zotarolimus-Eluting Stent: The ROLEX Study. EuroIntervention 2022, EIJ-D-22-00454. [Google Scholar] [CrossRef]
- Kubo, T.; Nakamura, N.; Matsuo, Y.; Okumoto, Y.; Wu, X.; Choi, S.-Y.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kitabata, H.; et al. Virtual Histology Intravascular Ultrasound Compared With Optical Coherence Tomography for Identification of Thin-Cap Fibroatheroma. Int. Heart J. 2011, 52, 175–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Vito, L.; Imola, F.; Gatto, L.; Romagnoli, E.; Limbruno, U.; Marco, V.; Picchi, A.; Micari, A.; Albertucci, M.; Prati, F.; et al. Limitations of OCT in identifying and quantifying lipid components: An in vivo comparison study with IVUS-NIRS. EuroIntervention 2017, 13, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Coronary Plaque Composition. J. Am. Coll. Cardiol. 2018, 72, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ren, X.; Vergallo, R.; Xing, L.; Yu, H.; Jia, H.; Soeda, T.; McNulty, I.; Hu, S.; Lee, H.; et al. Distinct Morphological Features of Ruptured Culprit Plaque for Acute Coronary Events Compared to Those With Silent Rupture and Thin-Cap Fibroatheroma: A Combined Optical Coherence Tomography and Intravascular Ultrasound Study. J. Am. Coll. Cardiol. 2014, 63, 2209–2216. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Maini, B.; Dixon, S.R.; Brilakis, E.S.; Grines, C.L.; Rizik, D.G.; Powers, E.R.; Steinberg, D.H.; Shunk, K.A.; Weisz, G.; et al. Detection of Lipid-Core Plaques by Intracoronary Near-Infrared Spectroscopy Identifies High Risk of Periprocedural Myocardial Infarction. Circ. Cardiovasc. Interv. 2011, 4, 429–437. [Google Scholar] [CrossRef]
- Puri, R.; Madder, R.D.; Madden, S.P.; Sum, S.T.; Wolski, K.; Muller, J.E.; Andrews, J.; King, K.L.; Kataoka, Y.; Uno, K.; et al. Near-Infrared Spectroscopy Enhances Intravascular Ultrasound Assessment of Vulnerable Coronary Plaque: A Combined Pathological and In Vivo Study. Arter. Thromb. Vasc. Biol. 2015, 35, 2423–2431. [Google Scholar] [CrossRef]
- Li, J.; Ma, T.; Mohar, D.; Steward, E.; Yu, M.; Piao, Z.; He, Y.; Shung, K.K.; Zhou, Q.; Patel, P.M.; et al. Ultrafast optical-ultrasonic system and miniaturized catheter for imaging and characterizing atherosclerotic plaques in vivo. Sci. Rep. 2015, 5, 18406. [Google Scholar] [CrossRef]
- Ughi, G.J.; Wang, H.; Gerbaud, E.; Gardecki, J.A.; Fard, A.M.; Hamidi, E.; Vacas-Jacques, P.; Rosenberg, M.; Jaffer, F.A.; Tearney, G.J. Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging. JACC Cardiovasc. Imaging 2016, 9, 1304–1314. [Google Scholar] [CrossRef]
- Liang, S.; Ma, T.; Jing, J.; Li, X.; Li, J.; Shung, K.K.; Zhou, Q.; Zhang, J.; Chen, Z. Trimodality imaging system and intravascular endoscopic probe: Combined optical coherence tomography, fluorescence imaging and ultrasound imaging. Opt. Lett. 2014, 39, 6652–6655. [Google Scholar] [CrossRef]
- Madder, R.D.; Puri, R.; Muller, J.E.; Harnek, J.; Götberg, M.; VanOosterhout, S.; Chi, M.; Wohns, D.; McNamara, R.; Wolski, K.; et al. Confirmation of the Intracoronary Near-Infrared Spectroscopy Threshold of Lipid-Rich Plaques That Underlie ST-Segment–Elevation Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2016, 36, 1010–1015. [Google Scholar] [CrossRef]
- Kang, S.-J.; Mintz, G.S.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Xu, K.; Goldstein, J.A.; Stone, G.W.; Muller, J.E.; et al. Combined IVUS and NIRS Detection of Fibroatheromas: Histopathological Validation in Human Coronary Arteries. JACC Cardiovasc. Imaging 2015, 8, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Torguson, R.; Spad, M.-A.; Garcia-Garcia, H.; Ware, J.; Wang, R.; Madden, S.; Shah, P.; Muller, J. The Lipid-Rich Plaque Study of vulnerable plaques and vulnerable patients: Study design and rationale. Am. Heart J. 2017, 192, 98–104. [Google Scholar] [CrossRef]
- Danek, B.A.; Karatasakis, A.; Karacsonyi, J.; Alame, A.; Resendes, E.; Kalsaria, P.; Nguyen-Trong, P.-K.J.; Rangan, B.V.; Roesle, M.; Abdullah, S.; et al. Long-term follow-up after near-infrared spectroscopy coronary imaging: Insights from the lipid cORe plaque association with CLinical events (ORACLE-NIRS) registry. Cardiovasc. Revascularization Med. 2017, 18, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kuku, K.O.; Singh, M.; Ozaki, Y.; Dan, K.; Chezar-Azerrad, C.; Waksman, R.; Garcia-Garcia, H.M. Near-Infrared Spectroscopy Intravascular Ultrasound Imaging: State of the Art. Front. Cardiovasc. Med. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of Culprit Lesion Morphology in Acute Myocardial Infarction: Ability of Optical Coherence Tomography Compared With Intravascular Ultrasound and Coronary Angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.-K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2009, 31, 401–415. [Google Scholar] [CrossRef]
- Cilingiroglu, M.; Oh, J.H.; Sugunan, B.; Kemp, N.J.; Kim, J.; Lee, S.; Ms, H.N.Z.; Escobedo, D.; Thomsen, S.; Milner, T.E.; et al. Detection of vulnerable plaque in a murine model of atherosclerosis with optical coherence tomography. Catheter. Cardiovasc. Interv. 2006, 67, 915–923. [Google Scholar] [CrossRef]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of Macrophage Content in Atherosclerotic Plaques by Optical Coherence Tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Phipps, J.E.; Vela, D.; Hoyt, T.; Halaney, D.L.; Mancuso, J.J.; Buja, L.M.; Asmis, R.; Milner, T.E.; Feldman, M.D. Macrophages and Intravascular OCT Bright Spots: A Quantitative Study. JACC Cardiovasc. Imaging 2015, 8, 63–72. [Google Scholar] [CrossRef]
- Reith, S.; Milzi, A.; Dettori, R.; Marx, N.; Burgmaier, M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: An optical coherence tomography study. Clin. Res. Cardiol. 2018, 107, 763–771. [Google Scholar] [CrossRef]
- Burgmaier, M.; Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S. Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS ONE 2018, 13, e0205984. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Shite, J.; Garcia-Garcia, H.M.; Shinke, T.; Watanabe, S.; Otake, H.; Matsumoto, D.; Tanino, Y.; Ogasawara, D.; Kawamori, H.; et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur. Heart J. 2008, 29, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A.; Matsumura, M.; Ali, Z.A.; Mintz, G.S.; Stone, G.W. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation: A Critical Appraisal. JACC Cardiovasc. Imaging 2017, 10, 1487–1503. [Google Scholar] [CrossRef]
- Nakano, M.; Yahagi, K.; Yamamoto, H.; Taniwaki, M.; Otsuka, F.; Ladich, E.R.; Joner, M.; Virmani, R. Additive Value of Integrated Backscatter IVUS for Detection of Vulnerable Plaque by Optical Frequency Domain Imaging: An Ex Vivo Autopsy Study of Human Coronary Arteries. JACC Cardiovasc. Imaging 2016, 9, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sheth, T.N.; Pinilla-Echeverri, N.; Mehta, S.R.; Courtney, B.K. First-in-Human Images of Coronary Atherosclerosis and Coronary Stents Using a Novel Hybrid Intravascular Ultrasound and Optical Coherence Tomographic Catheter. JACC Cardiovasc. Interv. 2018, 11, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kawashima, H.; Hara, H.; Gao, C.; Wang, R.; Kogame, N.; Takahashi, K.; Chichareon, P.; Modolo, R.; Tomaniak, M.; et al. Advances in IVUS/OCT and Future Clinical Perspective of Novel Hybrid Catheter System in Coronary Imaging. Front. Cardiovasc. Med. 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Kubo, T.; Kameyama, T.; Shimamura, K.; Terada, K.; Matsuo, Y.; Kitabata, H.; Shiono, Y.; Kashiwagi, M.; Kuroi, A.; et al. Clinical Utility of Combined Optical Coherence Tomography and Near-Infrared Spectroscopy for Assessing the Mechanism of Very Late Stent Thrombosis. JACC Cardiovasc. Imaging 2018, 11, 772–775. [Google Scholar] [CrossRef]
- Jaffer, F.A.; Libby, P.; Weissleder, R. Optical and Multimodality Molecular Imaging: Insights into Atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 1017–1024. [Google Scholar] [CrossRef]
- Abran, M.; Stähli, B.E.; Merlet, N.; Mihalache-Avram, T.; Mecteau, M.; Rhéaume, E.; Busseuil, D.; Tardif, J.-C.; Lesage, F. Validating a bimodal intravascular ultrasound (IVUS) and near-infrared fluorescence (NIRF) catheter for atherosclerotic plaque detection in rabbits. Biomed. Opt. Express 2015, 6, 3989–3999. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, J.W.; Shishkov, M.; Namati, E.; Morse, T.; Shubochkin, R.; McCarthy, J.R.; Ntziachristos, V.; E Bouma, B.; A Jaffer, F.; et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 2011, 17, 1680–1684. [Google Scholar] [CrossRef]
- Li, J.; Montarello, N.J.; Hoogendoorn, A.; Verjans, J.W.; Bursill, C.A.; Peter, K.; Nicholls, S.J.; McLaughlin, R.A.; Psaltis, P.J. Multimodality Intravascular Imaging of High-Risk Coronary Plaque. JACC Cardiovasc. Imaging 2022, 15, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.M.; Vacas-Jacques, P.; Hamidi, E.; Wang, H.; Carruth, R.W.; Gardecki, J.A.; Tearney, G.J. Optical coherence tomography—Near infrared spectroscopy system and catheter for intravascular imaging. Opt. Express 2013, 21, 30849–30858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jing, J.; Qu, Y.; Miao, Y.; Zhang, B.; Ma, T.; Yu, M.; Zhou, Q.; Chen, Z. Fully integrated optical coherence tomography, ultrasound, and indocyanine green-based fluorescence tri-modality system for intravascular imaging. Biomed. Opt. Express 2017, 8, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Kilic, Y.; Safi, H.; Bajaj, R.; Serruys, P.W.; Kitslaar, P.; Ramasamy, A.; Tufaro, V.; Onuma, Y.; Mathur, A.; Torii, R.; et al. The Evolution of Data Fusion Methodologies Developed to Reconstruct Coronary Artery Geometry From Intravascular Imaging and Coronary Angiography Data: A Comprehensive Review. Front. Cardiovasc. Med. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Popma, J.J.; Sonka, M.; Wahle, A.; Yeghiazarians, Y.; Maynard, C.; Kuntz, R.E.; Feldman, C.L. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: An in vivo serial study. Eur. Heart J. 2007, 28, 705–710. [Google Scholar] [CrossRef]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Clark, M.E.; Sonka, M.; Wahle, A.; Ilegbusi, O.J.; Yeghiazarians, Y.; Popma, J.J.; Orav, J.; et al. Effect of Endothelial Shear Stress on the Progression of Coronary Artery Disease, Vascular Remodeling, and In-Stent Restenosis in Humans: In Vivo 6-Month Follow-up Study. Circulation 2003, 108, 438–444. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Räber, L.; Sakellarios, A.; Ueki, Y.; Zanchin, T.; Koskinas, K.C.; Yamaji, K.; Taniwaki, M.; Heg, D.; Radu, M.D.; et al. Utility of Multimodality Intravascular Imaging and the Local Hemodynamic Forces to Predict Atherosclerotic Disease Progression. JACC Cardiovasc. Imaging 2019, 13, 1021–1032. [Google Scholar] [CrossRef]
- Ma, T.; Yu, M.; Li, J.; Munding, C.E.; Chen, Z.; Fei, C.; Shung, K.K.; Zhou, Q. Multi-frequency intravascular ultrasound (IVUS) imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015, 62, 97–107. [Google Scholar] [CrossRef]
- Martin, K.H.; Lindsey, B.D.; Ma, J.; Nichols, T.C.; Jiang, X.; Dayton, P.A. Ex Vivo Porcine Arterial and Chorioallantoic Membrane Acoustic Angiography Using Dual-Frequency Intravascular Ultrasound Probes. Ultrasound Med. Biol. 2016, 42, 2294–2307. [Google Scholar] [CrossRef]
- Wang, Z.; Martin, K.H.; Dayton, P.A.; Jiang, X. Real-time ultrasound angiography using superharmonic dual-frequency (2.25 MHz/30 MHz) cylindrical array: In vitro study. Ultrasonics 2018, 82, 298–303. [Google Scholar] [CrossRef]
- Yang, S.; Hoshino, M.; Yonetsu, T.; Zhang, J.; Hwang, D.; Shin, E.-S.; Doh, J.-H.; Nam, C.-W.; Wang, J.; Chen, S.; et al. Outcomes of Non-Ischaemic Coronary Lesions with High-Risk Plaque Characteristics on Coronary CT Angiography. EuroIntervention 2022, EIJ-D-22-00562. [Google Scholar] [CrossRef]

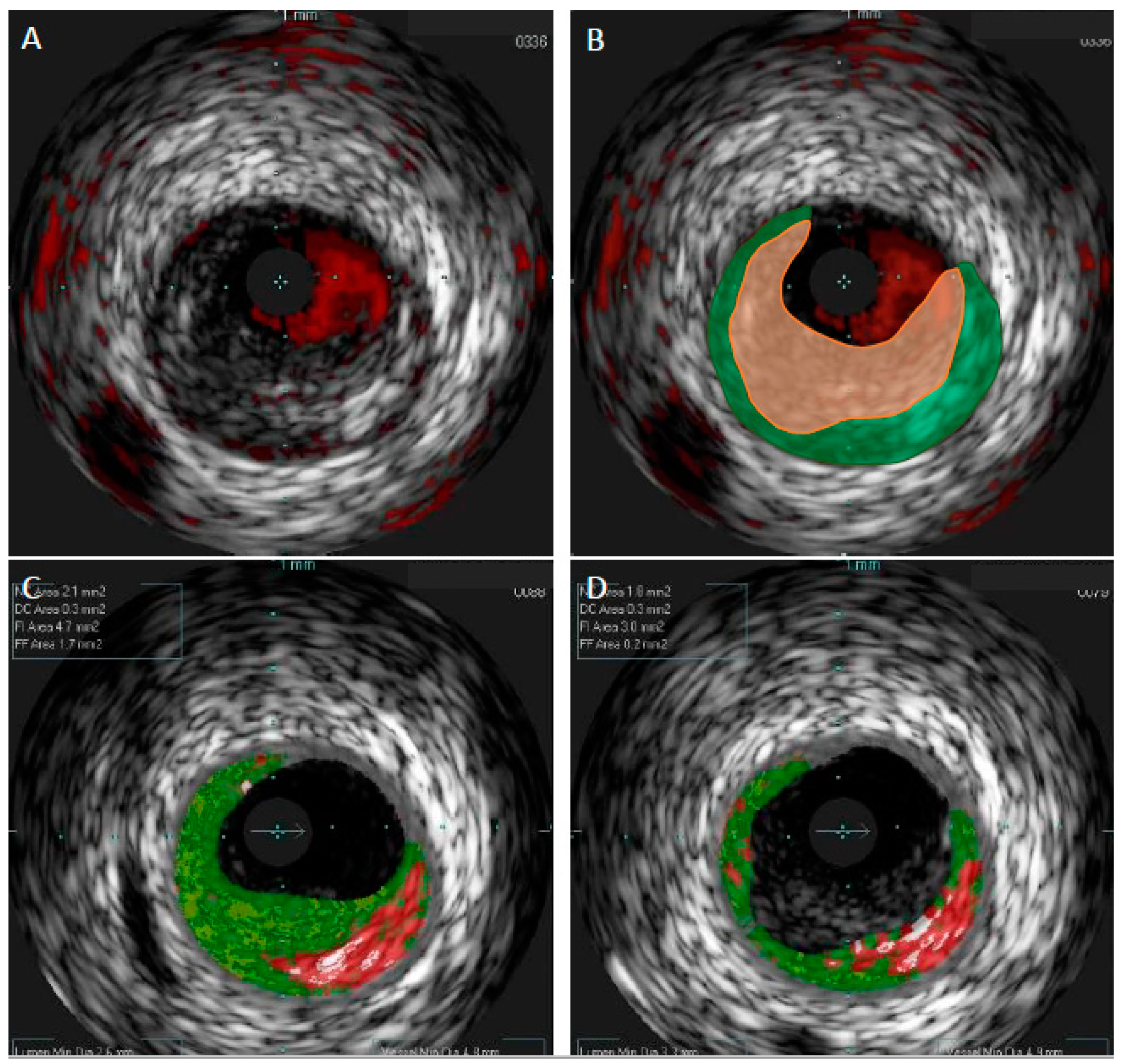
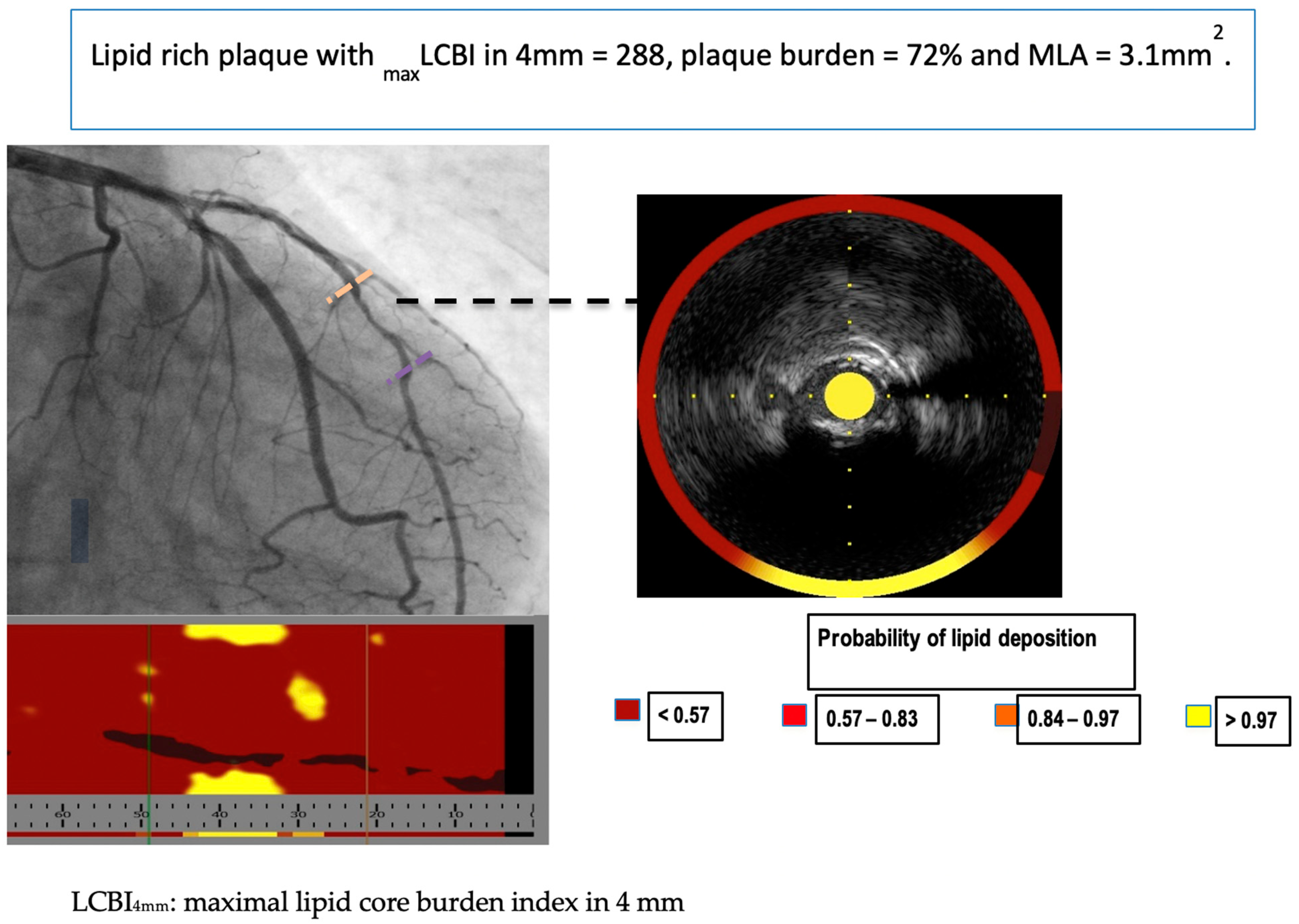

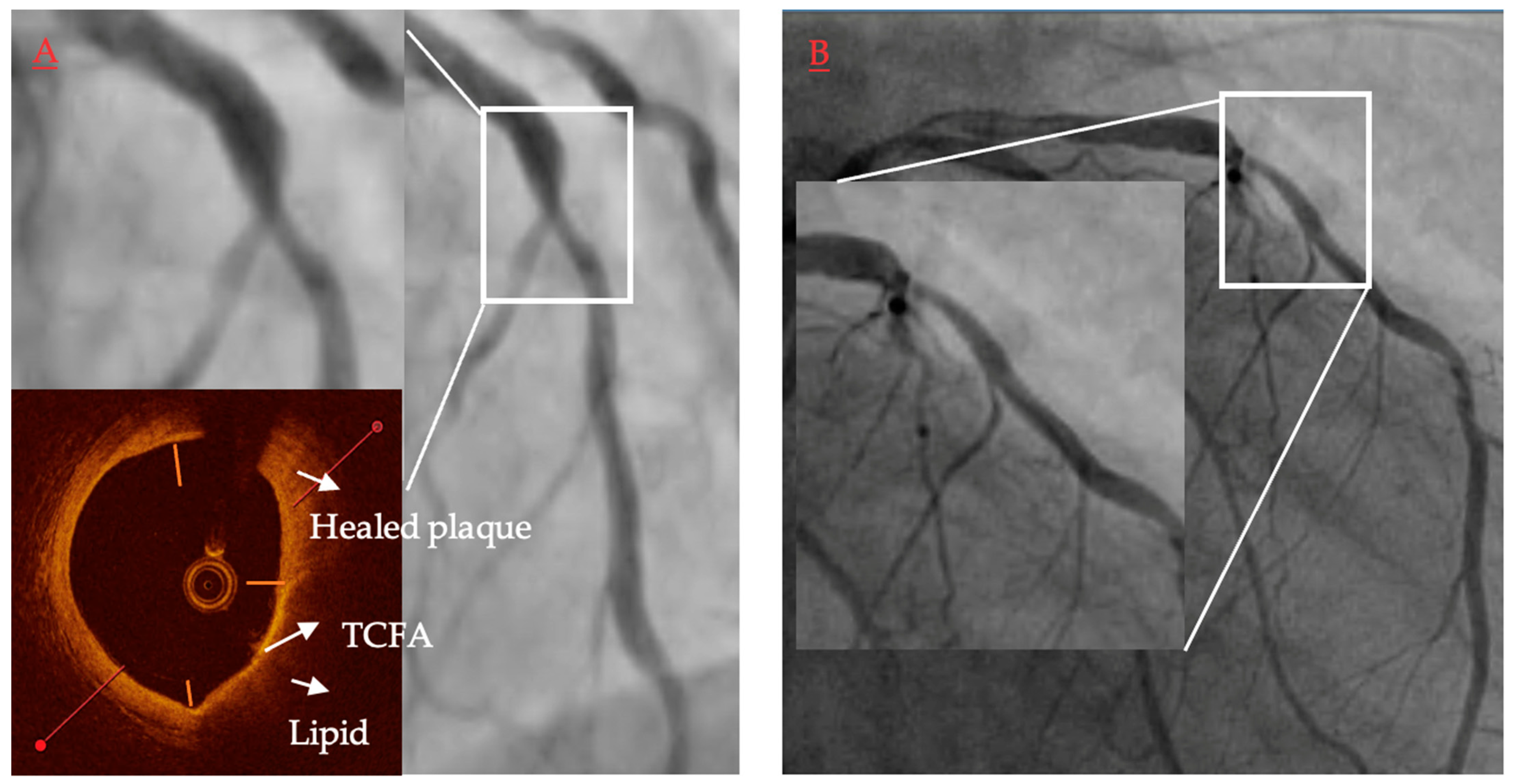
| Authors/Study/Publication Year | Modalities | Study Size | Study Objective | Main Results | Main Limitations |
|---|---|---|---|---|---|
| Rodriguez-Granillo, 2005 [36] | IVUS-VH | 55 patients | To assess the prevalence of intravascular ultrasound (IVUS)-derived thin-cap fibroatheroma (IDTCFA) and its relationship with the clinical presentation using spectral analysis of IVUS radiofrequency data. Definition of IDTCFA lesions—focal, necrotic-core-rich (≥10% of the cross-sectional area) plaques being in contact with the lumen, percent atheroma volume (PAV) ≥40%. | IVUS-VH identified IDTCFA as a more prevalent finding in ACS than in stable angina patients. ACS patients had a significantly higher incidence of IDTCFA than stable patients (0.7 (IQR 0.0 to 1.3) IDTCFA/cm vs. 0.2 (IQR 0.0 to 0.7) IDTCFA/cm, p = 0.031). | The lack of a direct comparison between IVUS-VH and histopathology |
| Stone, 2011 [23] PROSPECT [NCT00180466] | IVUS-VH | 697 patients (313 had TCFA) | To confirm that ACS arise from atheromas with certain histopathological characteristics, and that these characteristics are not necessarily dependent on the degree of angiographic stenosis at that site. | In multivariate analysis, the authors found that plaque burden ≥70%, TCFA and minimal lumen area ≤4.0 mm2 were independent predictors of non-culprit lesion related major adverse cardiac events in lesion-level analysis. Importantly, the rate of MACE increased from HR 3.90 (95% CI, 2.25–6.76) with TCFA alone to HR 11.05 (95% CI, 4.39–27.82) when combining all of the described plaque futures. | Only the proximal 6 to 8 cm of the coronary tree were examined. All 106 non-culprit lesions associated with recurrent events were evaluated with the use of baseline angiography, but only 55 of these lesions were seen on gray-scale ultrasonography and only 51 were seen on radiofrequency intravascular ultrasonography. |
| Calvert, 2011 [24] VIVA | IVUS-VH | 170 patients | TCFA identified by VH-IVUS are associated with major adverse cardiac events (MACE) in individual-plaque or whole-patient analysis. | The study showed that VH-IVUS TCFA was associated with MACE. The non-culprit lesion factors associated with non-restenotic MACE included VHTCFA (hazard ratio (HR): 7.53, p = 0.038) and plaque burden >70% (HR: 8.13, p = 0.011). VHTCFA (HR: 8.16, p = 0.007), plaque burden >70% (HR: 7.48, p < 0.001) and minimum luminal area <4.0 mm2 (HR: 2.91, p = 0.036) were associated with total MACE. | The definitions of TCFAs in VH-IVUS did not exactly match the histopathological definitions. VH-IVUS tended to overestimate the number of TCFAs compared to histology, and some histological ThCFAs were classified as VHTCFAs. |
| Brown, 2015 [22] | IVUS-VH, OCT | 258 ROI from 14 human hearts | The combination of VH-IVUS and OCT improves the identification of TCFA. | Combined VH-IVUS/OCT imaging markedly improved TCFA identification. The sensitivity, specificity and diagnostic accuracy for TCFA identification were 63.6%, 78.1% and 76.5% for VH-IVUS and 72.7%, 79.8% and 79.0% for OCT. Combining VH-defined fibroatheroma and fibrous cap thickness ≤85 μm over three continuous frames improved TCFA identification, with a diagnostic accuracy of 89.0%. | Small study size; small longitudinal mismatches between imaging modalities |
| Cheng, 2014 [25] ATHEROREMO | IVUS-GS, IVUS-VH | 581 patients | To investigate the prognostic value of the in vivo detection of high-risk coronary plaques by intravascular ultrasound (IVUS) in patients undergoing coronary angiography. | The study showed that presence of TCFA in non-culprit coronary artery is associated with greater incidence of death and ACS at 1 year follow-up. The presence of TCFA lesions was significantly associated with the composite of death or ACS only (present 7.5% vs. absent 3.0%; adjusted HR: 2.51, 95% CI: 1.15–5.49; p = 0.021). TCFA with a plaque burden of at least 70% were associated with a higher MACE rate both in the first 6 months (p = 0.011) and after 6 months (p < 0.001) of follow-up, while smaller TCFA lesions were only associated with a higher MACE rate after 6 months (p = 0.033). | The relatively small number of endpoints did not allow for the evaluation of whether adding IVUS imaging to a prognostic model with conventional risk factors would result in improved risk prediction. Missing repeat intracoronary imaging with IVUS virtual histology. |
| Fuji, 2015 [17] | IVUS-GS, OCT | 165 coronary arteries from 60 autopsy hearts | To assess the accuracy of optical coherence tomography (OCT), gray-scale intravascular ultrasound (IVUS), and their combination for detecting thin-cap fibroatheromas (TCFA). A total of 685 pairs of images of OCT and IVUS were compared with histology. | PPV increased from 41% to 69% after IVUS and OCT combination. The sensitivity, specificity, PPV, NPV and DA of the combined use of OCT and IVUS for characterizing TCFA using histology as a standard were 92%, 99%, 69%, 99% and 99%, respectively. | The low prevalence of TCFA in histology (2%) may affect the statistical power to assess the diagnostic accuracy of TCFA. |
| Prati, 2020 [37] CLIMA | IVOCT | 1003 | To explore the predictive value of multiple high-risk plaque features in the same coronary lesion (minimum lumen area (MLA), fibrous cap thickness (FCT), lipid arc circumferential extension and presence of optical coherence tomography (OCT)-defined macrophages). | At 1 year, the primary clinical endpoint was observed in 37 patients (3.7%). In a total of 1776 lipid plaques, presence of MLA < 3.5 mm2 (hazard ratio (HR) 2.1, 95% confidence interval (CI) 1.1–4.0), FCT < 75 µm (HR 4.7, 95% CI 2.4–9.0), lipid arc circumferential extension > 180° (HR 2.4, 95% CI 1.2–4.8) and OCT-defined macrophages (HR 2.7, 95% CI 1.2–6.1) were all associated with increased risk of the primary endpoint. The pre-specified combination of plaque features (simultaneous presence of the four OCT criteria in the same plaque) was observed in 18.9% of patients experiencing the primary endpoint and was an independent predictor of events (HR 7.54, 95% CI 3.1–18.6). OCT-based classification showed limited sensitivity (positive predictive value 19.4%), but high specificity (negative predictive value 96.9%) for the primary endpoint, and remained an independent predictor of 1 year events after correction for the other confounding variables. | The registry included patients with various clinical presentation and cardiovascular risk profiles uniquely pooled by the intraprocedural OCT assessment of proximal LAD. The combination of the four high-risk plaque features was uncommon. |
| Kedhi, 2021 [38] COMBINE | IVOCT | 482 | To study the impact of optical coherence tomography (OCT)-detected thin-cap fibroatheroma (TCFA) on the clinical outcomes of diabetes mellitus (DM) patients with fractional flow reserve (FFR)-negative lesions. | Among DM patients with ≥1 FFR-negative lesions, TCFA-positive patients represented 25% of this population and were associated with a five-fold higher rate of MACE despite the absence of ischaemia. The Cox regression multivariable analysis identified TCFA as the strongest predictor of major adverse clinical events (MACE) (hazard ratio 5.12; 95% confidence interval 2.12–12.34; p < 0.001). |
| IVUS vs. OCT | Comment | |
|---|---|---|
| Assessment of non-calcified and non-LM coronary plaques before stent implantation | Equal | OCT may provide more information regarding plaque composition (for example lipid plaque and optimal stent edge placement). |
| Assessment of calcified and non-LM coronary plaques before stent implantation | OCT better | Calcification obstructs penetration of the ultrasound (casting acoustic shadow). |
| Assessment of LM coronary plaques before stent implantation | IVUS better | OCT may be used in non-ostial LM lesions provided proper blood removal. |
| Optimalization after stent implantation | OCT better | Images from OCT due to high resolution may be easier to interpret provided proper blood removal (not possible in LM ostial lesions). |
| Spontaneous coronary dissection | IVUS better or equal | OCT may provide easier interpretation of SCAD and is used in clinical practice; however, contrast flush may propagate SCAD. |
| Stent failure | OCT | Higher resolution and easier interpretation with OCT. |
| Neoatherosclerosis | OCT | Higher resolution and easier interpretation with OCT. |
| Imaging in setting of ACS | OCT | OCT may provide information regarding the mechanism of ACS including plaque rapture, erosion or calcified nodule. |
| CTO | IVUS | OCT requires contrast flush, which is not possible in CTO. Moreover, when using OCT, it is not possible to provide continuous visualization of one chosen coronary artery. |
| CKD stage 4 | IVUS | OCT requires continuous contrast flush during pullback. |
| Authors/Publication Year/Study | Fused Imaging Modalities | Study Size | Objectives | Main Results | Main Limitations |
|---|---|---|---|---|---|
| Goldstein [75], 2011 COLOR Registry [NCT00831116] | NIRS-IVUS | 62 | Prospective identification of LCP with catheter-based near-infrared spectroscopy (NIRS) may predict an increased risk of periprocedural MI and facilitate development of preventive measures. | The primary finding of the study is that in patients with coronary artery disease, PCI of lesions with a large lipid core (maxLCBI4mm ≥ 500 by NIRS) is associated with a 50% risk of periprocedural MI (95% CI, 28–62), compared with only a 4.2% risk (95% CI, 0.8–11) for lesions without a large lipid core (maxLCBI4mm < 500 by NIRS). | The number, type, timing and frequency of biomarker determination were not standardized. A small sample size. |
| Kini, 2013, [44] YELLOW [NCT01567826)] | NIRS-IVUS | 86 patients | To determine the impact of short-term intensive statin therapy on intracoronary plaque lipid content. | The median reduction (95% confidence interval) in LCBI4mm max was significantly greater in the intensive versus standard group (−149.1 [−210.9 to −42.9] vs. 2.4 [−36.1 to 44.7]; p = 0.01). Short-term intensive statin therapy may reduce lipid content in obstructive lesions. | A small sample size and short duration of follow-up. The baseline LCBI was significantly higher in patients randomly allocated to intensive versus standard therapy. |
| Puri [76] 2015 | NIRS-IVUS | 116 coronary arteries of 51 autopsied hearts | To assess the relationships between intravascular ultrasound (IVUS)-derived PB and arterial remodeling with near-infrared spectroscopy (NIRS)-derived lipid content in ex vivo and in vivo human coronary arteries. | Lesion-based analyses demonstrated the highest LCBI and remodeling index within coronary fibroatheroma (P trend < 0.001 and 0.02 versus all plaque groups, respectively). Prediction models demonstrated similar abilities of PB, LCBI and the remodeling index for discriminating fibroatheroma (c indices: 0.675, 0.712, and 0.672, respectively). A combined PB + LCBI analysis significantly improved fibroatheroma detection accuracy (c index 0.77, p = 0.028 versus PB; net-reclassification index 43%, p = 0.003). | Small study size and on autopsied heart |
| Waksman [43] 2019 LRP Study NCT02033694 | NIRS-IVUS | 1271 patients | To investigate the relationship between LRPs detected by NIRS-intravascular ultrasound imaging at unstented sites and subsequent coronary events from new culprit lesions. | The 2-year cumulative incidence of NC-MACE was 9% (n = 103). The unadjusted hazard ratio (HR) for NC-MACE was 1.21 (95% CI 1.09–1.35; p = 0.0004) for each 100-unit increase in maxLCBI4mm and the adjusted HR was 1.18 (1.05–1.32; p = 0.0043). In patients with a maxLCBI4mm over 400, the unadjusted HR for NC-MACE was 2.18 (1.48–3.22; p < 0.0001) and the adjusted HR was 1.89 (1.26–2.83; p = 0.0021). | |
| Terada [46], 2021 PROSPECT II | NIRS-IVUS, OCT | 244 patients | To investigate the ability of combined near-infrared spectroscopy and intravascular ultrasound (NIRS-IVUS) to differentiate plaque rupture (PR), plaque erosion (PE) or calcified nodule (CN) in acute myocardial infarction (AMI). | NIRS-measured maxLCBI4mm was significantly largest in OCT-PR (705 (interquartile range (IQR): 545 to 854)), followed by OCT-CN (355 (IQR: 303 to 478)) and OCT-PE (300 (IQR: 126 to 357)) (p < 0.001). The NIRS-IVUS classification algorithm using plaque cavity, convex calcium and max LCBI4mm showed a sensitivity and specificity of 97% and 96% for identifying OCT-PR, 93% and 99% for OCT-PE, and 100% and 99% for OCT-CN, respectively. | Recognition of PR, PE and CN using OCT as a reference, without considering the intrinsic and insurmountable limitations of OCT technology. Aspiration thrombectomy and balloon angioplasty prior to imaging may have induced iatrogenic rupture of the fibrous cap and reduced the lipid composition of the PR. |
| Li [77] 2015 | IVUS-OCT | 50 human coronary arteries (in vitro) | To investigate the capability of recognition of vulnerable plaques using this IVUS-OCT technology. | Histology confirmed that TCFA and false TCFA can be differentiated using IVUS-OCT images. The full integration of the two complementary techniques of OCT and IVUS permits accurate evaluation of total plaque burden and plaque morphology by using an in vitro human cadaver study. | Limited study size and only on autopsied vessel |
| Ughi [78], 2016 | OCT-NIRAF | 12 patients | First clinical imaging of human coronary arteries in vivo using a multimodality OCT and near-infrared autofluorescence (NIRAF) intravascular imaging system and catheter. | High-quality intracoronary OCT and NIRAF image data (>50 mm pullback length) were successfully acquired without complication in all patients. In a substudy of 4 repeated pullbacks, NIRAF reproducibility was excellent with an average Pearson’s correlation coefficient of 0.925 ± 0.015. | Small study |
| Liang [79], 2014 | NIRF-OCT-IVUS | - | The study presented a trimodality imaging system and an intravascular endoscopic probe for the detection of early-stage atherosclerotic plaques. | The first ex vivo imaging of a normal New Zealand white rabbit aorta in which two model plaques had been planted inside the blood vessel wall. | Large dimension of probe Long imaging time |
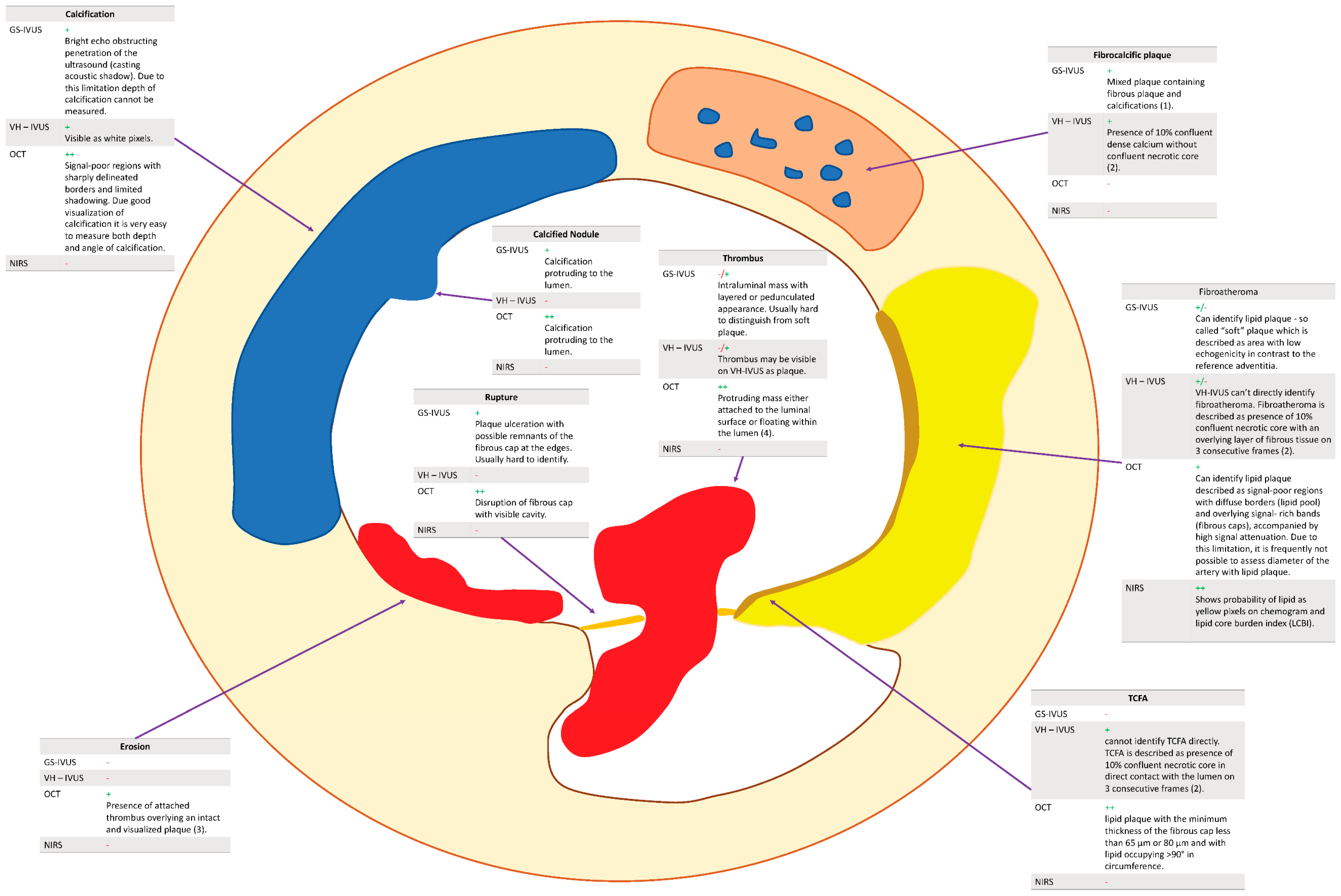
| GS-IVUS | VH-IVUS | NIRS | OCT | |
|---|---|---|---|---|
| Fibroatheroma | Can identify lipid plaque—so-called “soft” plaque—which is described as the area with low echogenicity in contrast to the reference adventitia. | VH-IVUS cannot directly identify fibroatheroma. Fibroatheroma is described as the presence of 10% confluent necrotic core with an overlying layer of fibrous tissue on three consecutive frames (2). | Shows the probability of lipid as yellow pixels on the chemogram and lipid core burden index (LCBI). | Can identify lipid plaque described as signal-poor regions with diffuse borders (lipid pool) and overlying signal-rich bands (fibrous caps), accompanied by high signal attenuation. Due to this limitation, it is frequently not possible to assess the diameter of the artery with lipid plaque. |
| Calcification | Bright echo obstructing penetration of the ultrasound (casting acoustic shadow). Due to this limitation, the depth of calcification cannot be measured. | Visible as white pixels. | NA | Signal-poor regions with sharply delineated borders and limited shadowing. Due to good visualization of calcification, it is very easy to measure both the depth and angle of calcification. |
| Fibrocalcific plaque | Mixed plaque containing fibrous plaque and calcifications (1). | Presence of 10% confluent dense calcium without confluent necrotic core (2). | NA | NA |
| Calcific nodule | Calcification protruding to the lumen. | NA | NA | Calcification protruding to the lumen. |
| TCFA | GS-IVUS does not have a resolution high enough to visualize TCFA. | VH-IVUS cannot identify TCFA directly. TCFA is described as the presence of 10% confluent necrotic core in direct contact with the lumen on three consecutive frames (2). | NA | Lipid plaque with a minimum thickness of the fibrous cap of less than 65 μm or 80 μm and with lipid occupying >90° in circumference. |
| Erosion | NA | NA | NA | Presence of attached thrombus overlying an intact and visualized plaque (3). |
| Rupture | Plaque ulceration with possible remnants of the fibrous cap at the edges. Usually hard to identify. | NA | NA | Disruption of fibrous cap with visible cavity. |
| Thrombus | Intraluminal mass with layered or pedunculated appearance. Usually hard to distinguish from soft plaque. | Thrombus may be visible on VH-IVUS as plaque. | NA | Protruding mass either attached to the luminal surface or floating within the lumen (4). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legutko, J.; Bryniarski, K.L.; Kaluza, G.L.; Roleder, T.; Pociask, E.; Kedhi, E.; Wojakowski, W.; Jang, I.-K.; Kleczynski, P. Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice. J. Clin. Med. 2022, 11, 6639. https://doi.org/10.3390/jcm11226639
Legutko J, Bryniarski KL, Kaluza GL, Roleder T, Pociask E, Kedhi E, Wojakowski W, Jang I-K, Kleczynski P. Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice. Journal of Clinical Medicine. 2022; 11(22):6639. https://doi.org/10.3390/jcm11226639
Chicago/Turabian StyleLegutko, Jacek, Krzysztof L. Bryniarski, Grzegorz L. Kaluza, Tomasz Roleder, Elzbieta Pociask, Elvin Kedhi, Wojciech Wojakowski, Ik-Kyung Jang, and Pawel Kleczynski. 2022. "Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice" Journal of Clinical Medicine 11, no. 22: 6639. https://doi.org/10.3390/jcm11226639
APA StyleLegutko, J., Bryniarski, K. L., Kaluza, G. L., Roleder, T., Pociask, E., Kedhi, E., Wojakowski, W., Jang, I.-K., & Kleczynski, P. (2022). Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice. Journal of Clinical Medicine, 11(22), 6639. https://doi.org/10.3390/jcm11226639






