Olfactory Evaluation in Clinical Medical Practice
Abstract
1. Introduction
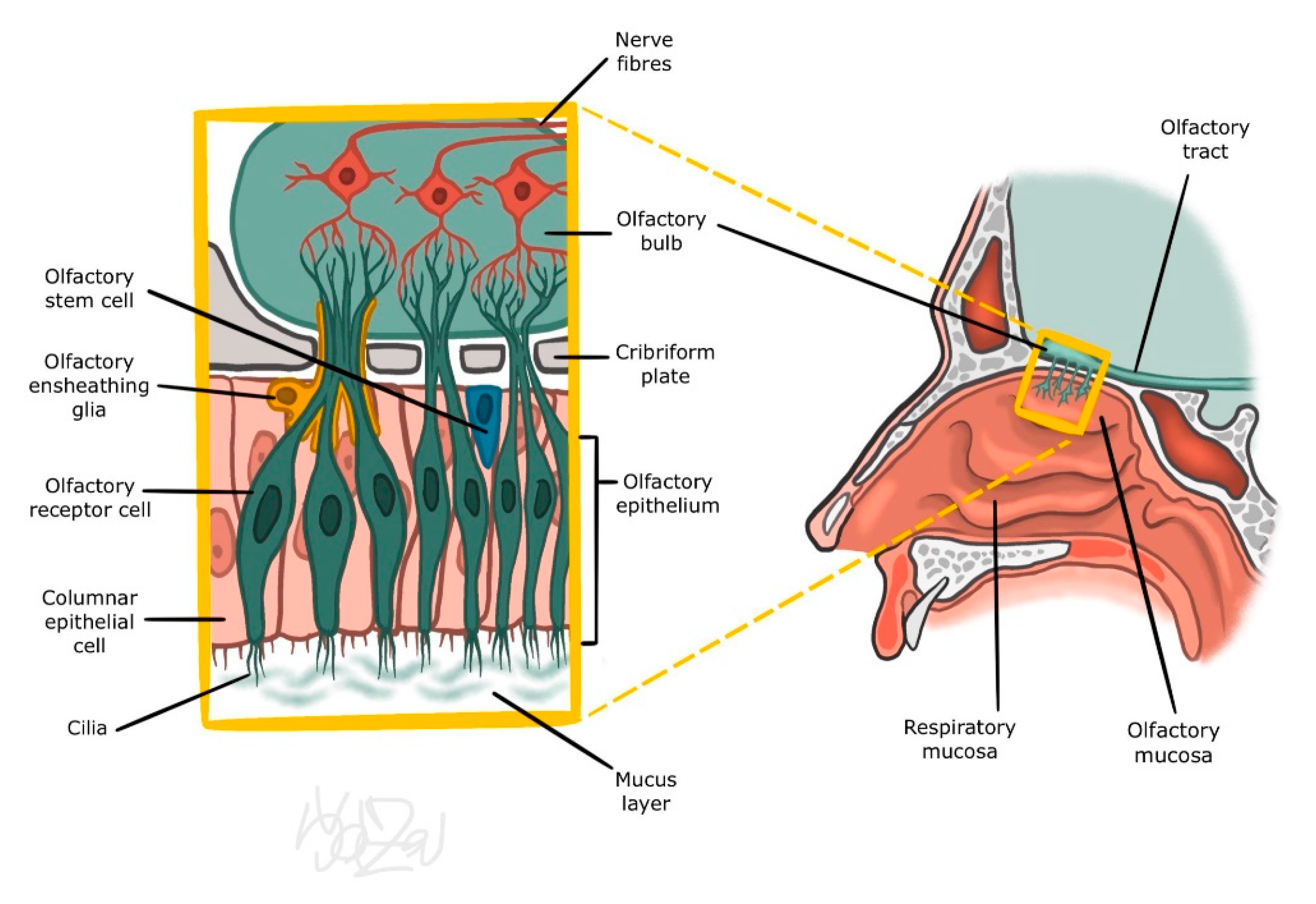
2. Nasal Mucosa
3. Physiology and Neurophysiology of the Olfaction
3.1. Peripheral Part of the Olfactory Organ
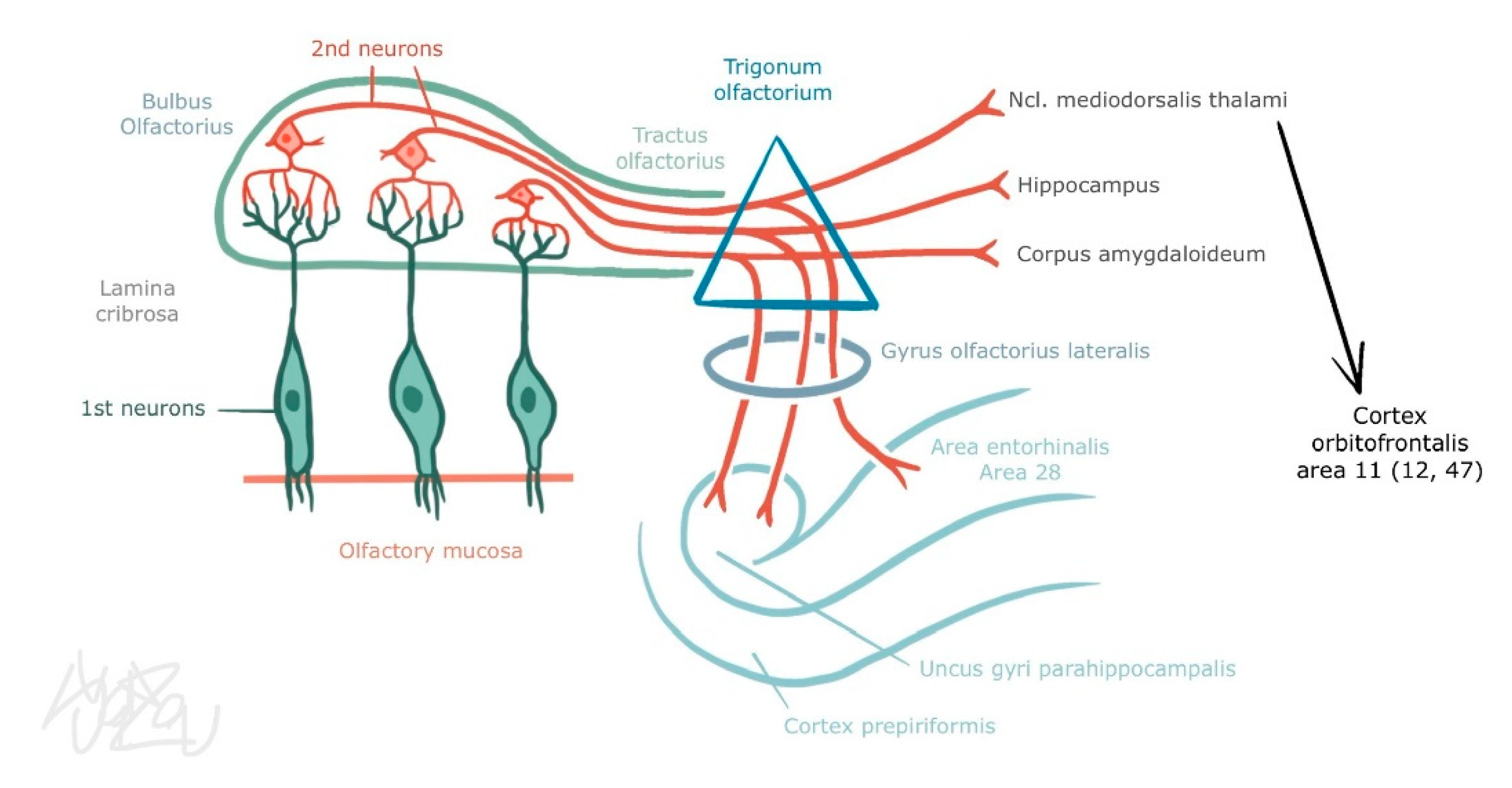
3.2. Central Part of the Olfactory Organ
3.3. Trigeminal Afferentation
4. Classification of Olfactory Disorders
- MB41.0 Anosmia
- MB41.1 Parosmia
- MB41.Z Disturbances of smell and taste, unspecified
5. Diseases Related to Olfactory Disorders
Nasal Microbiome and Olfactory Disorder
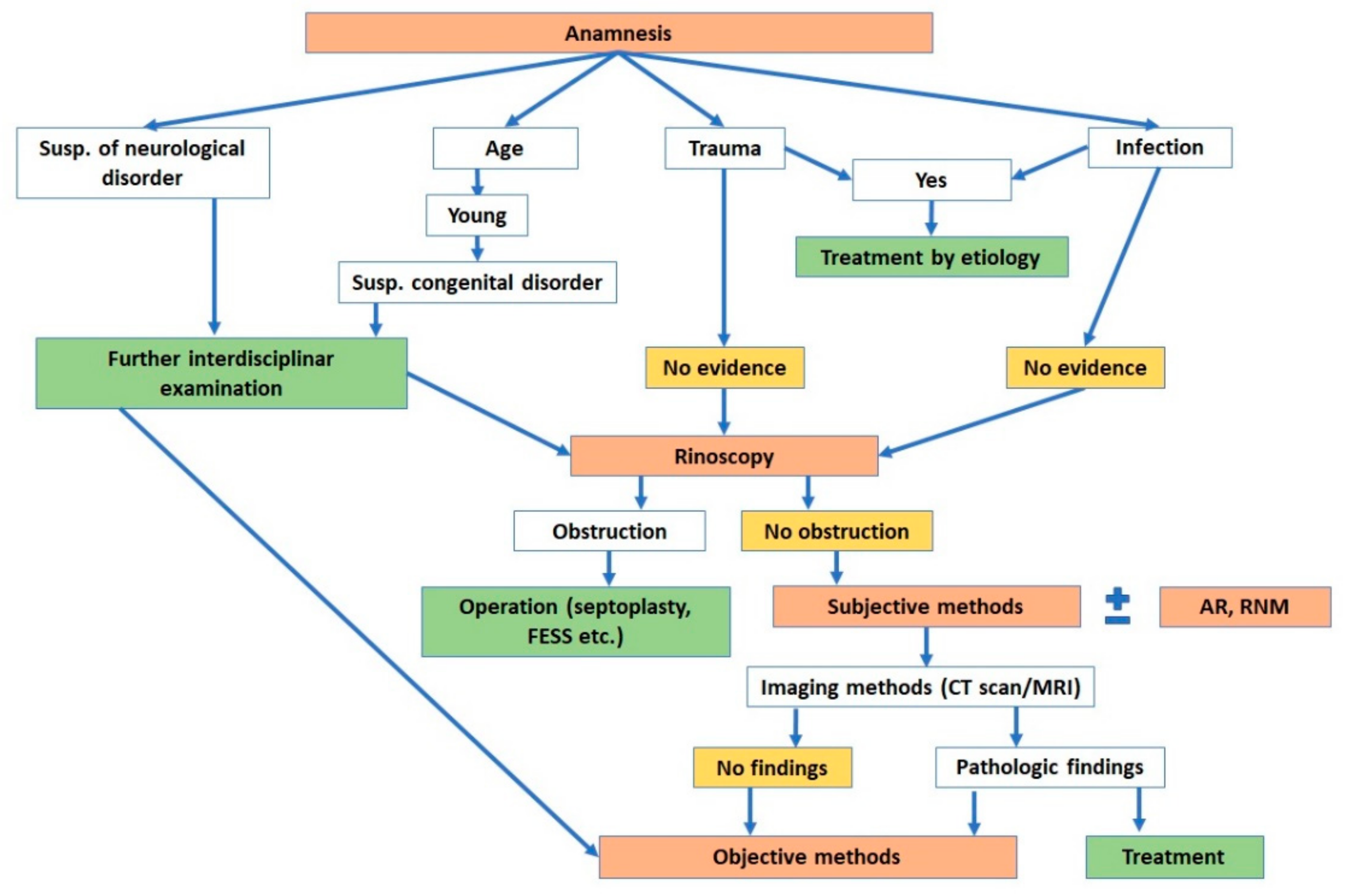
6. Clinical Methods of Olfactory Assessment: Our Algorithms
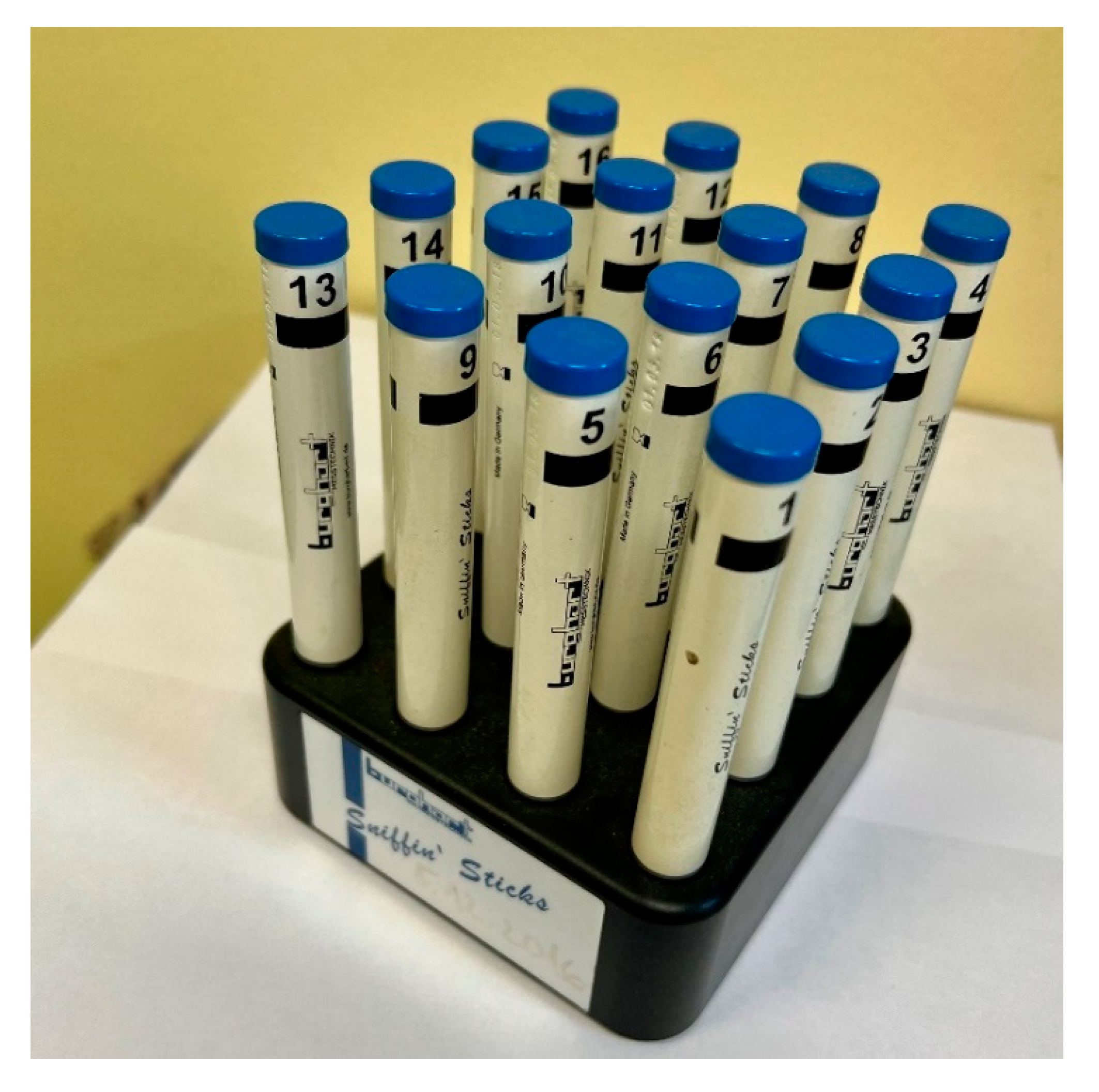
6.1. Subjective Methods
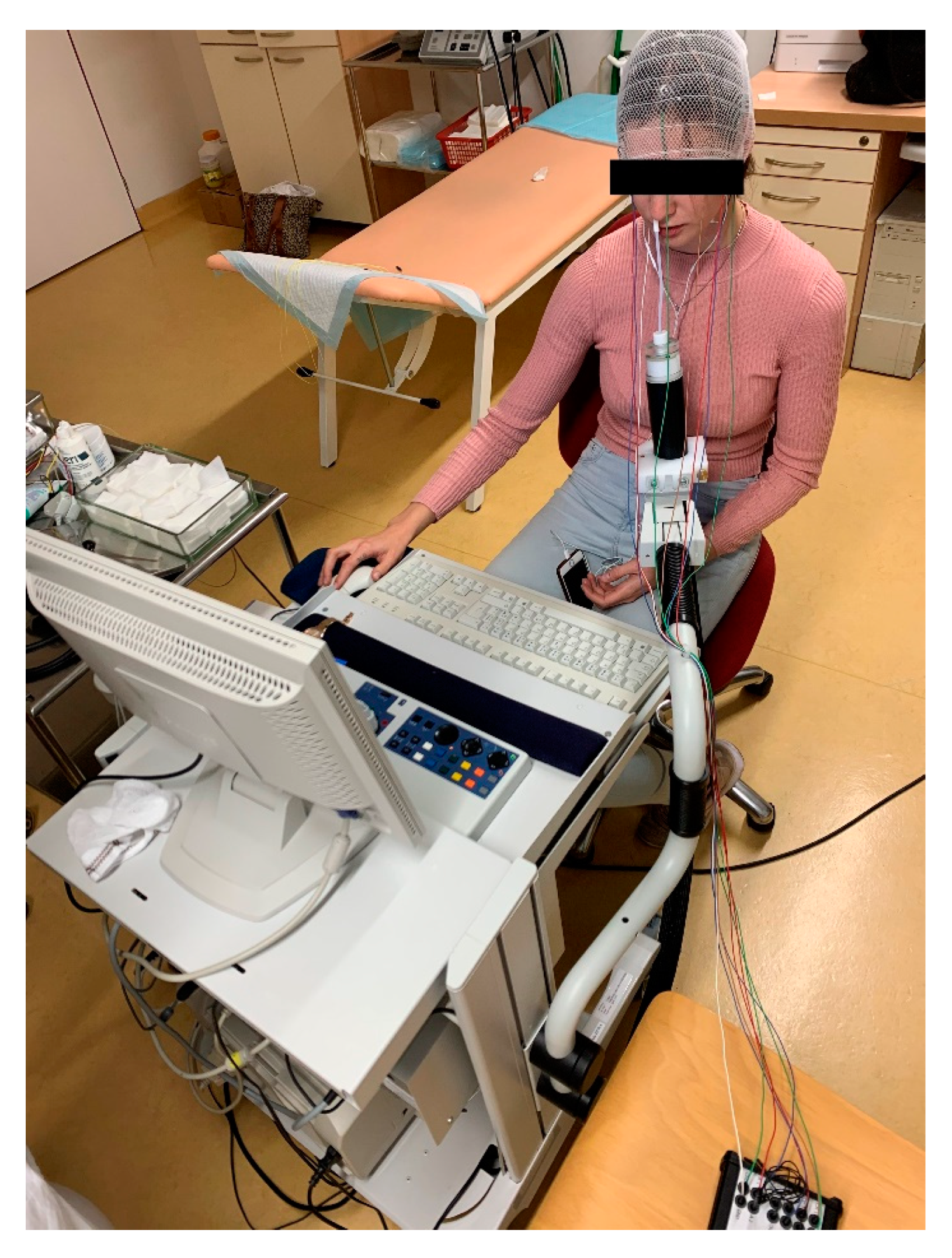
6.2. Objective Methods
7. Discussion
7.1. Discussion on Subjective Methods
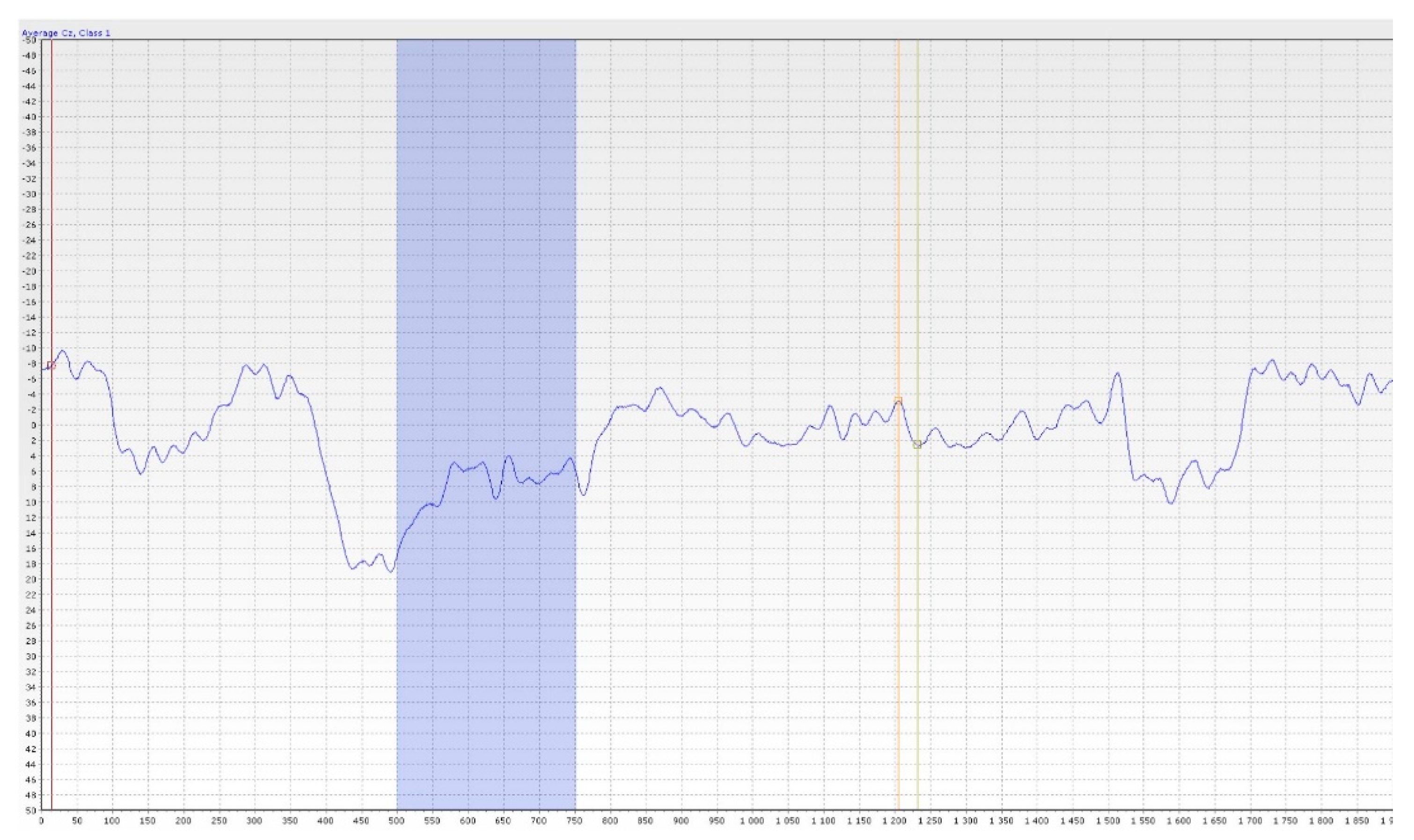
7.2. Discussion on Objective Olfactometry
8. Personal Experience
9. Leading Outlook for the Future
- -
- Detailed research on the objective assessment of olfactory loss in patients after COVID-19.
- -
- Detailed research on the objective assessment of olfactory loss in patients with Parkinson’s disease.
- -
- -
- Leading outlook for the future: in collaboration with neurosurgery—detailed research on the objective assessment of olfactory loss in patients before/after pituitary adenoma endoscopic surgery [42].
- -
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gizurarson, S. The Effect of Cilia and the Mucociliary Clearance on Successful Drug Delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, A.; Touhara, K. Enzymatic conversion of odorants in nasal mucus affects olfactory glomerular activation patterns and odor perception. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 16391–16398. [Google Scholar] [CrossRef] [PubMed]
- Escada, P. Localization and distribution of human olfactory mucosa in the nasal cavities. Acta Med. Port. 2013, 26, 200–207. [Google Scholar] [PubMed]
- Uraih, L.C.; Maronpot, R.R. Normal histology of the nasal cavity and application of special techniques. Environ. Health Perspect. 1990, 85, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, R.M. Regeneration and rewiring the olfactory bulb. Chem. Senses 2005, 30, i133–i134. [Google Scholar] [CrossRef]
- Kobayashi, M.; Costanzo, R.M. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem. Senses 2009, 34, 573–580. [Google Scholar] [CrossRef]
- Patin, A.; Pause, B.M. Human amygdala activations during nasal chemoreception. Neuropsychologia 2015, 78, 171–194. [Google Scholar] [CrossRef]
- Matsunaga, M.; Bai, Y.; Yamakawa, K.; Toyama, A.; Kashiwagi, M.; Fukuda, K.; Oshida, A.; Sanada, K.; Fukuyama, S.; Shinoda, J.; et al. Brain-immune interaction accompanying odor-evoked autobiographic memory. PLoS ONE 2013, 8, e72523. [Google Scholar] [CrossRef]
- Chen, D.; Dalton, P. The Effect of Emotion and Personality on Olfactory Perception. Chem. Senses 2005, 30, 345–351. [Google Scholar] [CrossRef]
- Pinto, J.M. Olfaction. Proc. Am. Thorac. Soc. 2011, 8, 46–52. [Google Scholar] [CrossRef]
- Frasnelli, J.; Schuster, B.; Hummel, T. Interactions between Olfaction and the Trigeminal System: What Can Be Learned from Olfactory Loss. Cereb. Cortex 2007, 17, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Kelly, C. Prevalence and persistence of smell and taste dysfunction in COVID-19; how should dental practices apply diagnostic criteria? BDJ Pract. 2021, 3, 22–23. [Google Scholar] [CrossRef]
- Schriever, V.A.; Zscheile, L.; Gellrich, J.; Hummel, T. Odor identification performance in children aged 3–6 years. Pediatr. Res. 2021, 89, 1304–1309. [Google Scholar] [CrossRef]
- Cameron, E.L. Olfactory perception in children. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, X.; Yao, L.Y.; Guo, Y.C.; Wei, Y.X. Study on the clinical characteristics of isolated congenital anosmia. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2021, 56, 442–446. (In Chinese) [Google Scholar] [CrossRef]
- Kovar, D.; Holy, R.; Voldrich, Z.; Fundova, P.; Astl, J. The Contribution of CT Navigation in Endoscopic Sinus Surgery: An Evaluation of Patient Postoperative Quality of Life and Olfaction Function Results. Otorinolaryngol. Foniatr. 2017, 66, 205–209. [Google Scholar]
- Kirschenbaum, D.; Imbach, L.L.; Ulrich, S.; Rushing, E.J.; Keller, E.; Reimann, R.R.; Frauenknecht, K.B.M.; Lichtblau, M.; Witt, M.; Hummel, T.; et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet 2020, 396, 366. [Google Scholar] [CrossRef]
- Vandersteen, C.; Payne, M.; Dumas, L.É.; Cancian, É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. J. Clin. Med. 2022, 11, 3275. [Google Scholar] [CrossRef]
- Burges Watson, D.L.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long COVID-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef]
- Howell, J.; Costanzo, R.M.; Reiter, E.R. Head trauma and olfactory function. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 39–45. [Google Scholar] [CrossRef]
- Landis, B.N.; Frasnelli, J.; Reden, J.; Lacroix, J.S.; Hummel, T. Differences between Orthonasal and Retronasal Olfactory Functions in Patients with Loss of the Sense of Smell. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Martinec Nováková, L.; Štěpánková, H.; Vodička, J.; Havlíček, J. Contribution of Olfactory Tests to Diagnosis of Neurodegenerative Diseases. Ceska Slov. Neurol. Neurochir. 2015, 78, 517–525. (In Czech) [Google Scholar]
- Doty, R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012, 46, 527–552. [Google Scholar] [CrossRef] [PubMed]
- Svačina, Š. Olfaction and gustation in diabetes. Vnitřní lékařství 2007, 53, 483–485. (In Czech) [Google Scholar]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Vent, J.; Robinson, A.M.; Gentry-Nielsen, M.J.; Conley, D.B.; Hallworth, R.; Leopold, D.A.; Kern, R.C. Pathology of the olfactory epithelium: Smoking and ethanol exposure. Laryngoscope 2004, 114, 1383–1388. [Google Scholar] [CrossRef]
- Kamel, U.F.; Maddison, P.; Whittaker, R. Impact of primary Sjögern’s syndrome on smell and taste: Effect on quality of life. Rheumatology 2009, 48, 1512–1514. [Google Scholar] [CrossRef]
- Wojciechowska, J.; Krajewski, W.; Krajewski, P.; Kręcicki, T. Granulomatosis with Polyangiitis in Otolaryngologist Practice: A Review of Current Knowledge. Clin. Exp. Otorhinolaryngol. 2016, 9, 8–13. [Google Scholar] [CrossRef]
- Steinbach, S.; Reindl, W.; Dempfle, A.; Schuster, A.; Wolf, P.; Hundt, W.; Huber, W. Smell and taste in inflammatory bowel disease. PLoS ONE 2013, 8, e73454. [Google Scholar] [CrossRef]
- Fundová, P.; Funda, D.P.; Kovář, D.; Holý, R.; Navara, M.; Tlaskalová-Hogenová, H. Increased expression of chemokine receptors CCR1 and CCR3 in nasal polyps: Molecular basis for recruitment of the granulocyte infiltrate. Folia Microbiol. 2013, 58, 219–224. [Google Scholar] [CrossRef]
- Corey, J.P.; Gungor, A.; Nelson, R.; Fredberg, J.; Lai, V. A comparison of the nasal cross-sectional areas and volumes obtained with acoustic rhinometry and magnetic resonance imaging. Otolaryngol. Head Neck Surg. 1997, 117, 349–354. [Google Scholar] [CrossRef]
- Vodička, J.; Pellant, A.; Chrobok, V. Screening of olfactory function using odourized markers. Rhinology 2007, 45, 164–168. [Google Scholar] [PubMed]
- Rombaux, P.; Collet, S.; Martinage, S.; Eloy, P.; Bertrand, B.; Negoias, S.; Hummel, T. Olfactory testing in clinical practise. B-ENT 2009, 5, 39–51. [Google Scholar] [PubMed]
- Muirhead, N.; Benjamin, E.; Saleh, H. Is the University of Pennsylvania Smell Identification Test (UPSIT) valid for the UK population? Otorhinolaryngologist 2013, 6, 99–103. [Google Scholar]
- Fornazieri, M.A.; Pinna, F.R.; Bezzera, T.F.P.; Antunes, M.B.; Voegels, R.L. Applicability of the university of pennsylvania smell identification test (SIT) in brazilians: Pilot study. Braz. J. Otorhinolaryngol. 2010, 76, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Lapid, H.; Hummel, T. Recording Odor-Evoked Response Potentials at the Human Olfactory Epithelium. Chem. Senses 2013, 38, 3–17. [Google Scholar] [CrossRef]
- Huart, C.; Legrain, V.; Hummel, T.; Rombaux, P.; Mouraux, A. Time-Frequency Analysis of Chemosensory Event-Related Potentials to Characterize the Cortical Representation of Odors in Humans. PLoS ONE 2012, 7, e33221. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Frey, S.; Freiburg, C.; Hörmann, K.; Zahnert, T.; Hummel, T. Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin. Neurophysiol. 2006, 117, 1367–1375. [Google Scholar] [CrossRef]
- Liu, J.; Pinto, J.M.; Yang, L.; Yao, L.; Miao, X.; Wei, Y. Evaluation of idiopathic olfactory loss with chemosensory event-related potentials and magnetic resonance imaging. Int. Forum. Allergy Rhinol. 2018, 8, 1315–1322. [Google Scholar] [CrossRef]
- Miao, X.; Yang, L.; Gu, H.; Ren, Y.; Chen, G.; Liu, J.; Wei, Y. Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. Eur. Arch. Otorhinolaryngol. 2015, 272, 1945–1953. [Google Scholar] [CrossRef]
- Biswas, K.; Wagner Mackenzie, B.; Ballauf, C.; Draf, J.; Douglas, R.G.; Hummel, T. Loss of bacterial diversity in the sinuses is associated with lower smell discrimination scores. Sci. Rep. 2020, 10, 16422. [Google Scholar] [CrossRef] [PubMed]
- Netuka, D.; Masopust, V.; Fundová, P.; Astl, J.; Školoudík, D.; Májovský, M.; Beneš, V. Olfactory Results of Endoscopic Endonasal Surgery for Pituitary Adenoma: A Prospective Study of 143 Patients. World Neurosurg. 2019, 129, e907–e914. [Google Scholar] [CrossRef] [PubMed]



| Quantitative | Qualitative |
|---|---|
| Hyposmia | Parosmia |
| Anosmia Hyperosmia | Phantosmia Specific anosmia |
| Conductive | Sensorineural |
|---|---|
| Loss of contact with olfactory region | Disorders of olfactory epithelium |
| Ventilation failure | Disorders of the olfactory pathway |
| Subjective | Objective |
|---|---|
| Sniffin‘ Sticks Test Odorized Markers Test (OMT) | Objective olfactometry |
| University of Pennsylvania Smell Identification Test (UPSIT) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Červený, K.; Janoušková, K.; Vaněčková, K.; Zavázalová, Š.; Funda, D.; Astl, J.; Holy, R. Olfactory Evaluation in Clinical Medical Practice. J. Clin. Med. 2022, 11, 6628. https://doi.org/10.3390/jcm11226628
Červený K, Janoušková K, Vaněčková K, Zavázalová Š, Funda D, Astl J, Holy R. Olfactory Evaluation in Clinical Medical Practice. Journal of Clinical Medicine. 2022; 11(22):6628. https://doi.org/10.3390/jcm11226628
Chicago/Turabian StyleČervený, Květoslav, Karla Janoušková, Kristýna Vaněčková, Šárka Zavázalová, David Funda, Jaromír Astl, and Richard Holy. 2022. "Olfactory Evaluation in Clinical Medical Practice" Journal of Clinical Medicine 11, no. 22: 6628. https://doi.org/10.3390/jcm11226628
APA StyleČervený, K., Janoušková, K., Vaněčková, K., Zavázalová, Š., Funda, D., Astl, J., & Holy, R. (2022). Olfactory Evaluation in Clinical Medical Practice. Journal of Clinical Medicine, 11(22), 6628. https://doi.org/10.3390/jcm11226628






