LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations
Abstract
1. Introduction
2. Global and National COPD Treatment Guidelines
3. Evidence for the Effectiveness and Safety of LABA/LAMA versus Other Therapies
3.1. Evidence for the Benefits of LABA/LAMA versus Monotherapies
3.2. Evidence for the Benefits of LABA/LAMA versus LABA/ICS
3.3. Comparison of LABA/LAMA versus Triple Therapy
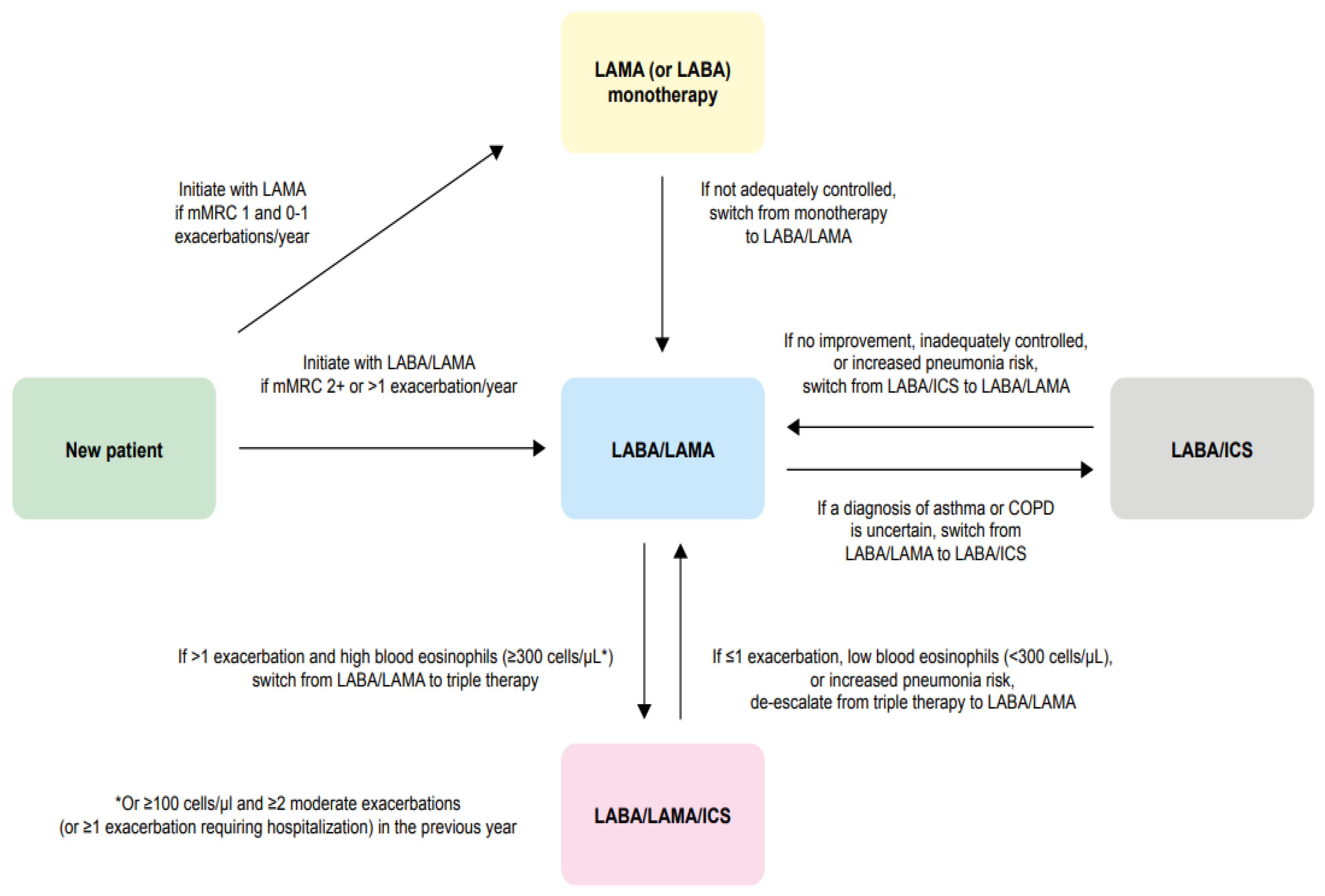
4. Summary of Recommendations for the Use of LABA/LAMA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: 2022 Report. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (accessed on 12 October 2022).
- Mayo Clinic. Shortness of Breath. Available online: https://www.mayoclinic.org/symptoms/shortness-of-breath/basics/definition/sym-20050890 (accessed on 30 March 2022).
- Hanania, N.A.; O’Donnell, D.E. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1127–1138. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.R.; Hass, N.; Liu, J.; Dreher, M. Daily Impact of COPD in Younger and Older Adults: Global Online Survey Results from over 1300 Patients. COPD 2020, 17, 419–428. [Google Scholar] [CrossRef]
- Schneider, L.P.; Furlanetto, K.C.; Rodrigues, A.; Lopes, J.R.; Hernandes, N.A.; Pitta, F. Sedentary Behaviour and Physical Inactivity in Patients with Chronic Obstructive Pulmonary Disease: Two Sides of the Same Coin? COPD 2018, 15, 432–438. [Google Scholar] [CrossRef]
- Hurst, J.R.; Gruffydd-Jones, K.; Biswas, M.; Guranlioglu, D.; Jenkins, M.; Stjepanovic, N.; Bamrara, A. Efficacy and Safety of LAMA/LABA Fixed-Dose Combination Therapies in Chronic Obstructive Pulmonary Disease: A Systematic Review of Direct and Indirect Treatment Comparisons. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1529–1543. [Google Scholar] [CrossRef]
- Singh, D. New combination bronchodilators for chronic obstructive pulmonary disease: Current evidence and future perspectives. Br. J. Clin. Pharmacol. 2015, 79, 695–708. [Google Scholar] [CrossRef]
- Nardini, S.; Camiciottoli, G.; Locicero, S.; Maselli, R.; Pasqua, F.; Passalacqua, G.; Pela, R.; Pesci, A.; Sebastiani, A.; Vatrella, A. COPD: Maximization of bronchodilation. Multidiscip. Respir. Med. 2014, 9, 50. [Google Scholar] [CrossRef]
- Sposato, B.; Petrucci, E.; Serafini, A.; Lena, F.; Lacerenza, L.G.; Montagnani, A.; Alessandri, M.; Cresti, A.; Scala, R.; Rogliani, P.; et al. Which LABA/LAMA should be chosen in COPD patients in real life? Pulm. Pharmacol. Ther. 2021, 71, 102076. [Google Scholar] [CrossRef]
- Malerba, M.; Foci, V.; Patrucco, F.; Pochetti, P.; Nardin, M.; Pelaia, C.; Radaeli, A. Single Inhaler LABA/LAMA for COPD. Front. Pharmacol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Cohen, J.S.; Miles, M.C.; Donohue, J.F.; Ohar, J.A. Dual therapy strategies for COPD: The scientific rationale for LAMA + LABA. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 785–797. [Google Scholar] [CrossRef]
- Rhee, C.K.; Yoshisue, H.; Lad, R. Fixed-Dose Combinations of Long-Acting Bronchodilators for the Management of COPD: Global and Asian Perspectives. Adv. Ther. 2019, 36, 495–519. [Google Scholar] [CrossRef]
- Nici, L.; Mammen, M.J.; Charbek, E.; Alexander, P.E.; Au, D.H.; Boyd, C.M.; Criner, G.J.; Donaldson, G.C.; Dreher, M.; Fan, V.S.; et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e56–e69. [Google Scholar] [CrossRef] [PubMed]
- Kardos, P.; Vogelmeier, C.; Worth, H.; Buhl, R.; Lossi, N.S.; Mailander, C.; Criee, C.P. A two-year evaluation of the ‘real life’ impact of COPD on patients in Germany: The DACCORD observational study. Respir. Med. 2017, 124, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Roman-Rodriguez, M.; Ribera, X.; Ritz, J.; Izquierdo, J.L.; On behalf of OPTI investigator’s group. Inhaled Corticosteroid Use Among COPD Patients in Primary Care in Spain. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Montes de Oca, M.; Menezes, A.M.; Wehrmeister, F.C.; Lopez Varela, M.V.; Mendoza, L.; Ramirez, L.; Miravitlles, M. Respiratory medication used in COPD patients from seven Latin American countries: The LASSYC study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Hanagama, M.; Ishida, M.; Sato, H.; Ono, M.; Yamanda, S.; Yamada, M.; Aizawa, H.; Yanai, M. Clinical characteristics and outcomes in Japanese patients with COPD according to the 2017 GOLD classification: The Ishinomaki COPD Network Registry. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3947–3955. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Chronic Obstructive Pulmonary Disease in over 16s: Diagnosis and Management NICE Guideline [NG115]. Available online: https://www.nice.org.uk/guidance/ng115/chapter/Recommendations#managing-stable-copd (accessed on 30 March 2022).
- Miravitlles, M.; Calle, M.; Molina, J.; Almagro, P.; Gomez, J.T.; Trigueros, J.A.; Cosio, B.G.; Casanova, C.; Lopez-Campos, J.L.; Riesco, J.A.; et al. Spanish COPD Guidelines (GesEPOC) 2021: Updated Pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022, 58, 69–81. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Buhl, R.; Burghuber, O.; Criee, C.P.; Ewig, S.; Godnic-Cvar, J.; Hartl, S.; Herth, F.; Kardos, P.; Kenn, K.; et al. Guideline for the Diagnosis and Treatment of COPD Patients—ISSUED by the German Respiratory Society and the German Atemwegsliga in Cooperation with the Austrian Society of Pneumology. Pneumologie 2018, 72, 253–308. [Google Scholar] [CrossRef]
- Cheng, S.L.; Lin, C.H. COPD Guidelines in the Asia-Pacific Regions: Similarities and Differences. Diagnostics 2021, 11, 1153. [Google Scholar] [CrossRef]
- Montes de Oca, M.; Lopez Varela, M.V.; Acuna, A.; Schiavi, E.; Rey, M.A.; Jardim, J.; Casas, A.; Tokumoto, A.; Torres Duque, C.A.; Ramirez-Venegas, A.; et al. ALAT-2014 Chronic Obstructive Pulmonary Disease (COPD) Clinical Practice Guidelines: Questions and answers. Arch. Bronconeumol. 2015, 51, 403–416. [Google Scholar] [CrossRef]
- Zatloukal, J.; Brat, K.; Neumannova, K.; Volakova, E.; Hejduk, K.; Kocova, E.; Kudela, O.; Kopecky, M.; Plutinsky, M.; Koblizek, V. Chronic obstructive pulmonary disease—Diagnosis and management of stable disease; a personalized approach to care, using the treatable traits concept based on clinical phenotypes. Position paper of the Czech Pneumological and Phthisiological Society. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2020, 164, 325–356. [Google Scholar] [CrossRef]
- Bourbeau, J.; Bhutani, M.; Hernandez, P.; Aaron, S.D.; Balter, M.; Beauchesne, M.-F.; D’Urzo, A.; Goldstein, R.; Kaplan, A.; Maltais, F.; et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD—2019 update of evidence. Can. J. Respir. Crit. Care Sleep Med. 2019, 3, 210–232. [Google Scholar] [CrossRef]
- Lung Foundation Australia. The COPD-X Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease 2020. Available online: https://copdx.org.au/wp-content/uploads/2021/02/COPDX-V2.62-June_Oct-2020-PUBLISHED.pdf (accessed on 19 April 2022).
- Chalmers, J.D.; Tebboth, A.; Gayle, A.; Ternouth, A.; Ramscar, N. Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: A retrospective study of UK general practice. NPJ Prim. Care Respir. Med. 2017, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Gruffydd-Jones, K.; Brusselle, G.; Jones, R.; Miravitlles, M.; Baldwin, M.; Stewart, R.; Rigazio, A.; Davis, E.; Keininger, D.L.; Price, D. Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: A real-world study. NPJ Prim. Care Respir. Med. 2016, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Rossi, A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir. Med. 2012, 106, 989–997. [Google Scholar] [CrossRef]
- Buhl, R.; Criee, C.P.; Kardos, P.; Vogelmeier, C.; Lossi, N.; Mailander, C.; Worth, H. A year in the life of German patients with COPD: The DACCORD observational study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1639–1646. [Google Scholar] [CrossRef]
- Miravitlles, M. GesEPOC 2021 and GOLD 2021. Closer Together or Further Apart? Arch. Bronconeumol. 2022, 58, 1–2. [Google Scholar] [CrossRef]
- Hizawa, N. LAMA/LABA vs ICS/LABA in the treatment of COPD in Japan based on the disease phenotypes. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1093–1102. [Google Scholar] [CrossRef][Green Version]
- Montes de Oca, M.; Lopez Varela, M.V.; Acuna, A.; Schiavi, E.; Casas, A.; Tokumoto, A.; Torres Duque, C.A.; Ramirez-Venegas, A.; Garcia, G.; Camelier, A.; et al. Incorporating New Evidence on Inhaled Medications in COPD. The Latin American Chest Association (ALAT) 2019. Arch. Bronconeumol. (Engl. Ed.) 2020, 56, 106–113. [Google Scholar] [CrossRef]
- Yang, I.A.; Brown, J.L.; George, J.; Jenkins, S.; McDonald, C.F.; McDonald, V.M.; Phillips, K.; Smith, B.J.; Zwar, N.A.; Dabscheck, E. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med. J. Aust. 2017, 207, 436–442. [Google Scholar] [CrossRef]
- Oba, Y.; Keeney, E.; Ghatehorde, N.; Dias, S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2018, 12, CD012620. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, W.C.; Huang, C.H.; Hsiang, Y.P.; Sheu, C.C.; Chen, Y.C.; Lin, M.C.; Chu, K.A.; Lee, C.H.; Wei, Y.F. LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937194. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Pai, V.; Aaron, S.D.; Nici, L.; Alhazzani, W.; Alexander, P.E. Dual LABA/LAMA Therapy versus LABA or LAMA Monotherapy for Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis in Support of the American Thoracic Society Clinical Practice Guideline. Ann. Am. Thorac. Soc. 2020, 17, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Braido, F.; Cazzola, M.; Clini, E.; Pelaia, G.; Rossi, A.; Scichilone, N.; Di Marco, F. LABA/LAMA fixed-dose combinations in patients with COPD: A systematic review. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3115–3130. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Price, D.; Anzueto, A.; Singh, D.; Altman, P.; Bader, G.; Patalano, F.; Fogel, R.; Kostikas, K. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Ora, J.; Puxeddu, E.; Cazzola, M.; Matera, M.G. LABA/LAMA combination in COPD: A meta-analysis on the duration of treatment. Eur. Respir. Rev. 2017, 26, 160043. [Google Scholar] [CrossRef]
- Calverley, P.M.A.; Anzueto, A.R.; Carter, K.; Gronke, L.; Hallmann, C.; Jenkins, C.; Wedzicha, J.; Rabe, K.F. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): A double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir. Med. 2018, 6, 337–344. [Google Scholar] [CrossRef]
- Calzetta, L.; Ora, J.; Cavalli, F.; Rogliani, P.; O’Donnell, D.E.; Cazzola, M. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: A pair-wise and network meta-analysis. Respir. Med. 2017, 129, 189–198. [Google Scholar] [CrossRef]
- Aziz, M.I.A.; Tan, L.E.; Wu, D.B.; Pearce, F.; Chua, G.S.W.; Lin, L.; Tan, P.T.; Ng, K. Comparative efficacy of inhaled medications (ICS/LABA, LAMA, LAMA/LABA and SAMA) for COPD: A systematic review and network meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3203–3231. [Google Scholar] [CrossRef]
- Mahler, D.A.; Decramer, M.; D’Urzo, A.; Worth, H.; White, T.; Alagappan, V.K.; Chen, H.; Gallagher, N.; Kulich, K.; Banerji, D. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: The BLAZE study. Eur. Respir. J. 2014, 43, 1599–1609. [Google Scholar] [CrossRef]
- Ichinose, M.; Nishimura, M.; Akimoto, M.; Kurotori, Y.; Zhao, Y.; de la Hoz, A.; Mishima, M. Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: Results from the DYNAGITO study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2147–2156. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Casaburi, R.; Frith, P.; Kirsten, A.; De Sousa, D.; Hamilton, A.; Xue, W.; Maltais, F. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur. Respir. J. 2017, 49, 1601348. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.T.; Karpel, J.; Bennett, N.; Clerisme-Beaty, E.; Gronke, L.; Voss, F.; Buhl, R. Effect of tiotropium and olodaterol on symptoms and patient-reported outcomes in patients with COPD: Results from four randomised, double-blind studies. NPJ Prim. Care Respir. Med. 2017, 27, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wedzicha, J.A.; Buhl, R.; Singh, D.; Vogelmeier, C.F.; de la Hoz, A.; Xue, W.; Anzueto, A.; Calverley, P.M.A. Tiotropium/Olodaterol Decreases Exacerbation Rates Compared with Tiotropium in a Range of Patients with COPD: Pooled Analysis of the TONADO((R))/DYNAGITO((R)) Trials. Adv. Ther. 2020, 37, 4266–4279. [Google Scholar] [CrossRef] [PubMed]
- Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Nakamura, S.; Anzai, T.; Hirata, K.; Ichinose, M. Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: Post hoc analysis of the VESUTO® study. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1789–1801. [Google Scholar] [CrossRef]
- Martinez, F.J.; Abrahams, R.A.; Ferguson, G.T.; Bjermer, L.; Gronke, L.; Voss, F.; Singh, D. Effects of baseline symptom burden on treatment response in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 181–194. [Google Scholar] [CrossRef]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Seki, T.; Anzai, T.; Nakamura, S.; Hirata, K. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1407–1419. [Google Scholar] [CrossRef]
- Price, D.; Ostrem, A.; Thomas, M.; Welte, T. Dual bronchodilation in COPD: Lung function and patient-reported outcomes—A review. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 141–168. [Google Scholar] [CrossRef]
- Maltais, F.; de la Hoz, A.; Casaburi, R.; O’Donnell, D. Effects of Tiotropium/Olodaterol on Activity-Related Breathlessness, Exercise Endurance and Physical Activity in Patients with COPD: Narrative Review with Meta-/Pooled Analyses. Adv. Ther. 2021, 38, 835–853. [Google Scholar] [CrossRef]
- Buhl, R.; Maltais, F.; Abrahams, R.; Bjermer, L.; Derom, E.; Ferguson, G.; Flezar, M.; Hebert, J.; McGarvey, L.; Pizzichini, E.; et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur. Respir. J. 2015, 45, 969–979. [Google Scholar] [CrossRef]
- Takahashi, K.; Uchida, M.; Kato, G.; Takamori, A.; Kinoshita, T.; Yoshida, M.; Tajiri, R.; Kojima, K.; Inoue, H.; Kobayashi, H.; et al. First-Line Treatment with Tiotropium/Olodaterol Improves Physical Activity in Patients with Treatment-Naive Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2115–2126. [Google Scholar] [CrossRef]
- Singh, D.; Ferguson, G.T.; Bolitschek, J.; Gronke, L.; Hallmann, C.; Bennett, N.; Abrahams, R.; Schmidt, O.; Bjermer, L. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir. Med. 2015, 109, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Urrutia, G.; Mathioudakis, A.G.; Ancochea, J. Efficacy and safety of tiotropium and olodaterol in COPD: A systematic review and meta-analysis. Respir. Res. 2017, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Labor, M.; Braido, F.; Bikov, A.; Lahousse, L.; Rogliani, P.; Baiardini, I. LABA/LAMA Fixed Dose Combination in Chronic Obstructive Pulmonary Disease: The Impact on Health-Related Quality of Life. Respiration 2018, 96, 370–381. [Google Scholar] [CrossRef]
- Beeh, K.M.; Westerman, J.; Kirsten, A.M.; Hebert, J.; Gronke, L.; Hamilton, A.; Tetzlaff, K.; Derom, E. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 32, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; Hamilton, A.; Voss, F.; Maleki-Yazdi, M.R. Dose Determination for a Fixed-Dose Drug Combination: A Phase II Randomized Controlled Trial for Tiotropium/Olodaterol Versus Tiotropium in Patients with COPD. Adv. Ther. 2019, 36, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A Systematic Review With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef]
- Maltais, F.; Aumann, J.L.; Kirsten, A.-M.; Nadreau, E.; Macesic, H.; Jin, X.; Hamilton, A.; O’Donnell, D.E. Dual bronchodilation with tiotropium/olodaterol further reduces activity-related breathlessness versus tiotropium alone in COPD. Eur. Respir. J. 2019, 53, 1802049. [Google Scholar] [CrossRef]
- Horita, N.; Goto, A.; Shibata, Y.; Ota, E.; Nakashima, K.; Nagai, K.; Kaneko, T. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2017, 2, CD012066. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest 2019, 155, 1158–1165. [Google Scholar] [CrossRef]
- Quint, J.K.; Montonen, J.; Esposito, D.B.; He, X.; Koerner, L.; Wallace, L.; de la Hoz, A.; Miravitlles, M. Effectiveness and safety of COPD maintenance therapy with tiotropium/olodaterol versus LABA/ICS in a US claims database. Adv. Ther. 2021, 38, 2249–2270. [Google Scholar] [CrossRef]
- Beeh, K.M.; Derom, E.; Echave-Sustaeta, J.; Gronke, L.; Hamilton, A.; Zhai, D.; Bjermer, L. The lung function profile of once-daily tiotropium and olodaterol via Respimat((R)) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler((R)) (ENERGITO((R)) study). Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Matera, M.G. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: A systematic review and meta-analysis. Eur. Respir. J. 2018, 52, 1801586. [Google Scholar] [CrossRef] [PubMed]
- Koarai, A.; Yamada, M.; Ichikawa, T.; Fujino, N.; Kawayama, T.; Sugiura, H. Triple versus LAMA/LABA combination therapy for patients with COPD: A systematic review and meta-analysis. Respir. Res. 2021, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Lloyd, D.R.; Kumar, S.; Ahmed, A.S.; Pai, V.; Kunadharaju, R.; Gupta, S.; Nici, L.; Aaron, S.D.; Alexander, P.E. Triple Therapy versus Dual or Monotherapy with Long-Acting Bronchodilators for Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2020, 17, 1308–1318. [Google Scholar] [CrossRef]
- Koarai, A.; Yamada, M.; Ichikawa, T.; Fujino, N.; Kawayama, T.; Sugiura, H. Triple versus LAMA/LABA combination therapy for Japanese patients with COPD: A systematic review and meta-analysis. Respir. Investig. 2022, 60, 90–98. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, J.; Liu, Y.; Lai, W.; Lin, C.; Qiu, K.; Wu, J.; Yao, W. Triple therapy in the management of chronic obstructive pulmonary disease: Systematic review and meta-analysis. BMJ 2018, 363, k4388. [Google Scholar] [CrossRef]
- Cabrera, C.; Quelen, C.; Ouwens, M.; Hedman, K.; Rigney, U.; Quint, J.K. Evaluating a Cox marginal structural model to assess the comparative effectiveness of inhaled corticosteroids versus no inhaled corticosteroid treatment in chronic obstructive pulmonary disease. Ann. Epidemiol. 2022, 67, 19–28. [Google Scholar] [CrossRef]
- Quint, J.; Montonen, J.; Singh, D.; Wachtel, H.; Attick, S.; Palli, S.; Frazer, M.; Willey, V.; Giessel, G. New insights into the optimal management of COPD: Extracts from CHEST 2021 annual meeting (October 17-20, 2021). Expert Rev. Respir. Med. 2022, 16, 485–493. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: Cohort study in real-world clinical practice. Chest 2020, 157, 846–855. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, J.; Jo, J.; Jang, E.J.; Lee, C.H. Comparisons of exacerbations and mortality among regular inhaled therapies for patients with stable chronic obstructive pulmonary disease: Systematic review and Bayesian network meta-analysis. PLoS Med. 2019, 16, e1002958. [Google Scholar] [CrossRef]
- Martinez, F.J.; Fabbri, L.M.; Ferguson, G.T.; Orevillo, C.; Darken, P.; Martin, U.J.; Reisner, C. Baseline Symptom Score Impact on Benefits of Glycopyrrolate/Formoterol Metered Dose Inhaler in COPD. Chest 2017, 152, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, J.F.; Jenkins, M.; Assam, P.N.; Wang, L.; Lipworth, B.J. Glycopyrrolate/formoterol fumarate metered dose inhaler for maintenance-naive patients with chronic obstructive pulmonary disease: A post-hoc analysis of the randomized PINNACLE trials. Respir. Res. 2020, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.T.; Flezar, M.; Korn, S.; Korducki, L.; Gronke, L.; Abrahams, R.; Buhl, R. Efficacy of Tiotropium + Olodaterol in Patients with Chronic Obstructive Pulmonary Disease by Initial Disease Severity and Treatment Intensity: A Post Hoc Analysis. Adv. Ther. 2015, 32, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; de la Hoz, A.; Xue, W.; Singh, D.; Ferguson, G.T. Efficacy of Tiotropium/Olodaterol Compared with Tiotropium as a First-Line Maintenance Treatment in Patients with COPD Who Are Naive to LAMA, LABA and ICS: Pooled Analysis of Four Clinical Trials. Adv. Ther. 2020, 37, 4175–4189. [Google Scholar] [CrossRef]
- Singh, D.; Maleki-Yazdi, M.R.; Tombs, L.; Iqbal, A.; Fahy, W.A.; Naya, I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1413–1424. [Google Scholar] [CrossRef]
- Muro, S.; Yoshisue, H.; Kostikas, K.; Olsson, P.; Gupta, P.; Wedzicha, J.A. Indacaterol/glycopyrronium versus tiotropium or glycopyrronium in long-acting bronchodilator-naive COPD patients: A pooled analysis. Respirology 2020, 25, 393–400. [Google Scholar] [CrossRef]
- Rabe, K.F.; Chalmers, J.D.; Miravitlles, M.; Kocks, J.W.H.; Tsiligianni, I.; de la Hoz, A.; Xue, W.; Singh, D.; Ferguson, G.T.; Wedzicha, J. Tiotropium/Olodaterol Delays Clinically Important Deterioration Compared with Tiotropium Monotherapy in Patients with Early COPD: A Post Hoc Analysis of the TONADO((R)) Trials. Adv. Ther. 2021, 38, 579–593. [Google Scholar] [CrossRef]
- Maltais, F.; Bjermer, L.; Kerwin, E.M.; Jones, P.W.; Watkins, M.L.; Tombs, L.; Naya, I.P.; Boucot, I.H.; Lipson, D.A.; Compton, C.; et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: The EMAX randomised trial. Respir. Res. 2019, 20, 238. [Google Scholar] [CrossRef]
- Donohue, J.F.; Worsley, S.; Zhu, C.Q.; Hardaker, L.; Church, A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir. Med. 2015, 109, 870–881. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Paggiaro, P.L.; Dorca, J.; Sliwinski, P.; Mallet, M.; Kirsten, A.M.; Beier, J.; Seoane, B.; Segarra, R.M.; Leselbaum, A. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: A phase 3 COPD study. Eur. Respir. J. 2016, 48, 1030–1039. [Google Scholar] [CrossRef]
- Roman-Rodriguez, M.; Kaplan, A. GOLD 2021 Strategy Report: Implications for Asthma-COPD Overlap. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Andreu, I.; Romero, Y.; Sitjar, S.; Altes, A.; Anton, E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br. J. Gen. Pract. 2012, 62, e68–e75. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, S.; Kim, J.-H.; Kim, S.-H.; Lee, T.; Yoon, S.-Y.; Kim, M.-H.; Moon, J.-Y.; Yang, M.-S.; Jung, J.-W. A randomized, noninferiority trial comparing ICS+ LABA with ICS+ LABA+ LAMA in asthma-COPD overlap (ACO) treatment: The ACO Treatment with Optimal Medications (ATOMIC) study. J. Allergy Clin. Immunol. Pract. 2021, 9, 1304–1311.e1302. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Ritondo, B.L.; de Marco, P.; Cazzola, M.; Rogliani, P. Evaluating triple ICS/LABA/LAMA therapies for COPD patients: A network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies. Expert Rev. Respir. Med. 2021, 15, 143–152. [Google Scholar] [CrossRef]
- Miravitlles, M.; Verhamme, K.; Calverley, P.M.A.; Dreher, M.; Bayer, V.; Gardev, A.; de la Hoz, A.; Wedzicha, J.; Price, D. A pooled analysis of mortality in patients with COPD receiving dual bronchodilation with and without additional inhaled corticosteroid. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 545–558. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Triple Inhaler versus Dual Bronchodilator Therapy in COPD: Real-World Effectiveness on Mortality. COPD 2022, 19, 1–9. [Google Scholar] [CrossRef]
- Vanfleteren, L.; Fabbri, L.M.; Papi, A.; Petruzzelli, S.; Celli, B. Triple therapy (ICS/LABA/LAMA) in COPD: Time for a reappraisal. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3971. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Laska, I.F.; Franssen, F.M.E.; Janssens, W.; Pavord, I.; Rigau, D.; McDonnell, M.J.; Roche, N.; Sin, D.D.; Stolz, D.; et al. Withdrawal of inhaled corticosteroids in COPD: A European Respiratory Society guideline. Eur. Respir. J. 2020, 55, 2000351. [Google Scholar] [CrossRef]
- Magnussen, H.; Disse, B.; Rodriguez-Roisin, R.; Kirsten, A.; Watz, H.; Tetzlaff, K.; Towse, L.; Finnigan, H.; Dahl, R.; Decramer, M.; et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014, 371, 1285–1294. [Google Scholar] [CrossRef]
- Avdeev, S.; Aisanov, Z.; Arkhipov, V.; Belevskiy, A.; Leshchenko, I.; Ovcharenko, S.; Shmelev, E.; Miravitlles, M. Withdrawal of inhaled corticosteroids in COPD patients: Rationale and algorithms. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1267–1280. [Google Scholar] [CrossRef]
- Chapman, K.R.; Hurst, J.R.; Frent, S.M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, H.R.; Wing, K.; Douglas, I.; Kiddle, S.J.; Quint, J.K. Inhaled Corticosteroid Withdrawal and Change in Lung Function in Primary Care Chronic Obstructive Pulmonary Disease Patients in England. Ann. Am. Thorac. Soc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Auladell-Rispau, A.; Monteagudo, M.; Vazquez-Niebla, J.C.; Mohammed, J.; Nunez, A.; Urrutia, G. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev. 2021, 30, 210075. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St Rose, E.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Monteagudo, M.; Nunez, A.; Solntseva, I.; Dhalwani, N.; Booth, A.; Barrecheguren, M.; Lambrelli, D.; Miravitlles, M. Treatment Pathways Before and After Triple Therapy in COPD: A Population-based Study in Primary Care in Spain. Arch. Bronconeumol. (Engl. Ed.) 2021, 57, 205–213. [Google Scholar] [CrossRef]
| LABA/LAMA | Device | Approved Dose | Frequency of Administration |
|---|---|---|---|
| Tiotropium/olodaterol | Respimat® | 2.5/2.5 µg * | Once daily |
| Aclidinium/formoterol | Genuair® | 340/12 µg † | Twice daily |
| 400/12 µg ‡ | Twice daily | ||
| Umeclidinium/vilanterol | Ellipta® | 55/22 µg § | Once daily |
| 62.5/25 µg ‖ | Once daily | ||
| Glycopyrronium/indacaterol | Breezhaler® | 85/43 µg ¶ | Once daily |
| Neohaler® | 27.5/15.6 µg ** | Twice daily | |
| Glycopyrronium/formoterol fumarate | Aerosphere® | 7.2/5 μg †† | Twice daily |
| 9/4.8 μg ‡‡ | Twice daily |
| Guideline | Dyspnea, Infrequent Exacerbations | Dyspnea, Frequent Exacerbations |
|---|---|---|
| GOLD [1] | Initial treatment
| Initial treatment
|
| ATS [13] |
|
|
| NICE [18] |
|
|
| Spain [19,30] |
|
|
| Germany [20] |
|
|
| Japan [21,31] |
|
|
| Latin America (ALAT) [32] |
|
|
| Czech Republic [23] |
|
|
| Canada [24] |
|
|
| Australia and New Zealand (TSANZ) [33] |
|
|
| LABA/LAMA versus | Lung Function | Dyspnea | Exacerbations | Exercise Tolerance | Health/ Functional Status/ Quality of Life | Pneumonia |
|---|---|---|---|---|---|---|
| LAMA | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] |
| Calzetta Eur Respir Rev 2017 MA [39] | Calzetta Eur Respir Rev 2017 MA [39] | Calverley Lancet Respir Med 2018 RCT [40] | Calzetta Respir Med 2017 MA [41] | Calzetta Eur Respir Rev 2017 MA [39] | Oba Cochrane Library 2018 SR/MA [34] | |
| Aziz Int J Chron Obstruct Pulmon Dis 2018 SR/MA [42] | Mahler Eur Respir J 2014 RCT [43] | Ichinose Int J Chron Obstruct Pulmon Dis 2018 RCT [44] | O’Donnell Eur Respir J 2017 PRCT [45] | Ferguson NPJ Prim Care Respir Med 2017 PRCT [46] | ||
| Mahler Eur Respir J 2014 RCT [43] | Ferguson NPJ Prim Care Respir Med 2017 PRCT [46] | Wedzicha Adv Ther 2020 PRCT [47] | Minakata Int J Chron Obstruct Pulmon Dis 2019 PRCT [48] | Martinez Int J Chron Obstruct Pulmon Dis 2019 PRCT [49] | ||
| Martinez Int J Chron Obstruct Pulmon Dis 2019 PRCT [49] | Martinez Int J Chron Obstruct Pulmon Dis 2019 PRCT [49] | Chen Ther Adv Respir Dis 2020 SR/MA [35] | Ichinose Int J Chron Obstruct Pulmon Dis 2018 RCT [50] | Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | ||
| Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | Maltais Adv Ther 2021 MA/PRCT [52] | Buhl Eur Respir J 2015 PRCT [53] | ||
| Buhl Eur Respir J 2015 PRCT [53] | O’Donnell Eur Respir J 2017 PRCT [45] | Takahashi Int J Chron Obstruct Pulmon Dis 2020 RCT [54] | Singh Respir Med 2015 PRCT [55] | |||
| Singh Respir Med 2015 PRCT [55] | Miravitlles Respir Res 2017 SR/MA [56] | Labor Respiration 2018 SR [57] | ||||
| Beeh Pulm Pharmacol Ther 2015 RCT [58] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | Miravitlles Respir Res 2017 SR/MA [56] | ||||
| Maltais Adv Ther 2019 RCT [59] | Takahashi Int J Chron Obstruct Pulmon Dis 2020 RCT [54] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | ||||
| Miravitlles Respir Res 2017 SR/MA [56] | Calzetta Chest 2016 SR/MA [60] | Calzetta Chest 2016 SR/MA [60] | ||||
| Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | ||||
| Calzetta Chest 2016 SR/MA [60] | Maltais Eur Respir J 2019 RCT [61] | |||||
| O’Donnell Eur Resp J 2017 PRCT [45] | ||||||
| Ichinose Int J Chron Obstruct Pulmon Dis 2018 RCT2 [50] | ||||||
| Maltais Adv Ther 2021 MA/PRCT [52] | ||||||
| Takahashi Int J Chron Obstruct Pulmon Dis 2020 RCT [54] | ||||||
| LABA | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Oba Cochrane Library 2018 SR/MA [34] |
| Calzetta Eur Respir Rev 2017 MA [39] | Calzetta Eur Respir Rev 2017 MA [39] | Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | O’Donnell Eur Respir J 2017 PRCT [45] | Calzetta Eur Respir Rev 2017 MA [39] | ||
| Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | Ferguson NPJ Prim Care Respir Med 2017 PRCT [46] | Ferguson NPJ Prim Care Respir Med 2017 PRCT [46] | ||||
| Beeh Pulm Pharmacol Ther 2015 RCT [58] | Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | Price Int J Chron Obstruct Pulmon Dis 2017 SR [51] | ||||
| Miravitlles Respir Res 2017 SR/MA [56] | Miravitlles Respir Res 2017 SR/MA [56] | Miravitlles Respir Res 2017 SR/MA [56] | ||||
| Calzetta Chest 2016 SR/MA [60] | Calzetta Chest 2016 SR/MA [60] | Calzetta Chest 2016 SR/MA [60] | ||||
| O’Donnell Eur Respir J 2017 PRCT [45] | O’Donnell Eur Respir J 2017 PRCT [45] | Labor Respiration 2018 SR [57] | ||||
| Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | Mammen et al. Ann Am Thorac Soc 2020 aSR/MA [36] | |||||
| LABA/ICS | Horita Cochrane Database Syst Rev 2017 CR [62] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Horita Cochrane Database Syst Rev 2017 CR [62] | Horita Cochrane Database Syst Rev 2017 CR [62] | Suissa Chest 2019 RWS [63] | |
| Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Miravitlles Respir Res 2017 SR/MA [56] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Rogliani Int J Chron Obstruct Pulmon Dis 2018 SR [37] | Quint Adv Ther 2021 RWS [64] | ||
| Aziz Int J Chron Obstruct Pulmon Dis 2018 SR/MA [42] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | Miravitlles Respir Res 2017 SR/MA [56] | Horita Cochrane Database Syst Rev 2017 CR [62] | ||
| Beeh Int J Chron Obstruct Pulmon Dis 2016 RCT [65] | Quint Adv Ther 2021 RWS [64] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | |||
| Miravitlles Respir Res 2017 SR/MA [56] | Suissa Chest 2019 RWS [63] | |||||
| Rodrigo Int J Chron Obstruct Pulmon Dis 2017 SR/MA [38] | ||||||
| Triple therapy | Cazzola Eur Respir J 2018 SR/MA [66] | Koarai Respir Res 2021 SR/MA [67] | Cazzola Eur Respir J 2018 SR/MA [66] | Koarai Respir Res 2021 SR/MA [67] | Mammen Annals ATS 2020 bSR/MA [68] | |
| Koarai Respir Res 2021 SR/MA [67] | Mammen Annals ATS 2020 bSR/MA [68] | Koarai Respir Res 2021 SR/MA [67] | Koarai Respir Investig 2022 SR/MA [69] | Zheng The BMJ 2018 SR/MA [70] | ||
| Koarai Respir Investig 2022 SR/MA [69] | Cabrera Ann Epidemiol 2022 RWS [71] | Zheng The BMJ 2018 SR/MA [70] | Quint Expert Rev Respir Med 2022 RWS [72] | |||
| Zheng The BMJ 2018 SR/MA [70] | Quint Expert Rev Respir Med 2022 RWS [72] | Koarai Respir Res 2021 SR/MA [67] | ||||
| Suissa Chest 2020 RWS [73] | Suissa Chest 2020 RWS [73] | |||||
| Koarai Respir Investig 2022 SR/MA [69] | Cazzola Eur Respir J 2018 SR/MA [66] | |||||
| Lee PLOS Med 2019 SR/MA [74] | Koarai Respir Investig 2022 SR/MA [69] | |||||
| Mammen Annals ATS 2020 bSR/MA [68] | Lee PLOS Med 2019 SR/MA [74] | |||||
| Zheng The BMJ 2018 SR/MA [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miravitlles, M.; Kawayama, T.; Dreher, M. LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations. J. Clin. Med. 2022, 11, 6623. https://doi.org/10.3390/jcm11226623
Miravitlles M, Kawayama T, Dreher M. LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations. Journal of Clinical Medicine. 2022; 11(22):6623. https://doi.org/10.3390/jcm11226623
Chicago/Turabian StyleMiravitlles, Marc, Tomotaka Kawayama, and Michael Dreher. 2022. "LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations" Journal of Clinical Medicine 11, no. 22: 6623. https://doi.org/10.3390/jcm11226623
APA StyleMiravitlles, M., Kawayama, T., & Dreher, M. (2022). LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations. Journal of Clinical Medicine, 11(22), 6623. https://doi.org/10.3390/jcm11226623







