False versus True Statin Intolerance in Patients with Peripheral Artery Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Definition of Statin Treatment
2.3. Evaluation of Statin Intolerance
2.4. Follow-Up in the Lipid Outpatient Clinic and Definition of Clinical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics and Identification of Patients with Possible or Probable Statin Intolerance
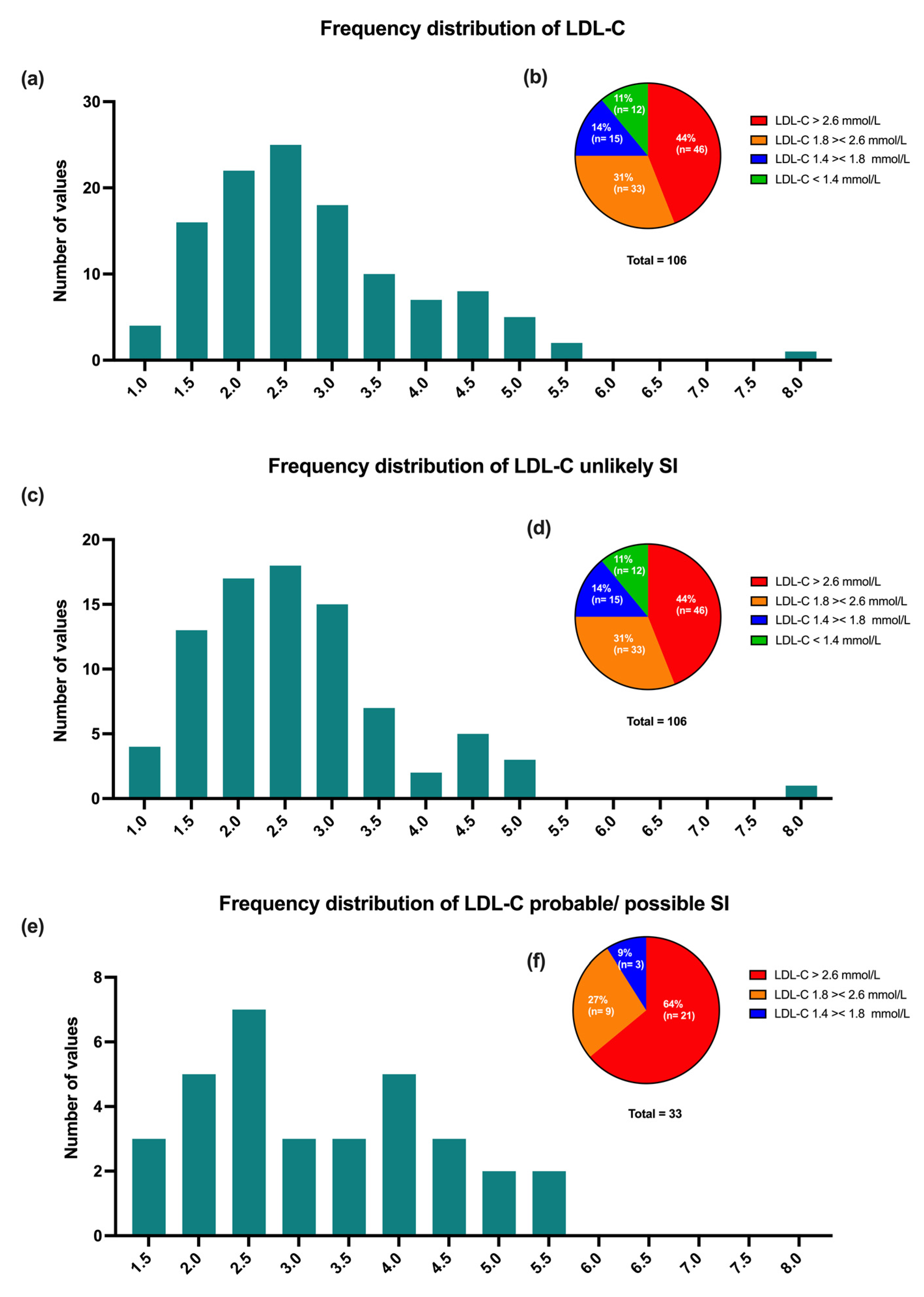
3.2. Statin Therapy and Lipid Profile at Baseline
3.3. Lipid-Lowering Therapy over Time
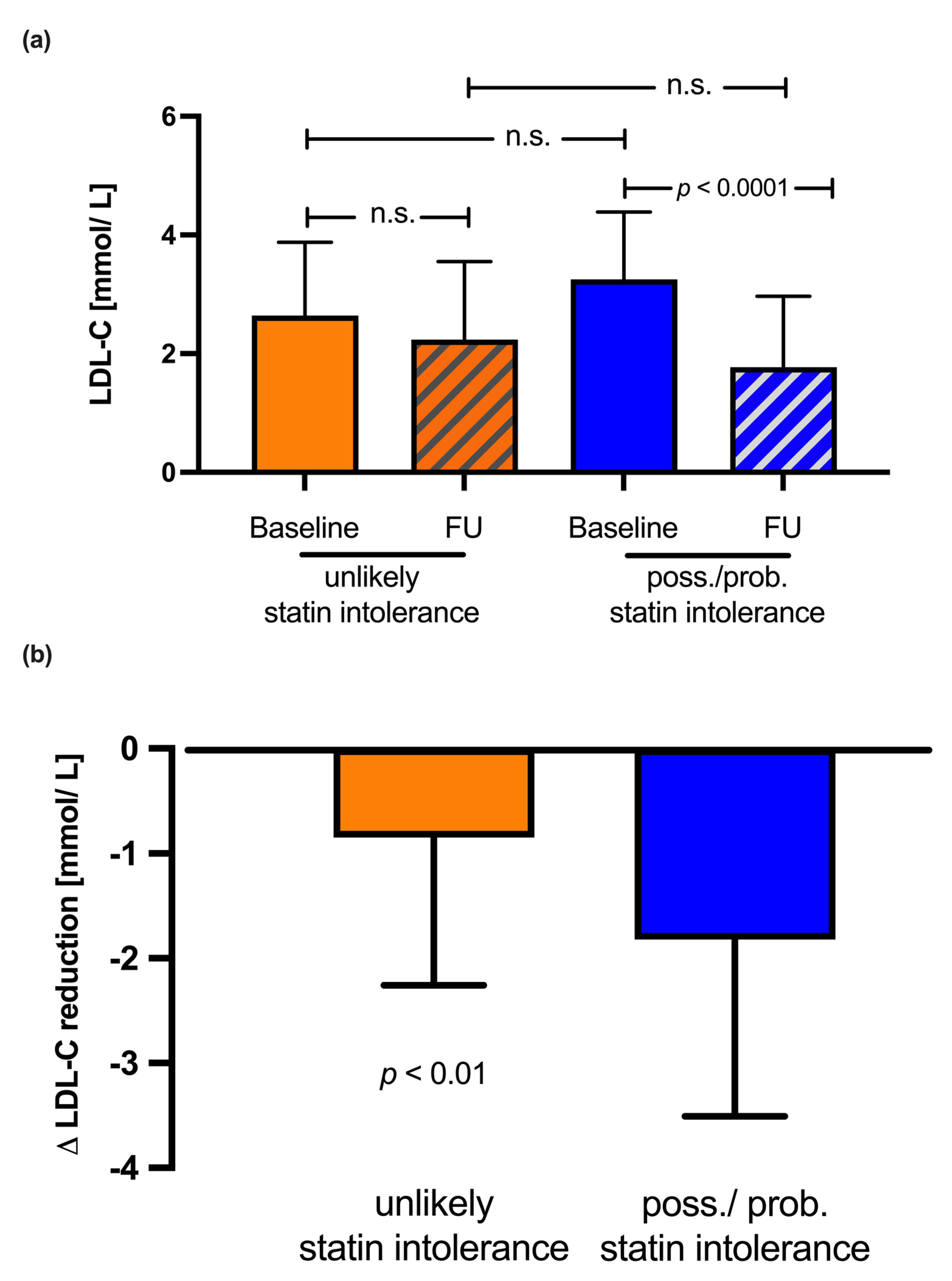
3.4. LDL-C Levels over Time
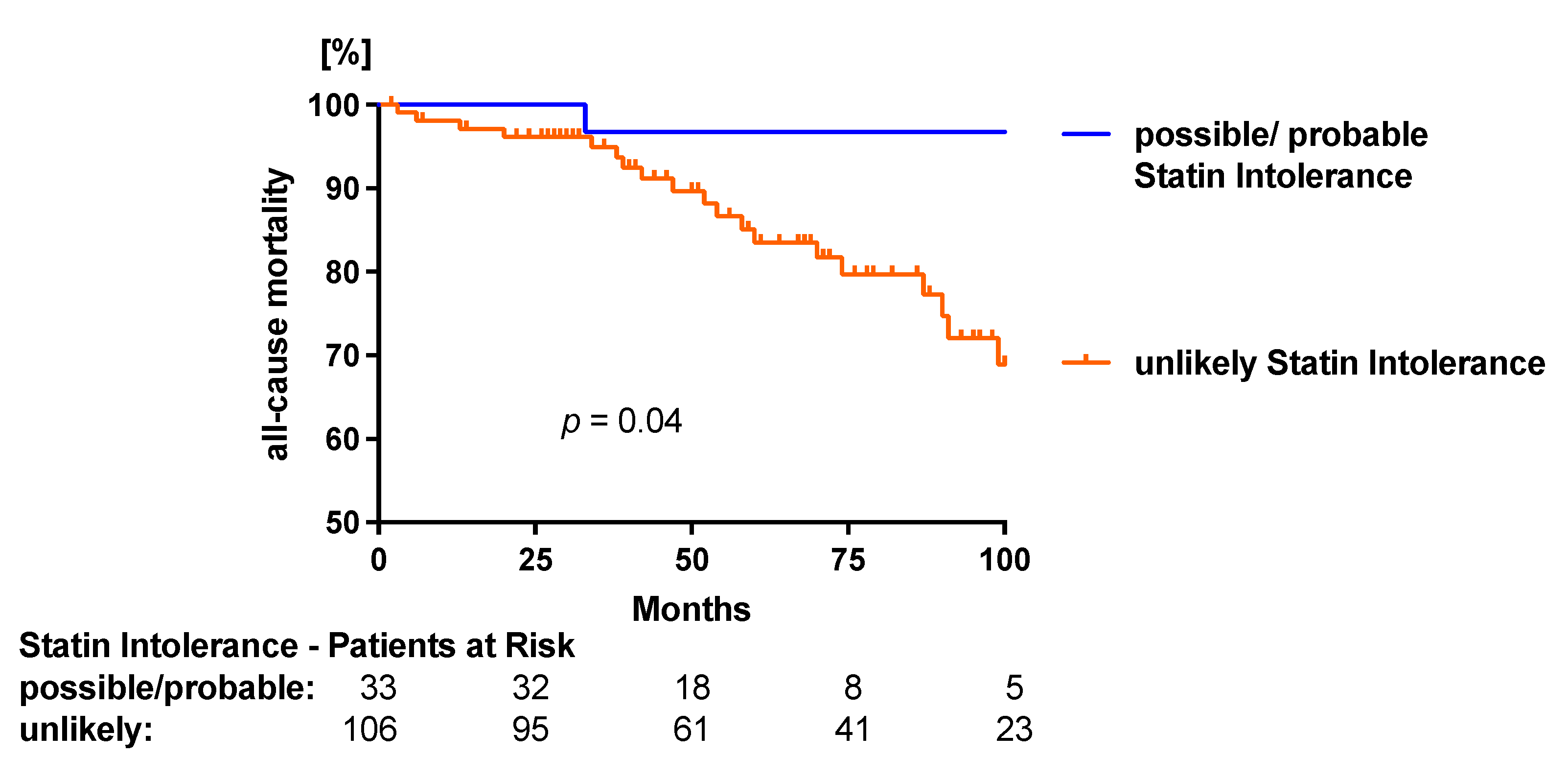
3.5. Clinical Endpoints
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Drozda, J.P.; Ferguson, T.B.; Jneid, H.; Krumholz, H.M.; Nallamothu, B.K.; Olin, J.W.; Ting, H.H. 2015 ACC/AHA Focused Update of Secondary Prevention Lipid Performance Measures. A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 2016, 67, 558–587. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 1—Full Report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of Dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- Cacoub, P.P.; Abola, M.T.B.; Baumgartner, I.; Bhatt, D.L.; Creager, M.A.; Liau, C.-S.; Goto, S.; Röther, J.; Steg, P.G.; Hirsch, A.T. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis 2009, 204, e86–e92. [Google Scholar] [CrossRef]
- Vonbank, A.; Agewall, S.; Kjeldsen, K.P.; Lewis, B.S.; Torp-Pedersen, C.; Ceconi, C.; Funck-Brentano, C.; Kaski, J.C.; Niessner, A.; Tamargo, J.; et al. Comprehensive efforts to increase adherence to statin therapy. Eur. Heart J. 2017, 38, 2473–2479. [Google Scholar] [CrossRef]
- Vonbank, A.; Drexel, H.; Agewall, S.; Lewis, B.S.; Dopheide, J.F.; Kjeldsen, K.; Ceconi, C.; Savarese, G.; Rosano, G.; Wassmann, S.; et al. Reasons for Disparity in Statin Adherence Rates between Clinical Trials and Real World Observations. A Review. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 230–236. [Google Scholar] [CrossRef]
- Chodick, G.; Shalev, V.; Gerber, Y.; Heymann, A.D.; Silber, H.; Simah, V.; Kokia, E. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: A population-based retrospective cohort study in Israel. Clin. Ther. 2008, 30, 2167–2179. [Google Scholar] [CrossRef]
- Jackevicius, C.A.; Mamdani, M.; Tu, J.V. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002, 288, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Khan, H.; Heydon, E.; Shroufi, A.; Fahimi, S.; Moore, C.; Stricker, B.; Mendis, S.; Hofman, A.; Mant, J.; et al. Adherence to cardiovascular therapy: A meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013, 34, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Goff, D.C., Jr.; Lloyd-Jones, D.M.; Smith, S.C., Jr. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Lautsch, D.; Ferrieres, J.; Kastelein, J.; Drexel, H.; Horack, M.; Brudi, P.; Vanneste, B.; Bramlage, P.; Chazelle, F.; et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis 2016, 255, 200–209. [Google Scholar] [CrossRef]
- Task Force, M. Guidelines ESCCfP, Societies ESCNC. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Baker, S.K.; Jacobson, T.A.; Kopecky, S.L.; Parker, B.A. The National Lipid Association’s Muscle Safety Expert P. An assessment by the Statin Muscle Safety Task Force: 2014 update. J. Clin. Lipidol. 2014, 8, S58–S71. [Google Scholar] [CrossRef]
- Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Cole, S.M.; Keadle, J.; Chipkin, S.; Pescatello, L.S.; Simpson, K.; White, C.M.; et al. Effect of statins on skeletal muscle function. Circulation 2013, 127, 96–103. [Google Scholar] [CrossRef]
- Chen, D.C.; Singh, G.D.; Armstrong, E.J.; Waldo, S.W.; Laird, J.R.; Amsterdam, E.A. Long-Term Comparative Outcomes of Patients With Peripheral Artery Disease with and Without Concomitant Coronary Artery Disease. Am. J. Cardiol. 2017, 119, 1146–1152. [Google Scholar] [CrossRef]
- Welten, G.M.; Schouten, O.; Hoeks, S.E.; Chonchol, M.; Vidakovic, R.; van Domburg, R.T.; Bax, J.J.; van Sambeek, M.R.; Poldermans, D. Long-Term Prognosis of Patients With Peripheral Arterial Disease: A Comparison in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2008, 51, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Dopheide, J.F.; Adam, L.; Wiedmer, S.; Kaspar, M.; Silbernagel, G.; Baumgartner, I.; Drexel, H. Alirocumab in Patients With Polyvascular Disease and Recent Acute Coronary Syndrome: ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 74, 1167–1176. [Google Scholar]
- Kato, E.T.; Cannon, C.P.; Blazing, M.A.; Bohula, E.; Guneri, S.; White, J.A.; Murphy, S.A.; Park, J.; Braunwald, E.; Giugliano, R.P. Efficacy and Safety of Adding Ezetimibe to Statin Therapy Among Women and Men: Insight From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). J. Am. Heart Assoc. 2017, 6, e006901. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Dopheide, J.F.; Papac, L.; Schindewolf, M.; Baumgartner, I.; Drexel, H. Poor attainment of lipid targets in patients with symptomatic peripheral artery disease. J. Clin. Lipidol. 2018, 12, 711–717. [Google Scholar] [CrossRef]
- Dopheide, J.F.; Veit, J.; Ramadani, H.; Adam, L.; Papac, L.; Vonbank, A.; Kaspar, M.; Rastan, A.; Baumgartner, I.; Drexel, H. Adherence to Statin Therapy Favours Survival of Patients with Symptomatic Peripheral Artery Disease. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 7, 263–270. [Google Scholar] [CrossRef]
- Tobert, J.A.; Newman, C.B. The nocebo effect in the context of statin intolerance. J. Clin. Lipidol. 2016, 10, 739–747. [Google Scholar] [CrossRef]
- Barsky, A.J.; Saintfort, R.; Rogers, M.P.; Borus, J.F. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002, 287, 622–627. [Google Scholar] [CrossRef]
- Gupta, A.; Thompson, D.; Whitehouse, A.; Collier, T.; Dahlof, B.; Poulter, N.; Collin, R.; Sever, P. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): A randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017, 389, 2473–2481. [Google Scholar]
- Rosenson, R.S.; Baker, S.; Banach, M.; Borow, K.M.; Braun, L.T.; Bruckert, E.; Brunham, L.R.; Catapano, A.L.; Elam, M.B.; Mancini, G.J.; et al. Optimizing Cholesterol Treatment in Patients With Muscle Complaints. J. Am. Coll. Cardiol. 2017, 70, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Plutzky, J.; Skentzos, S.; Morrison, F.; Mar, P.; Shubina, M.; Turchin, A. Discontinuation of statins in routine care settings: A cohort study. Ann. Intern. Med. 2013, 158, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Brinton, E.A.; Ito, M.K.; Jacobson, T.A. Understanding Statin Use in America and Gaps in Patient Education (USAGE): An internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 2012, 6, 208–215. [Google Scholar] [CrossRef]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Begaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Phillips, C.O.; Foody, J.M.; Wang, Y.; Mangalmurti, S.; Ko, D.T.; Krumholz, H.M. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation 2006, 114, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
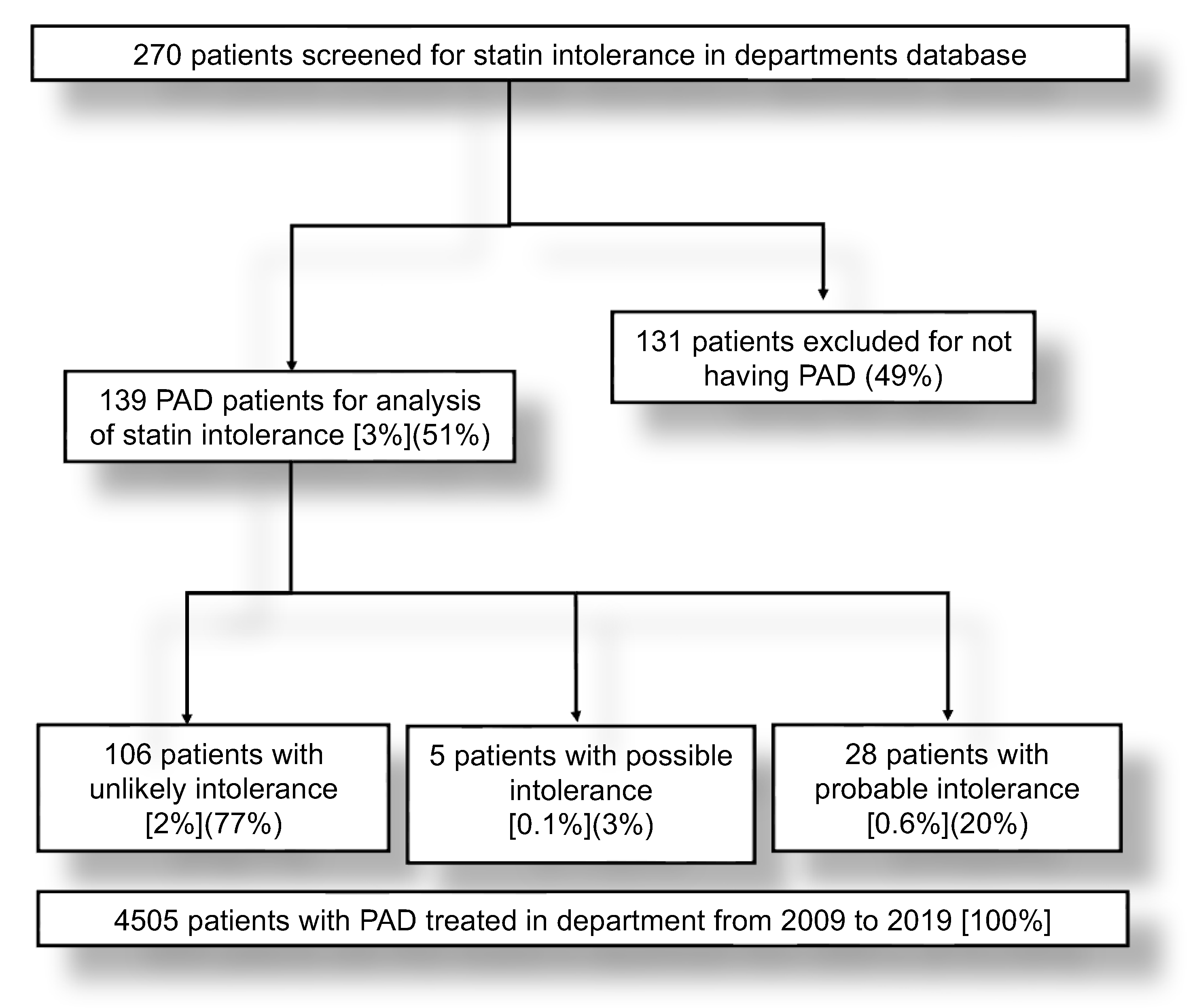
| Baseline | All PAD Patients (n = 139) | Unlikely SI (n = 106) | Probable/Possible SI (n = 33) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 66.4 ± 9.9 | 68.1 ± 9.7 | 60.8 ± 8.7 | 0.001 |
| Female Sex, n (%) | 38 (27) | 28 (26) | 10 (30) | 0.66 |
| Current smoker, n (%) | 43 (31) | 30 (28) | 13 (39) | 0.28 |
| Diabetes, n (%) | 48 (35) | 41 (39) | 7 (21) | 0.09 |
| HbA1c [%] | 6.19 ± 1.01 | 6.23 ± 10.7 | 6.07 ± 0.87 | 0.86 |
| Hypertension, n (%) | 122 (88) | 91 (86) | 31 (94) | 0.31 |
| Chronic Kidney disease (CKD), n (%) | 84 (60) | 73 (69) | 18 (55) | 0.14 |
| Stage 2 | 54 (64) | 44 (42) | 12 (36) | 0.69 |
| Stage 3 | 25 (18) | 21 (20) | 5 (15) | 0.62 |
| Stage 4 | 5 (6) | 8 (8) | 1 (3) | 0.67 |
| Stage 5 | 0 (0) | 0 (0) | 0 (0) | |
| Severity of PAD (Fontaine stage) | ||||
| I, n (%) | 49 (35) | 40 (38) | 9 (27) | 0.3 |
| II a, n (%) | 27 (19) | 18 (17) | 9 (27) | 0.21 |
| II b, n (%) | 43 (31) | 32 (30) | 11 (33) | 0.83 |
| III, n (%) | 8 (6) | 7 (7) | 1 (3) | 0.68 |
| IV, n (%) | 12 (9) | 9 (8) | 3 (9) | 0.99 |
| Creatinine, [µmol/L] | 109.30 ± 87.40 | 118.10 ± 99.40 | 85.30 ± 28.15 | 0.03 |
| eGFR [mL/min/1.73 m2] | 67.87 ± 21.12 | 69.25 ± 22.14 | 77.52 ± 15.53 | 0.07 |
| Total Cholesterol [mmol/L] | 4.50 ± 1.23 | 4.35 ± 1.19 | 4.89 ± 1.28 | 0.04 |
| HDL Cholesterol [mmol/L] | 1.19 ± 0.36 | 1.22 ± 0.38 | 1.12 ± 0.29 | 0.22 |
| Non-HDL [mmol/L] | 3.28 ± 1.26 | 2.69 ± 1.55 | 3.77 ± 1.25 | <0.001 |
| LDL Cholesterol [mmol/L] | 2.79 ± 1.18 | 2.62 ± 1.15 | 3.22 ± 1.14 | 0.01 |
| Triglycerides [mmol/L] | 2.11 ± 1.34 | 2.00 ± 1.35 | 2.39 ± 1.29 | 0.13 |
| ALAT [U/L] | 24.12 ± 17.40 | 22.70 ± 18.17 | 27.25 ± 15.10 | 0.06 |
| ASAT [U/L] | 25.21 ± 17.02 | 23.36 ± 17.26 | 29.31 ± 16.22 | 0.06 |
| Creatine Kinase [U/L] | 106.70 ± 109.10 | 102.40 ± 113.50 | 114.40 ± 97.78 | 0.30 |
| Baseline | Final | Baseline | Final | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n= 139) | All (n= 139) | p | Unlikely SI (n = 106) | Probable/ Possible SI (n = 33) | p | Unlikely SI (n = 106) | Probable/ Possible SI (n = 33) | p | |
| Statin therapy, n (%) | 119 (86) | 63 (45) | <0.0001 | 87(82) | 32 (97) | 0.04 | 56 (53) | 7 (21) | 0.23 |
| Statin dose/d (mg ± SD) | 57.8 ± 34.9 | 50.8 ± 32.0 | 0.16 | 57.0 ± 32.9 | 58.7 ± 40.7 | 0.83 | 52.5 ± 32.9 | 37.1 ± 21.4 | 0.28 |
| Statin intensity, n (%) | |||||||||
| High, n (%) | 72 (61) | 23 (37) | 0.003 | 53 (61) | 19 (59) | 0.99 | 22 (39) | 1 (14) | 0.41 |
| Moderate, n (%) | 45 (38) | 38 (60) | 0.005 | 33 (38) | 12 (38) | 0.99 | 32 (57) | 6 (86) | 0.23 |
| Low, n (%) | 2 (2) | 2 (3) | 0.61 | 1 (1) | 1 (3) | 0.47 | 2 (4) | 0 (0) | 0.99 |
| Lipophil Statin, n (%) | 106 (58) | 28 (44) | <0.0001 | 80 (87) | 26 (69) | 0.11 | 23 (41) | 5 (71) | 0.23 |
| Hydrophil Statin, n (%) | 76 (42) | 35 (56) | 0.07 | 54 (62) | 22 (69) | 0.16 | 33 (31) | 2 (6) | 0.003 |
| Ezetimib, n (%) | 26 (19) | 35 (25) | 0.25 | 19 (18) | 5 (15) | 0.80 | 26 (25) | 9 (27) | 0.82 |
| +Statin, n (%) | 24 (92) | 18 (51) | <0.001 | 19 (100) | 5 (100) | 0.80 | 16 (62) | 2 (22) | 0.06 |
| +PCSK-9i, n (%) | 0 (0) | 3 (9) | <0.0001 | 0 (0) | 0 (0) | 0(0) | 3 (33) | 0.01 | |
| PCSK-9i, n (%) | 1 (1) | 25 (18) | <0.0001 | 1 (1) | 0 (0) | 0.99 | 7 (7) | 18 (55) | <0.0001 |
| +Statin, n (%) | 1 (100) | 5 (20) | 0.23 | 1 (100) | 0 (0) | 0.99 | 3 (43) | 2 (11) | 0.11 |
| +Ezetimib, n (%) | 0 (0) | 3 (12) | 0.99 | 0 (0) | 0 (0) | 0 (0) | 3 (17) | 0.53 | |
| +Statin + Ezetimib, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dopheide, J.F.; Gillmann, P.; Spirk, D.; Khorrami Borozadi, M.; Adam, L.; Drexel, H. False versus True Statin Intolerance in Patients with Peripheral Artery Disease. J. Clin. Med. 2022, 11, 6619. https://doi.org/10.3390/jcm11226619
Dopheide JF, Gillmann P, Spirk D, Khorrami Borozadi M, Adam L, Drexel H. False versus True Statin Intolerance in Patients with Peripheral Artery Disease. Journal of Clinical Medicine. 2022; 11(22):6619. https://doi.org/10.3390/jcm11226619
Chicago/Turabian StyleDopheide, Jörn F., Patrick Gillmann, David Spirk, Meisam Khorrami Borozadi, Luise Adam, and Heinz Drexel. 2022. "False versus True Statin Intolerance in Patients with Peripheral Artery Disease" Journal of Clinical Medicine 11, no. 22: 6619. https://doi.org/10.3390/jcm11226619
APA StyleDopheide, J. F., Gillmann, P., Spirk, D., Khorrami Borozadi, M., Adam, L., & Drexel, H. (2022). False versus True Statin Intolerance in Patients with Peripheral Artery Disease. Journal of Clinical Medicine, 11(22), 6619. https://doi.org/10.3390/jcm11226619







