Association of Whole-Heart Myocardial Mechanics by Transthoracic Echocardiography with Presence of Late Gadolinium Enhancement by CMR in Non-Ischemic Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- ischemic coronary disease (ICD). All patients underwent invasive coronary angiography. ICD was defined as a history of myocardial infarction, revascularization, and the presence of epicardial coronary artery diameter stenosis > 50%;
- primary valvular heart disease;
- chronic kidney disease (eGFR < 30 mL/min/1.73 m2);
- under the age of 18;
- a poor echocardiographic or CMR image quality;
- inflammatory myocardial disease;
- previous pulmonary embolism;
- peripartum cardiomyopathy;
- toxic damage (alcohol, drugs).
2.2. 2D Echocardiographic Analysis
2.2.1. Standard Echocardiographic Parameters
2.2.2. Speckle-Tracking Echocardiography
2.3. Reproducibility of Myocardial Strain Measurements
2.4. CMR Imaging Protocol and Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eijgenraam, T.R.; Silljé, H.H.W.; de Boer, R.A. Current understanding of fibrosis in genetic cardiomyopathies. Trends Cardiovasc. Med. 2020, 30, 353–361. [Google Scholar] [CrossRef]
- Cojan-Minzat, B.O.; Zlibut, A.; Agoston-Coldea, L. Non-ischemic dilated cardiomyopathy and cardiac fibrosis. Heart Fail. Rev. 2021, 26, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.G.; Barison, A.; Aquaro, G.D.; Pingitore, A.; Mariotti, R.; Balbarini, A.; Passino, C.; Lombardi, M.; Emdin, M. Myocardial delayed enhancement in paucisymptomatic nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2012, 157, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association between mid-wall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef]
- Leyva, F.; Taylor, R.J.; Foley, P.; Umar, F.; Mulligan, L.J.; Patel, K.; Stegemann, B.; Haddad, T.; Smith, R.E.; Prasad, S.K. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1659–1667. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 19, 10944. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhang, L.; Tian, F.; Wang, B.; Xie, Y.; Sun, W.; Sun, Z.; Yang, Y.; Lv, Q.; et al. Assessment of Myocardial Fibrosis Using Two-Dimensional and Three-Dimensional Speckle Tracking Echocardiography in Dilated Cardiomyopathy with Advanced Heart Failure. J. Card. Fail. 2021, 27, 651–661. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995. World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Lehrke, S.; Lossnitzer, D.; Schöb, M.; Steen, H.; Merten, C.; Kemmling, H.; Pribe, R.; Ehlermann, P.; Zugck, C.; Korosoglou, G.; et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: Prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 727–732. [Google Scholar] [CrossRef]
- Looi, J.L.; Edwards, C.; Armstrong, G.P.; Scott, A.; Patel, H.; Hart, H.; Christiansen, J.P. Characteristics and prognostic importance of myocardial fibrosis in patients with dilated cardiomyopathy assessed by contrast-enhanced cardiac magnetic resonance imaging. Clin. Med. Insights Cardiol. 2010, 4, 129–134. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; De Lazzari, M.; Zorzi, A.; Migliore, F.; Zilio, F.; Calore, C.; Vettor, G.; Tona, F.; Tarantini, G.; Cacciavillani, L.; et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm 2014, 11, 856–863. [Google Scholar] [CrossRef]
- Iles, L.; Pfluger, H.; Lefkovits, L.; Butler, M.J.; Kistler, P.M.; Kaye, D.M.; Taylor, A.J. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J. Am. Coll. Cardiol. 2011, 57, 821–828. [Google Scholar] [CrossRef]
- Choi, E.Y.; Choi, B.W.; Kim, S.A.; Rhee, S.J.; Shim, C.Y.; Kim, Y.J.; Kang, S.M.; Ha, J.W.; Chung, N. Patterns of late gadolinium enhancement are associated with ventricular stiffness in patients with advanced non-ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2014, 11, 573–580. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late gadolinium enhancement and the Risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: Systematic review and meta-analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef]
- Leong, D.P.; Chakrabarty, A.; Shipp, N.; Molaee, P.; Madsen, P.L.; Joerg, L.; Sullivan, T.; Worthley, S.G.; De Pasquale, C.G.; Sanders, P.; et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: Insights from cardiovascular magnetic resonance and echocardiography. Eur. Heart J. 2012, 33, 640–648. [Google Scholar] [CrossRef]
- Cameli, M.; Mondillo, S.; Righini, F.M.; Lisi, M.; Dokollari, A.; Lindqvist, P.; Maccherini, M.; Henein, M. Left Ventricular Deformation and Myocardial Fibrosis in Patients with Advanced Heart Failure Requiring Transplantation. J. Card. Fail. 2016, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gozdzik, A.; Letachowicz, K.; Grajek, B.B.; Plonek, T.; Obremska, M.; Jsinski, M.; Gzdzik, W. Application of strain and other echocardiographic parameters in the evaluation of early and long-term clinical outcomes after cardiac surgery revascularization. BMC Cardiovasc. Disord. 2019, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Lisi, M.; Cameli, M.; Righini, F.M.; Malandrino, A.; Tacchini, D.; Focardi, M.; Tsioulpas, C.; Bernazzali, S.; Tanganelli, P.; Maccherini, M.; et al. RV Longitudinal Deformation Correlates with Myocardial Fibrosis in Patients with End-Stage Heart Failure. JACC Cardiovasc. Imaging 2015, 8, 514–522. [Google Scholar] [CrossRef]
- Bogarapu, S.; Puchalski, M.D.; Everitt, M.D.; Williams, R.V.; Weng, H.Y.; Menon, S.C. Novel Cardiac Magnetic Resonance Feature Tracking (CMR-FT) analysis for detection of myocardial fibrosis in pediatric hypertrophic cardiomyopathy. Pediatric Cardiol. 2016, 37, 663–673. [Google Scholar] [CrossRef]
- Raafs, A.G.; Boscutti, A.; Henkens, M.T.H.M.; van den Broek, W.W.A.; Verdonschot, J.A.J.; Weerts, J.; Stolfo, D.; Nuzzi, V.; Manca, P.; Hazebroek, M.R.; et al. Global Longitudinal Strain is Incremental to Left Ventricular Ejection Fraction for the Prediction of Outcome in Optimally Treated Dilated Cardiomyopathy Patients. J. Am. Heart Assoc. 2022, 11, e024505. [Google Scholar] [CrossRef]
- Oliver, W.; Matthews, G.; Ayers, C.R.; Garg, S.; Gupta, S.; Neeland, I.J.; Drazner, M.H.; Berry, J.D.; Matulevicius, S.; de Lemos, J.A. Factors Associated with Left Atrial Remodeling in the General Population. Circ. Cardiovasc. Imaging 2017, 10, 5047. [Google Scholar] [CrossRef]
- Mohseni-Badalabadi, R.; Mehrabi-Pari, S.; Hosseinsabet, A. Evaluation of the left atrial function by two-dimensional speckle-tracking echocardiography in diabetic patients with obesity. Int. J. Cardiovasc. Imaging 2020, 36, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Olsen, F.J.; Christensen, L.M.; Krieger, D.W.; Højberg, S.; Høst, N.; Karlsen, F.M.; Svendsen, J.H.; Christensen, H.; Biering-Sørensen, T. Relationship between left atrial strain, diastolic dysfunction and subclinical atrial fibrillation in patients with cryptogenic stroke: The SURPRISE echo substudy. Int. J. Cardiovasc. Imaging 2019, 36, 79–89. [Google Scholar] [CrossRef]
- Ozturk, O.; Ozturk, U.; Ozturk, S. Assessment of right atrial function with speckle tracking echocardiography after percutaneous closure of an atrial septal defect. Rev. Port. Cardiol. 2017, 36, 895–900. [Google Scholar] [CrossRef]
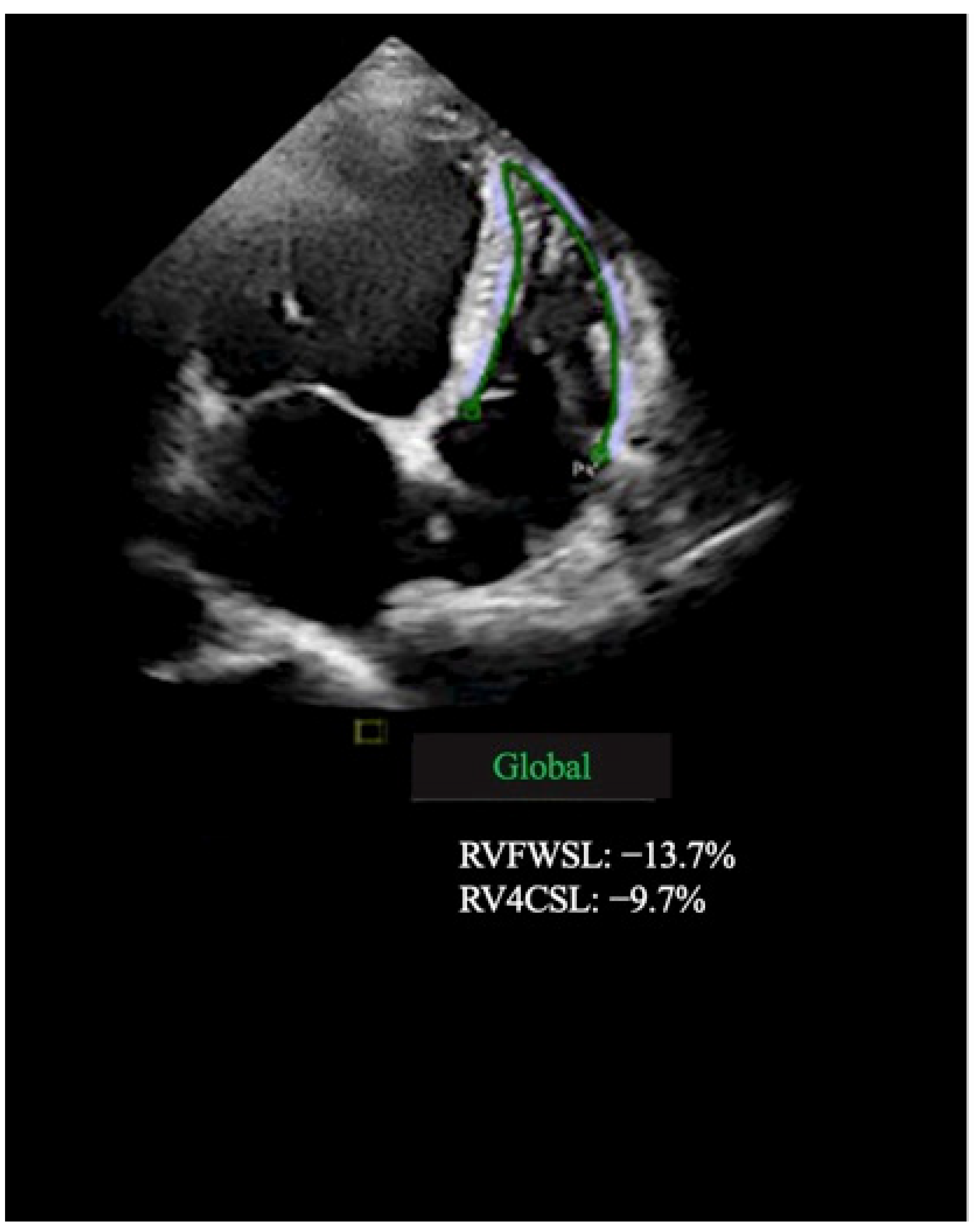
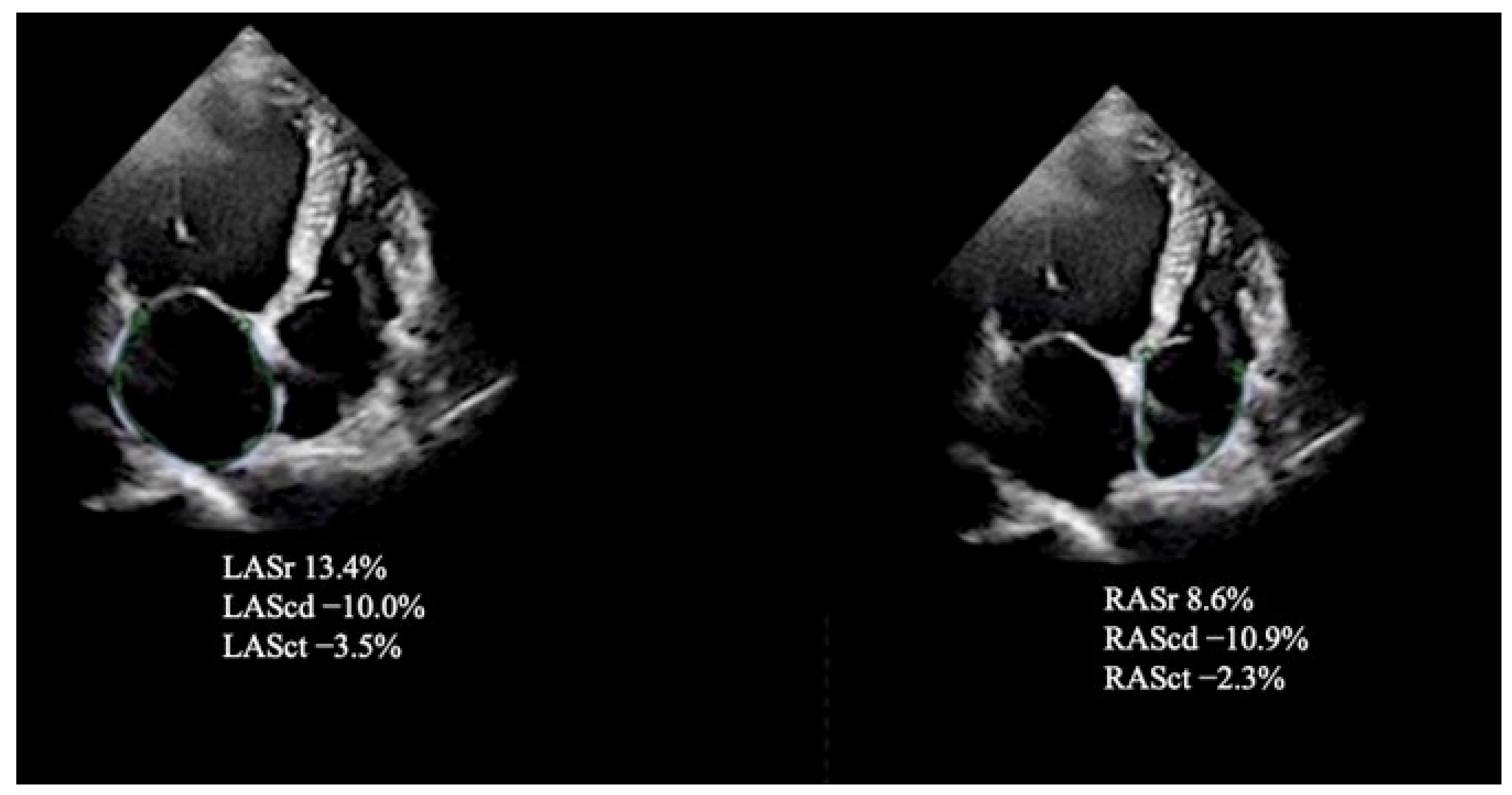

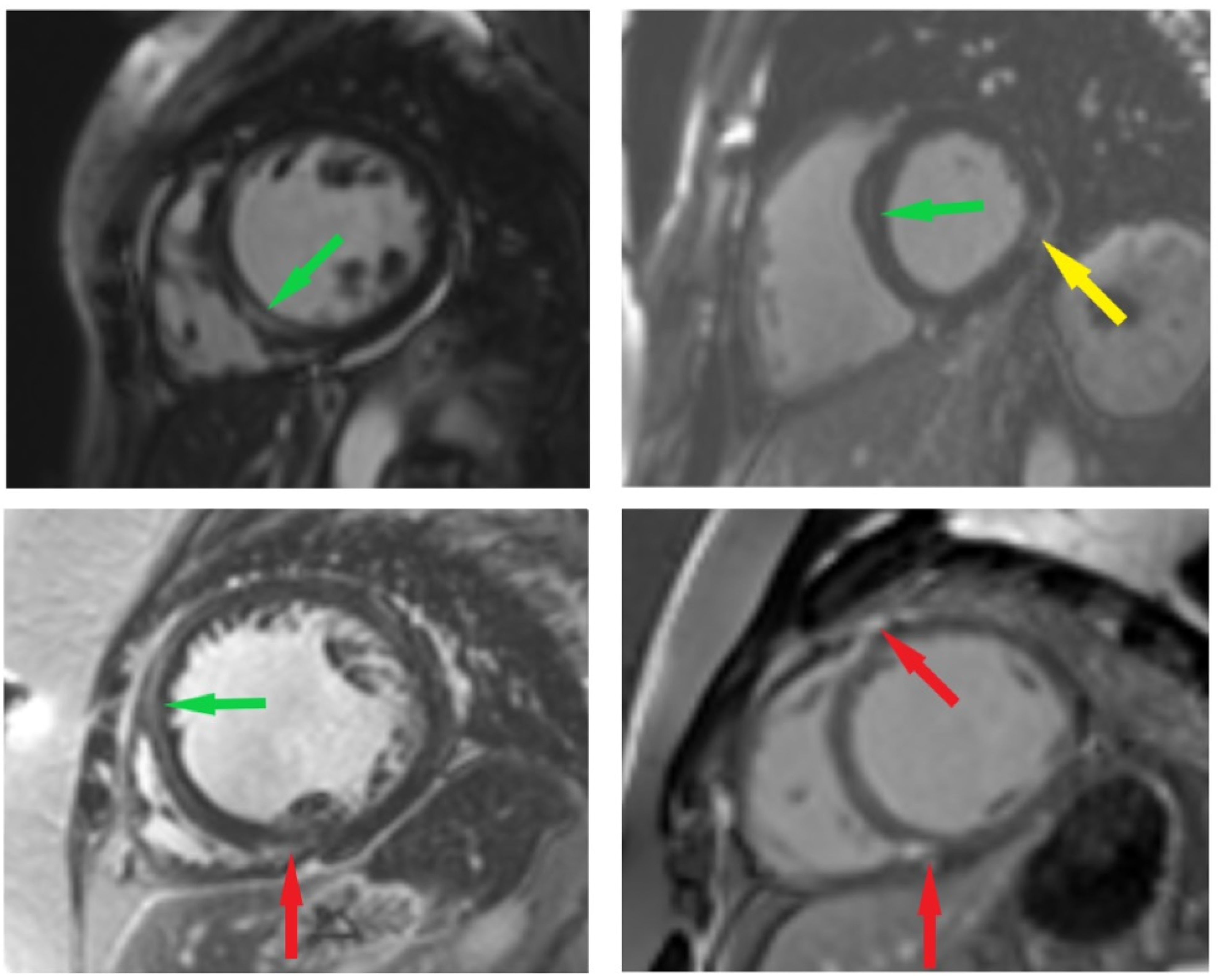
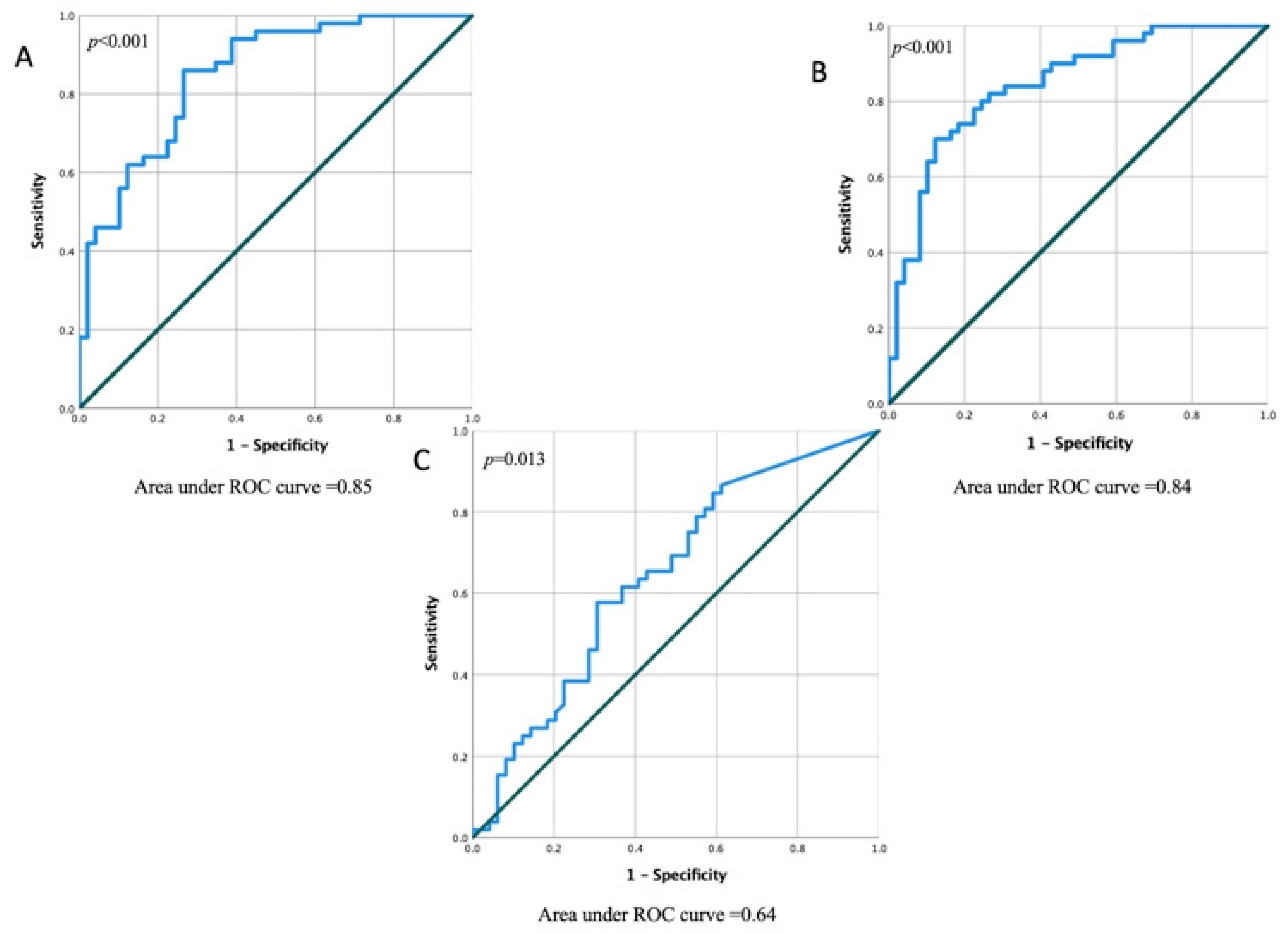
| Variables | Fibrosis Positive (LGE+) n = 51 | Fibrosis Negative (LGE) n = 50 | p Value (Fibrosis Positive vs. Fibrosis Negative) |
|---|---|---|---|
| Age, y | 50.7 ± 10.8 | 50.3 ± 10.9 | 0.832 |
| Males, n (%) | 37 (74) | 33 (67.3) | 0.467 |
| BSA, m2 | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.725 |
| Heart rate, beat/min | 82.3 ± 18.2 | 77.3 ± 16.01 | 0.160 |
| Systolic blood pressure, mmHg | 124.1 ± 12.8 | 126.6 ± 13.9 | 0.353 |
| Dyslipidemia, n (%) | 21 (42) | 19 (38.8) | 0.838 |
| Smoking, (%) | 23 (46) | 20 (40.8) | 0.686 |
| VT/VF, n (%) | 26 (52) | 7 (14.3) | <0.001 |
| LBBB, n (%) | 26 (52) | 18 (36.7) | 0.158 |
| AF/AFL, n (%) | 22 (44) | 19 (38.8) | 0.685 |
| HF symptoms > 3 months, n (%) | 33 (66) | 39 (79.6) | 0.176 |
| QRS duration, ms | 122.6 ± 30.6 | 119.9 ± 29.1 | 0.657 |
| NYHA class III-IV, n (%) | 34 (68) | 23 (46.9) | 0.045 |
| BNP, ng/L | 1540.2 ± 651.6 | 980.1 ± 714.7 | 0.106 |
| Variables | Fibrosis Positive (LGE+) n = 51 | Fibrosis Negative (LGE) n = 50 | p Value (Fibrosis Positive vs. Fibrosis Negative) |
|---|---|---|---|
| IVS, mm | 9.5 ± 1.3 | 9.9 ± 1.1 | 0.118 |
| PW, mm | 9.6 ± 1.4 | 9.8 ± 1.1 | 0.484 |
| LVESD, mm | 58.8 ± 7.5 | 53.1 ± 7.5 | <0.001 |
| LVESDi, mm/m2 | 29.1 ± 4.6 | 26.0 ± 3.9 | <0.001 |
| LVEDD, mm | 66.7 ± 6.8 | 62.7 ± 5.0 | 0.001 |
| LVEDDi, mm/m2 | 33.1 ± 1 | 30.7 ± 3.5 | 0.003 |
| LA, mm | 38.7 ± 4.6 | 37.8 ± 4.3 | 0.989 |
| LAAi, mm/m2 | 19.8 ± 3.2 | 19.2 ± 2.8 | 0.988 |
| LVEDV, mL | 256.9 ± 80.6 | 209.7 ± 56.4 | <0.001 |
| LVEDVi, mL/m2 | 126.8 ± 41.1 | 103.1 ± 30.3 | <0.001 |
| LVESV, mL | 188.9 ± 70.9 | 135.7 ± 45.3 | <0.001 |
| LVESVi, mL/m2 | 96.8 ± 41.8 | 68.2 ± 25.2 | <0.001 |
| GLS, % | −7.1 ± 2.1 | −10.3 ± 2.5 | <0.001 |
| GCS, % | −11.1 ± 3.4 | −16.9 ± 4.2 | <0.001 |
| GRS, % | 15.5 ± 7.8 | 25.7 ± 7.8 | <0.001 |
| LVEF, % | 22.1 ± 6.0 | 33.6 ± 7.3 | <0.001 |
| RV free wall LS, % | −17.3 ± 2.9 | −19.0 ± 2.9 | 0.008 |
| RV GLS, % | −9.5 ± 3.5 | −13.2 ± 4.2 | 0.007 |
| RV FAC, % | 30.1 ± 5.9 | 33.5 ± 5.1 | 0.002 |
| LAV, mL | 129.4 ± 62.3 | 104.2 ± 63.5 | 0.05 |
| LAVi, mL/m2 | 63.4 ± 32.1 | 49.8 ± 25.1 | 0.021 |
| LAScd, % | −10.7 ± 3.2 | −16.0 ± 4.1 | <0.001 |
| LASr, % | 19.1 ± 6.1 | 27.4 ± 6.0 | <0.001 |
| LASct, % | −8.1 ± 2.6 | −11.6 ± 4.9 | <0.001 |
| RAV, mL | 84.3 ± 24.1 | 75.9 ± 24.4 | 0.091 |
| RAVi, mL/m2 | 41.3 ± 10.8 | 36.8 ± 9.4 | 0.031 |
| RAScd, % | −13.5 ± 5.1 | −18.0 ± 4.8 | <0.001 |
| RASr, % | 26.9 ± 6.4 | 31.6 ± 5.1 | <0.001 |
| RASct, % | −10.4 ± 6.9 | −14.6 ± 3.1 | <0.001 |
| Fibrosis | ||
|---|---|---|
| rs | p | |
| GLS, % | −0.586 | <0.001 |
| GCS, % | −0.609 | <0.001 |
| GRS, % | 0.553 | <0.001 |
| LVEF, % | 0.662 | <0.001 |
| RV free wall LS, % | −0.244 | 0.015 |
| RV FAC, % | 0.274 | 0.006 |
| RV GLS, % | 0.282 | 0.005 |
| LAScd, % | −0.570 | 0.001 |
| LASr, % | 0.588 | 0.001 |
| LASct, % | −0.409 | 0.001 |
| RAScd, % | −0.420 | 0.001 |
| RASr, % | 0.379 | 0.001 |
| RASct, % | −0.369 | 0.001 |
| Parameter | Exp (B) | 95% CI | p |
|---|---|---|---|
| LV GLS, % | 0.637 | 0.494–0.821 | <0.001 |
| LV GCS, % | 0.828 | 0.715–0.958 | 0.011 |
| LV GRS, % | 1.002 | 0.911–1.103 | 0.058 |
| LVEF, % | 1.231 | 1.015–1.492 | 0.051 |
| RV free wall LS, % | 1.102 | 0.804–1.508 | 0.547 |
| RV FAC, % | 1.016 | 0.867–1.191 | 0.842 |
| RV GLS, % | 0.982 | 0.974–1.032 | 0.876 |
| LAScd, % | 0.988 | 0.978–1.123 | 0.064 |
| LASr, % | 1.120 | 1.010–1.242 | 0.031 |
| LASct, % | 1.009 | 0.732–1.391 | 0.069 |
| RAScd, % | 1.051 | 0.841–1.313 | 0.058 |
| RASr, % | 1.083 | 0.907–1.292 | 0.379 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mėlinytė-Ankudavičė, K.; Bučius, P.; Mizarienė, V.; Lapinskas, T.; Šakalytė, G.; Plisienė, J.; Jurkevičius, R. Association of Whole-Heart Myocardial Mechanics by Transthoracic Echocardiography with Presence of Late Gadolinium Enhancement by CMR in Non-Ischemic Dilated Cardiomyopathy. J. Clin. Med. 2022, 11, 6607. https://doi.org/10.3390/jcm11226607
Mėlinytė-Ankudavičė K, Bučius P, Mizarienė V, Lapinskas T, Šakalytė G, Plisienė J, Jurkevičius R. Association of Whole-Heart Myocardial Mechanics by Transthoracic Echocardiography with Presence of Late Gadolinium Enhancement by CMR in Non-Ischemic Dilated Cardiomyopathy. Journal of Clinical Medicine. 2022; 11(22):6607. https://doi.org/10.3390/jcm11226607
Chicago/Turabian StyleMėlinytė-Ankudavičė, Karolina, Paulius Bučius, Vaida Mizarienė, Tomas Lapinskas, Gintarė Šakalytė, Jurgita Plisienė, and Renaldas Jurkevičius. 2022. "Association of Whole-Heart Myocardial Mechanics by Transthoracic Echocardiography with Presence of Late Gadolinium Enhancement by CMR in Non-Ischemic Dilated Cardiomyopathy" Journal of Clinical Medicine 11, no. 22: 6607. https://doi.org/10.3390/jcm11226607
APA StyleMėlinytė-Ankudavičė, K., Bučius, P., Mizarienė, V., Lapinskas, T., Šakalytė, G., Plisienė, J., & Jurkevičius, R. (2022). Association of Whole-Heart Myocardial Mechanics by Transthoracic Echocardiography with Presence of Late Gadolinium Enhancement by CMR in Non-Ischemic Dilated Cardiomyopathy. Journal of Clinical Medicine, 11(22), 6607. https://doi.org/10.3390/jcm11226607






