Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Outcomes
2.4. Eligibility Criteria
2.5. Study Selection
2.6. Data Extraction
2.7. Risk of Bias Assessment
2.8. Data Synthesis
3. Results
3.1. Search Results
3.2. Baseline Characteristics of Included Studies
3.3. Risk of Bias
3.4. Meta-Analysis Outcomes [PVP-I + NSPT vs. NSPT Alone]
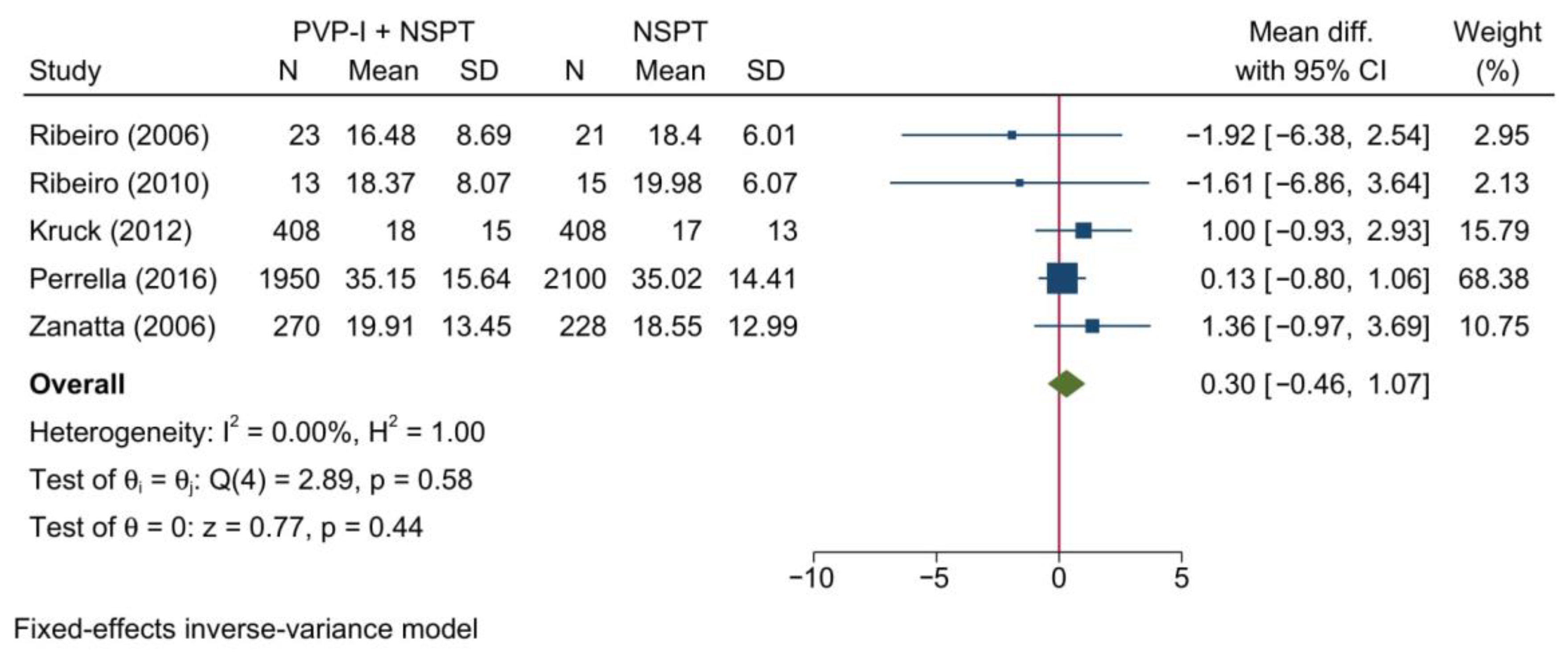
3.4.1. Probing Depth of Pockets
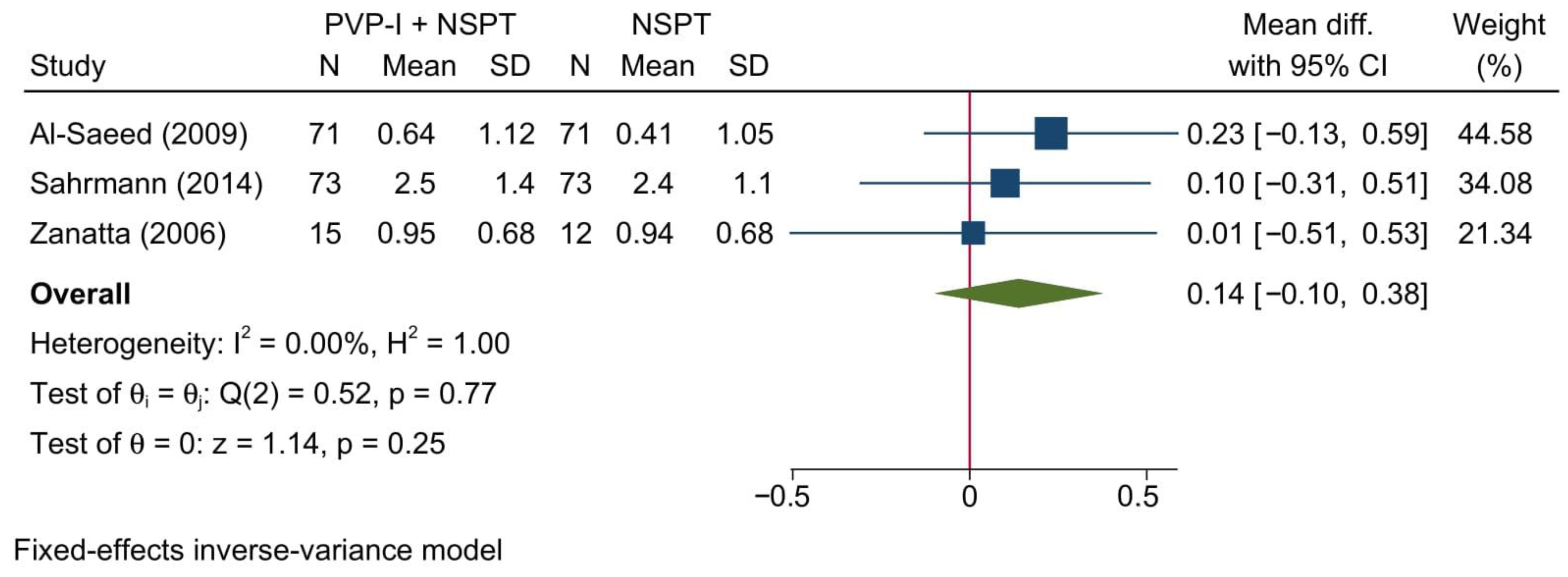
3.4.2. Plaque Index
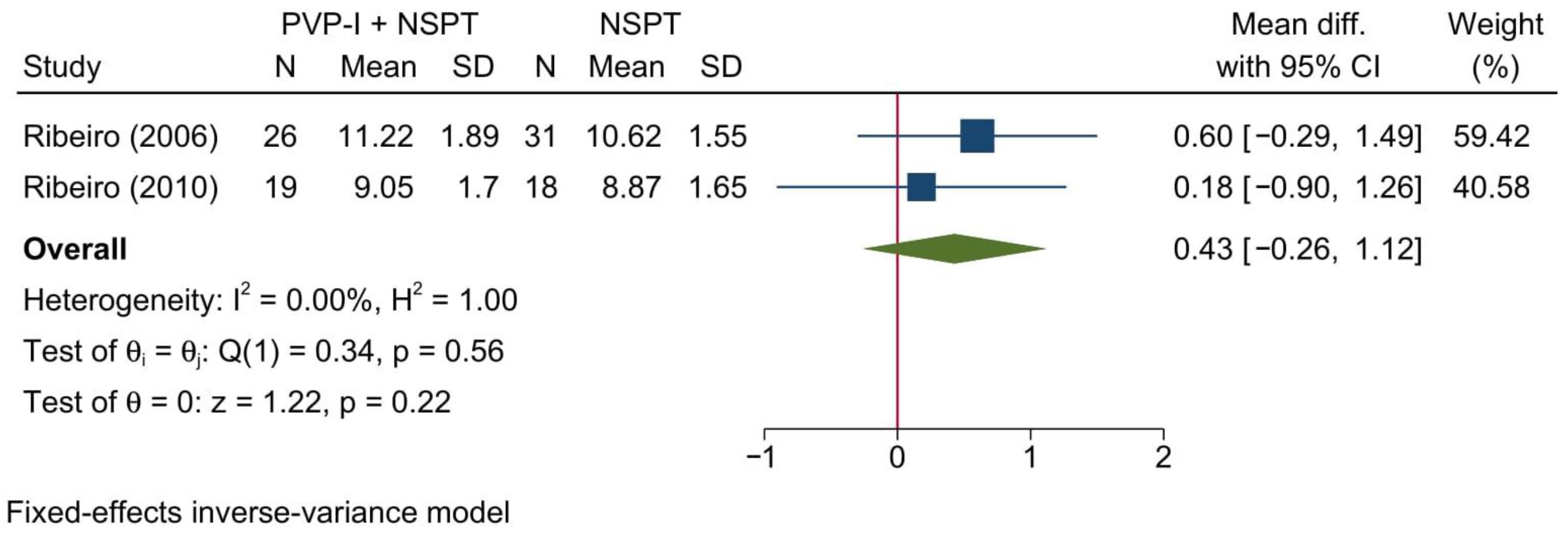
3.4.3. Clinical Attachment Level
3.4.4. Relative Horizontal Attachment Level
3.4.5. Bleeding on Probing
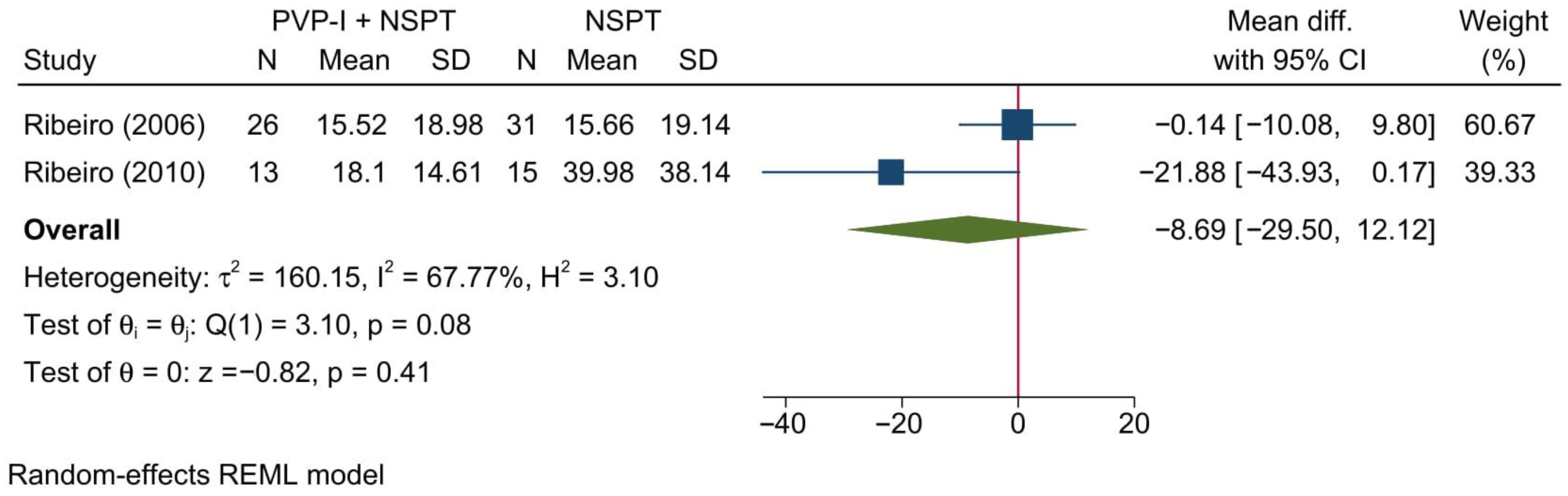
3.4.6. Gingival Recession
3.4.7. Position of Gingival Margin
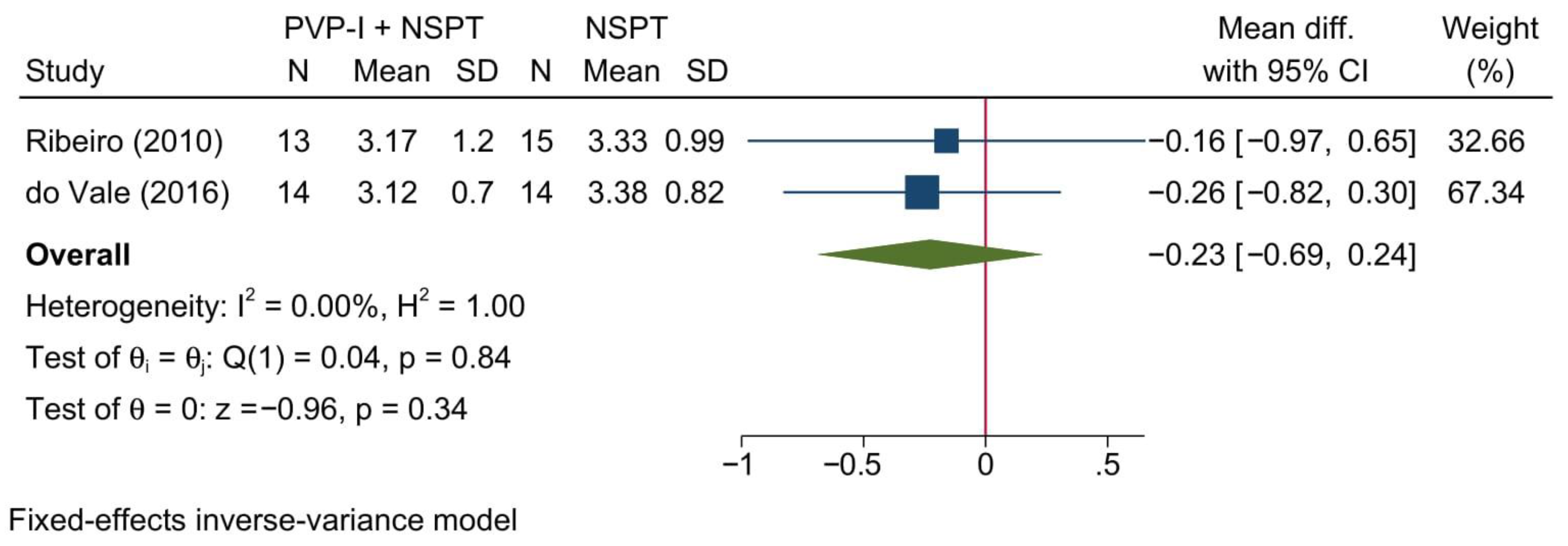
3.4.8. Biochemical Parameter: BAPNA
3.5. Meta-Analysis Outcomes [NaOCl + NSPT vs. NSPT Alone]
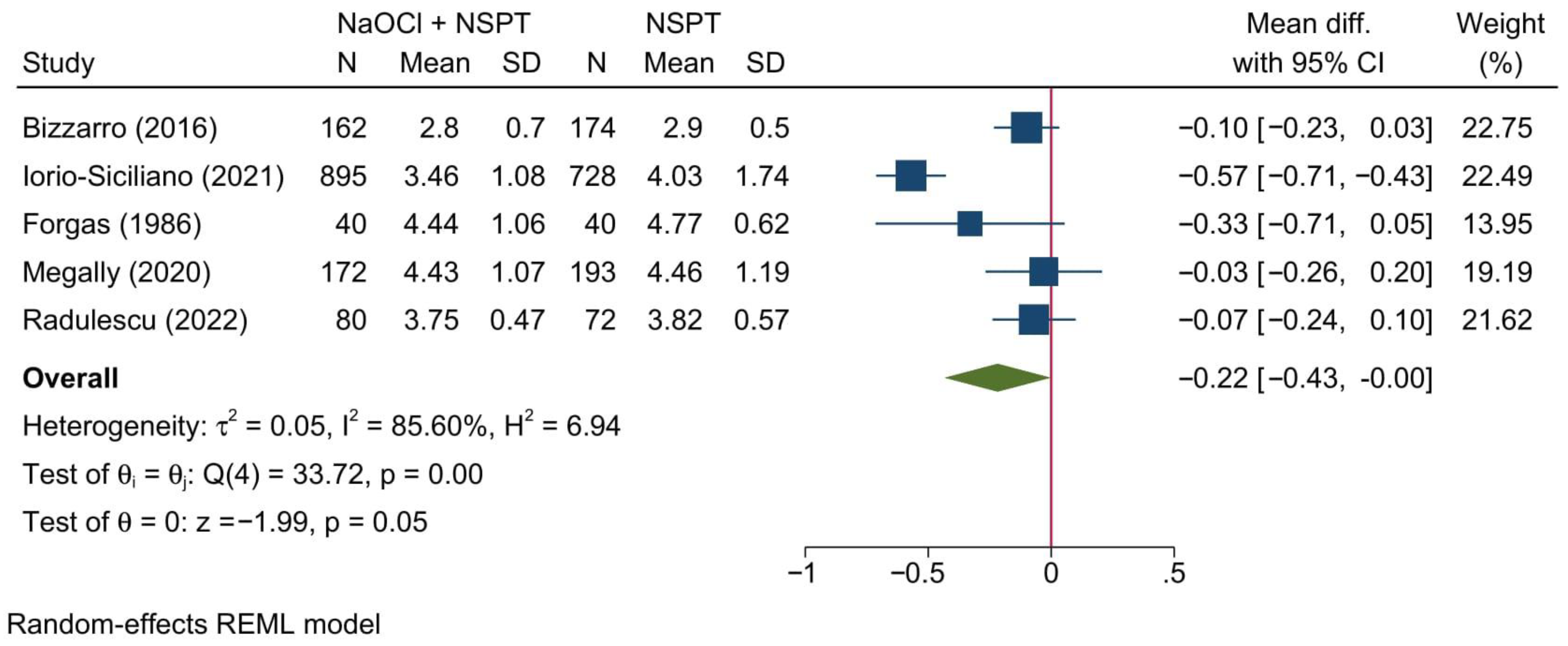
3.5.1. Probing Depth of Pockets
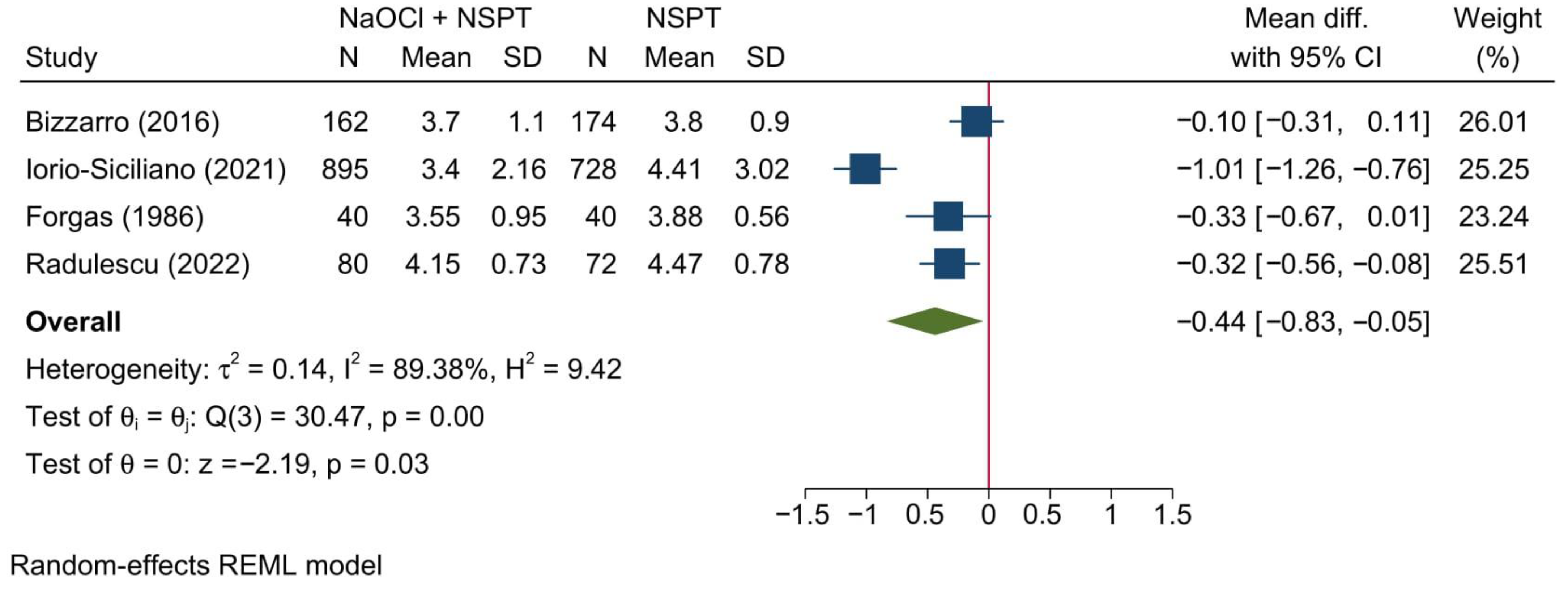
3.5.2. Clinical Attachment Level
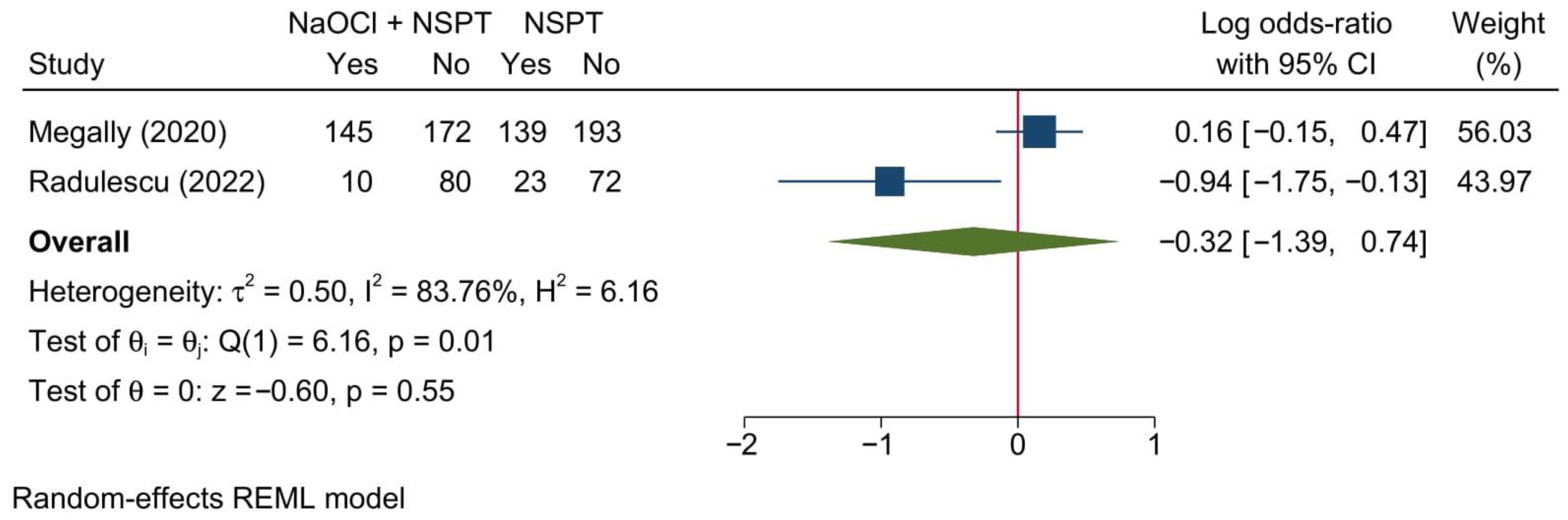
3.5.3. Bleeding on Probing
3.5.4. Gingival Recession
3.5.5. Other Parameters
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Orlandi, M.; Muñoz Aguilera, E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J. Clin. Periodontol. 2022, 49, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Seymour, R.A.; Heasman, P.A. Current concepts in periodontal pathogenesis. Dent. Update 2004, 31, 570–572, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Ma, X.; Yang, B.; Liu, Y.; Xie, Y.; Wang, X.; Yuan, W.; Ma, J. Periodontitis pathogen Porphyromonas gingivalis promotes pancreatic tumorigenesis via neutrophil elastase from tumor-associated neutrophils. Gut Microbes 2022, 14, 2073785. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.; Felton, J.; Brunsvold, M.; Kornman, K.S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 1988, 239, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Armitage, G.C.; Cullinan, M.P. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 12–27. [Google Scholar] [CrossRef]
- Murphy, K.G.; Gunsolley, J.C. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann. Periodontol. 2003, 8, 266–302. [Google Scholar] [CrossRef]
- Kebschull, M.; Chapple, I. Evidence-based, personalised and minimally invasive treatment for periodontitis patients—The new EFP S3-level clinical treatment guidelines. Br. Dent. J. 2020, 229, 443–449. [Google Scholar] [CrossRef]
- Cho-Yan Lee, J.; Mattheos, N.; Nixon, K.C.; Ivanovski, S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin. Oral Implant. Res. 2012, 23, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S., Jr.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontology 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Karapetsa, D.; Mardas, N.; Leow, N.; Donos, N. Surgical treatment of the residual periodontal pocket. Periodontology 2000 2018, 76, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.; Rodrigues, R.C.; Andrade Junior, C.V.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M. Antimicrobial peptides and periodontal disease. J. Clin. Periodontol. 2011, 38, 126–141. [Google Scholar] [CrossRef]
- Li, S.; Schmalz, G.; Schmidt, J.; Krause, F.; Haak, R.; Ziebolz, D. Antimicrobial peptides as a possible interlink between periodontal diseases and its risk factors: A systematic review. J. Periodontal Res. 2018, 53, 145–155. [Google Scholar] [CrossRef]
- Martin-Cabezas, R.; Davideau, J.L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef]
- Mulhall, H.; DiChiara, J.M.; Deragon, M.; Iyer, R.; Huck, O.; Amar, S. Akkermansia muciniphila and Its Pili-Like Protein Amuc_1100 Modulate Macrophage Polarization in Experimental Periodontitis. Infect. Immun. 2020, 89. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K. Making Use of Lasers in Periodontal Treatment: A New Gold Standard? Photomed. Laser Surg. 2017, 10, 513–514. [Google Scholar] [CrossRef]
- Gonzalez, S.; Cohen, C.L.; Galván, M.; Alonaizan, F.A.; Rich, S.K.; Slots, J. Gingival bleeding on probing: Relationship to change in periodontal pocket depth and effect of sodium hypochlorite oral rinse. J. Periodontal Res. 2015, 50, 397–402. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, F.M.; Tai, K.W.; Chou, M.Y. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 446–450. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, R.; Chiappe, V.; Gómez, M.; Romanelli, H.; Slots, J. Effects of 0.05% sodium hypochlorite oral rinse on supragingival biofilm and gingival inflammation. Int. Dent. J. 2012, 62, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Galván, M.; Gonzalez, S.; Cohen, C.L.; Alonaizan, F.A.; Chen, C.T.; Rich, S.K.; Slots, J. Periodontal effects of 0.25% sodium hypochlorite twice-weekly oral rinse. A pilot study. J. Periodontal Res. 2014, 49, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, K.; Nietzsche, S.; Ender, C.; Sculean, A.; Eick, S. In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin. Oral Investig. 2016, 20, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, E.; Machiulskiene, V.; Eliezer, M.; Sculean, A. Sodium Hypochlorite as an Adjunct to Nonsurgical Treatment of Periodontitis: A Systematic Review. Oral Health Prev. Dent. 2020, 18, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; De Luca, M.; Gerosa, R. Chemical stability of heated sodium hypochlorite endodontic irrigants. J. Endod. 1998, 24, 432–434. [Google Scholar] [CrossRef]
- Sirtes, G.; Waltimo, T.; Schaetzle, M.; Zehnder, M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J. Endod. 2005, 31, 669–671. [Google Scholar] [CrossRef]
- Forabosco, A.; Spinato, S.; Grandi, T.; Prini, M. A comparative study between different techniques in non-surgical periodontal treatment. Minerva Stomatol. 2006, 55, 289–296. [Google Scholar]
- Hoang, T.; Jorgensen, M.G.; Keim, R.G.; Pattison, A.M.; Slots, J. Povidone-iodine as a periodontal pocket disinfectant. J. Periodontal Res. 2003, 38, 311–317. [Google Scholar] [CrossRef]
- Rosling, B.; Hellström, M.K.; Ramberg, P.; Socransky, S.S.; Lindhe, J. The use of PVP-iodine as an adjunct to non-surgical treatment of chronic periodontitis. J. Clin. Periodontol. 2001, 28, 1023–1031. [Google Scholar] [CrossRef]
- Del Peloso Ribeiro, E.; Bittencourt, S.; Ambrosano, G.M.; Nociti, F.H., Jr.; Sallum, E.A.; Sallum, A.W.; Casati, M.Z. Povidone-iodine used as an adjunct to non-surgical treatment of furcation involvements. J. Periodontol. 2006, 77, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Koshy, G.; Kawashima, Y.; Kiji, M.; Nitta, H.; Umeda, M.; Nagasawa, T.; Ishikawa, I. Effects of single-visit full-mouth ultrasonic debridement versus quadrant-wise ultrasonic debridement. J. Clin. Periodontol. 2005, 32, 734–743. [Google Scholar] [CrossRef]
- Leonhardt, A.; Bergström, C.; Krok, L.; Cardaropoli, G. Healing following ultrasonic debridement and PVP-iodine in individuals with severe chronic periodontal disease: A randomized, controlled clinical study. Acta Odontol. Scand. 2006, 64, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, G.M.; Bittencourt, S.; Nociti, F.H., Jr.; Sallum, E.A.; Sallum, A.W.; Casati, M.Z. Periodontal debridement with povidone-iodine in periodontal treatment: Short-term clinical and biochemical observations. J. Periodontol. 2006, 77, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Forgas, L.B.; Gound, S. The effects of antiformin-citric acid chemical curettage on the microbial flora of the periodontal pocket. J. Periodontol. 1987, 58, 153–158. [Google Scholar] [CrossRef]
- Megally, A.; Zekeridou, A.; Cancela, J.; Giannopoulou, C.; Mombelli, A. Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: Randomized clinical trial of 12 months. Clin. Oral Investig. 2020, 24, 201–209. [Google Scholar] [CrossRef]
- Al-Saeed, M.Y.; Babay, N. The use of povidone-iodine and hydrogen peroxide mixture as an adjunct to non-surgical treatment of slight to moderate chronic periodontitis. Saudi Dent. J. 2009, 21, 127–133. [Google Scholar] [CrossRef]
- Bizzarro, S.; Van der Velden, U.; Loos, B.G. Local disinfection with sodium hypochlorite as adjunct to basic periodontal therapy: A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 778–788. [Google Scholar] [CrossRef]
- Do Vale, H.F.; Casarin, R.C.; Taiete, T.; Bovi Ambrosano, G.M.; Ruiz, K.G.; Nociti, F.H., Jr.; Sallum, E.A.; Casati, M.Z. Full-mouth ultrasonic debridement associated with povidone iodine rinsing in GAgP treatment: A randomised clinical trial. Clin. Oral Investig. 2016, 20, 141–150. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.; Lasserre, J.; Toma, S. Evaluation of multiple subgingival irrigations with 10% povidone-iodine after scaling and root planing: A randomized clinical trial. Quintessence Int. 2021, 52, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Krück, C.; Eick, S.; Knöfler, G.U.; Purschwitz, R.E.; Jentsch, H.F. Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: A pilot randomized trial. J. Periodontol. 2012, 83, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Perrella, F.A.; Rovai, E.D.S.; De Marco, A.C.; Santamaria, M.P.; Feres, M.; de Figueredo, L.C.; Kerbauy, W.D.; Amorim, J.B.O. Clinical and Microbiological Evaluation of Povidone-Iodine 10% as an Adjunct to Nonsurgical Periodontal Therapy in Chronic Periodontitis: A Randomized Clinical Trial. J. Int. Acad. Periodontol. 2016, 18, 109–119. [Google Scholar]
- Radulescu, V.; Boariu, M.I.; Rusu, D.; Roman, A.; Surlin, P.; Voicu, A.; Didilescu, A.C.; Jentsch, H.; Siciliano, V.I.; Ramaglia, L.; et al. Clinical and microbiological effects of a single application of sodium hypochlorite gel during subgingival re-instrumentation: A triple-blind randomized placebo-controlled clinical trial. Clin. Oral Investig. 2022. [Google Scholar] [CrossRef]
- Ribeiro Edel, P.; Bittencourt, S.; Sallum, E.A.; Sallum, A.W.; Nociti, F.H., Jr.; Casati, M.Z. Non-surgical instrumentation associated with povidone-iodine in the treatment of interproximal furcation involvements. J. Appl. Oral Sci. Rev. FOB 2010, 18, 599–606. [Google Scholar] [CrossRef][Green Version]
- Sahrmann, P.; Imfeld, T.; Ronay, V.; Attin, T.; Schmidlin, P.R. Povidone-iodine gel and solution as adjunct to ultrasonic debridement in nonsurgical periodontitis treatment: An RCT pilot study. Quintessence Int. 2014, 45, 281–290. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Van der Weijden, G.; Hioe, K. A systematic review of the effectiveness of self-performed mechanical plaque removal in adults with gingivitis using a manual toothbrush. J. Clin. Periodontol. 2005, 32, 214–228. [Google Scholar] [CrossRef]
- Estrela, C.; Sydney, G.B.; Bammann, L.L.; Felippe Junior, O. Estudo do efeito biológico do pH na atividade de enzimática de bacterias anaeróbicas. Rev. Fac. Odontol. Bauru 1994, 2, 31–38. [Google Scholar]
- Sahrmann, P.; Puhan, M.A.; Attin, T.; Schmidlin, P.R. Systematic review on the effect of rinsing with povidone-iodine during nonsurgical periodontal therapy. J. Periodontal Res. 2010, 45, 153–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef] [PubMed]
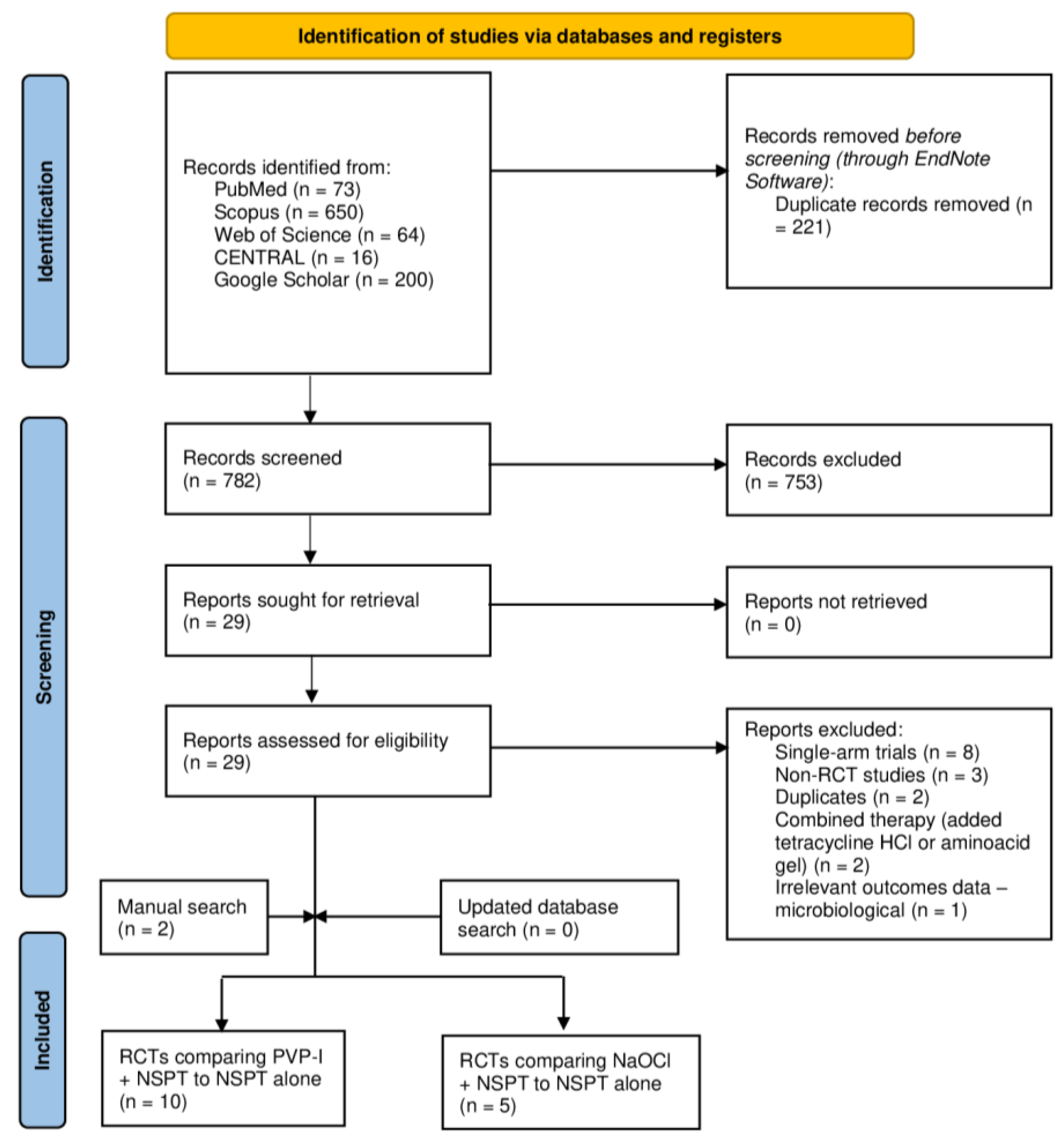
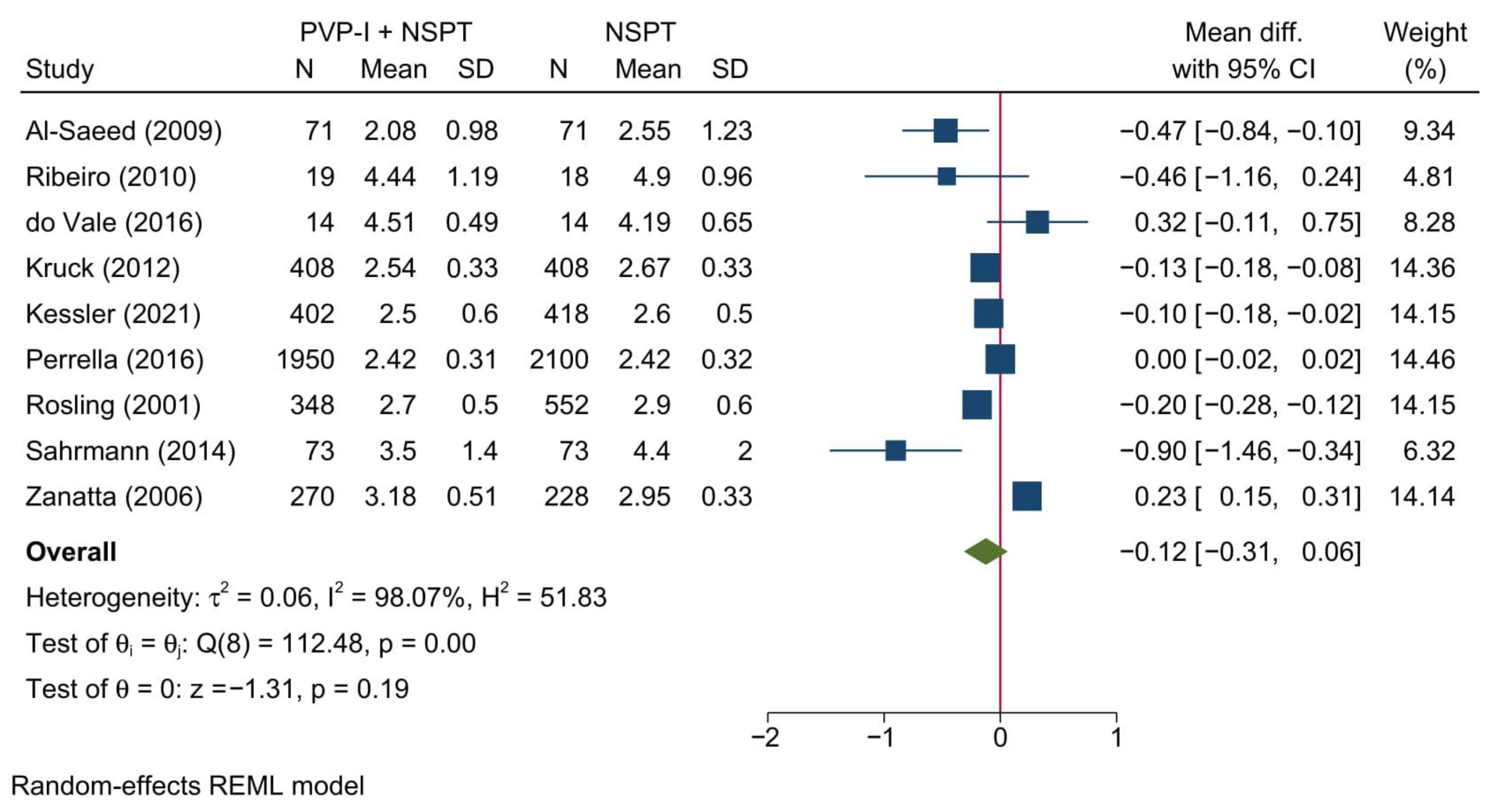
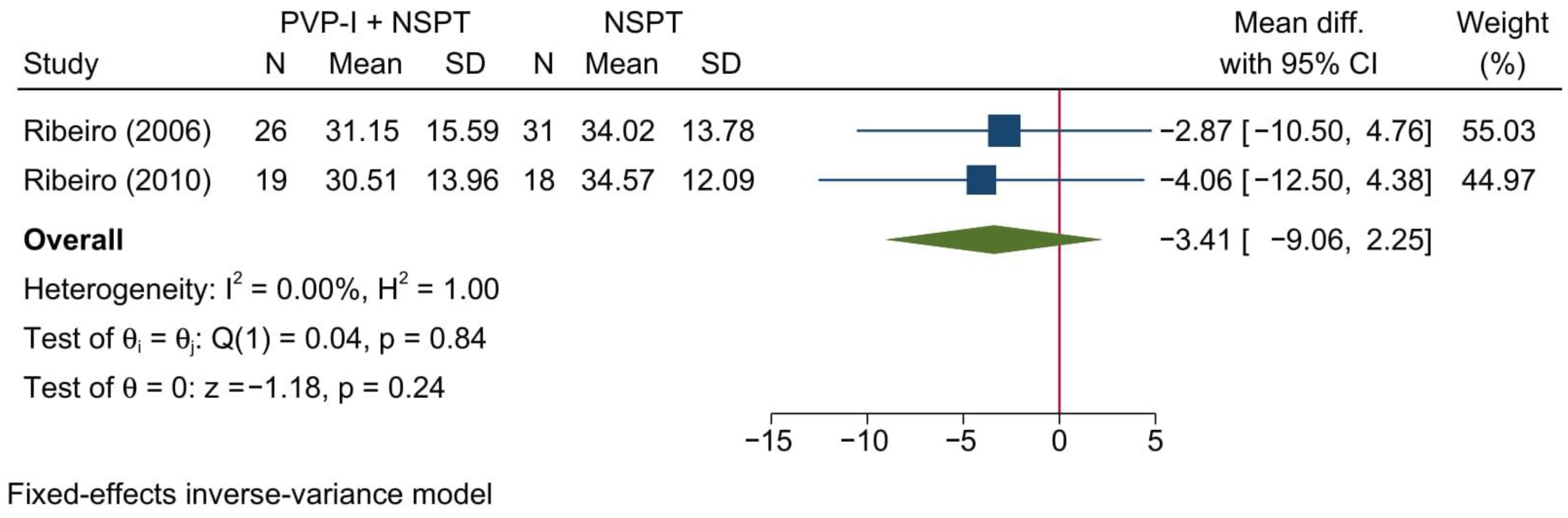
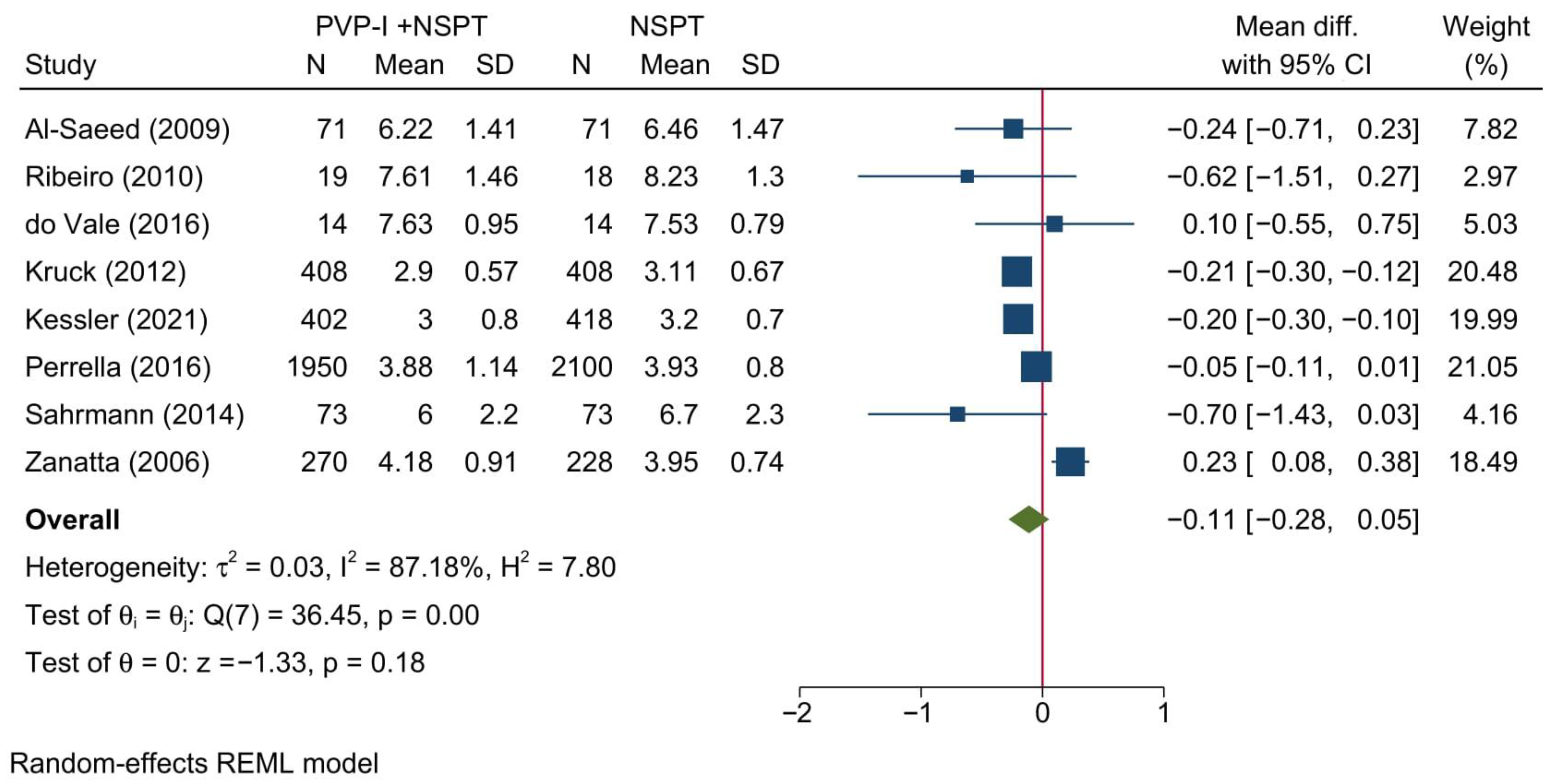


| Author (YOP) | Country | Design | Sample (n) | Periodontitis | Intervention | Control | Gender [Male] | Age | Follow-up (months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | Chronicity | n | Sites | Description | n | Sites | Description | Intervention | Control | Intervention | Control | |||||||
| n (%) | n (%) | Mean | SD | Mean | SD | |||||||||||||
| PVP-I + NSPT vs. NSPT Alone | ||||||||||||||||||
| Al-Saeed (2009) [38] | Saudi Arabia | Single-blinded RCT | 26 | Slight to Moderate [n = 13] | Chronic [n = 13] | - | 71 | PVP-I + ultrasonic irrigation + root planning | - | 71 | Ultrasonic irrigation + root planning alone | - | - | - | - | - | - | 3 |
| Ribeiro (2006) [31] | Brazil | Single-blinded, parallel-arm RCT | 44 | - | - | 23 | 26 | PVP-I + subgingival instrumentation | 21 | 31 | subgingival instrumentation | 11 (47.83%) | 9 (42.86%) | 42.96 | - | 42.52 | - | 6 |
| Ribeiro (2010) [46] | Brazil | Parallel-arm RCT | 28 | - | - | 13 | 19 | PVP-I + subgingival instrumentation | 15 | 18 | subgingival instrumentation | 6 (46.15%) | 7 (47.37%) | 43.74 | - | 42.96 | - | 6 |
| do Vale (2016) [40] | Brazil | Parallel-arm RCT | 34 | Aggressive [n = 34] | Chronic [n = 34] | 14 | 14 | FMUD + PVPI | 14 | 14 | FMUD + SS | 2 (14.29%) | 5 (35.71%) | 28.54 | 4.14 | 28.57 | 4.59 | 6 |
| Kessler (2021) [42] | Belgium | Single-blinded RCT | 34 | - | Chronic [n = 17] | 17 | 402 | SRP + PVP-I | 17 | 418 | SRP + SS | 9 (52.94%) | 9 (52.94%) | 51.8 | - | 51.8 | - | 12 |
| Kruck (2012) [43] | Germany | RCT | 51 | Moderate [n = 51] | Chronic [n = 51] | 17 | 408 | SRP + PVP-I | 17 | 408 | SRP + NaCl | 8 (47.06%) | 14 (41.18%) | 50.13 | 9.74 | 51.82 | 10.61 | 6 |
| Perrella (2016) [44] | Brazil | RCT | 29 | - | Chronic [n = 29] | 14 | 1950 | SRP + PVP-I | 15 | 2100 | SRP + irrigation with saline solution | 5 (35.71%) | 7 (46.67%) | 43.93 | 3.13 | 44.87 | 4.41 | 3 |
| Rosling (2001) [30] | USA | RCT | 223 | Advanced destructive [n = 223] | Chronic [n = 223] | 58 | 348 | Non-surgical ultrasonic instrumentation + PVP-I | 92 | 552 | Non-surgical ultrasonic instrumentation | 29 (50.6%) | 52 (56.60%) | 44.2 | 8.8 | 44.5 | 8.6 | 12 |
| Sahrmann (2014) [47] | Switzerland | RCT | 11 | Severe [n = 11] | Chronic [n = 11] | 11 | 73 | SRP + PVP-I | 11 | 73 | SRP + water | 9 (81.81%) | 9 (81.81%) | 48.9 | - | 48.9 | - | 3 |
| Zanatta (2006) [34] | Brazil | Single-blinded, parallel-arm RCT | 40 | Moderate [n = 40] | Chronic [n = 40] | 15 | 270 | Non-surgical ultrasonic instrumentation + PVP-I | 13 | 228 | Root planing + NaCl irrigation | - | - | 42 | - | 40 | - | 3 |
| NaOCl + NSPT vs. NSPT Alone | ||||||||||||||||||
| Bizzarro (2016) [39] | Netherlands, | RCT | 110 | - | - | 27 | 162 | Basic periodontal therapy + NaOCl | 29 | 174 | Basic periodontal therapy + Saline | 15 (55.00%) | 11 (44.00%) | 47.7 | 11.2 | 46.9 | 8.5 | 12 |
| Iorio-Siciliano (2021) [41] | Italy | RCT | 37 | Severe [n = 37] | Chronic [n = 37] | 18 | 895 | MINST and NaOCl gel application | 19 | 728 | MINST alone | 6 (33.33%) | 10 (52.63%) | 53.3 | 9.8 | 48.5 | 6.5 | 6 |
| Forgas (1986) [36] | USA | RCT | 20 | Moderate [n = 20] | - | 10 | 40 | SRP + NaOCl | 10 | 40 | 6 (60.00%) | 6 (60.00%) | Both groups [mean = 38.8] | 3 | ||||
| Megally (2020) [37] | Switzerland | RCT | 32 | - | - | 16 | 172 | NaOCl gel + ultrasonic debridement | 16 | 193 | ultrasonic debridement only | 7 (43.75%) | 6 (37.50%) | 61.7 | 9.8 | 62.1 | 8.8 | 12 |
| Radulescu (2022) [45] | Romania | Triple-blinded RCT | 62 | Stage III-IV [n = 62] | - | 21 | 84 | NaOCl gel + mechanical re-instrumentation | 21 | 84 | Placebo gel + mechanical re-instrumentation | 10 (50.00%) | 12 (66.66%) | 44.6 | 9.86 | 50.61 | 9.31 | 12 |
| Author (YOP) | Randomization | Deviations from Intended Interventions | Missing Outcomes Data | Outcome Measurement | Selective Reporting | Overall |
|---|---|---|---|---|---|---|
| PVP-I + NSPT vs. NSPT Alone | ||||||
| Al-Saeed (2009) [38] | Some concerns | Low | High | Low | Some concerns | High |
| Del Peloso Ribeiro (2006) [31] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Del Peloso Ribeiro (2010) [36] | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| do Vale (2016) [40] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Kruck (2012) [43] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Kessler (2021) [42] | Some concerns | High | Some concerns | Low | Some concerns | High |
| Perrella (2016) [44] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Rosling (2001) [30] | Some concerns | High | Low | Low | Some concerns | High |
| Sahrmann (2014) [47] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Zanatta (2006) [34] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| NaOCl + NSPT vs. NSPT Alone | ||||||
| Bizzarro (2016) [39] | Low | Low | Low | Low | Some concerns | Some concerns |
| Iorio-Siciliano (2021) [41] | Low | Low | Low | Low | Low | Some concerns |
| Forgas (1986) [36] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Megally (2020) [37] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Radulescu (2022) [45] | Low | Low | Low | Low | Some concerns | Some concerns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mobadder, M.; Nammour, S.; Grzech-Leśniak, Z.; Grzech-Leśniak, K. Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6593. https://doi.org/10.3390/jcm11216593
El Mobadder M, Nammour S, Grzech-Leśniak Z, Grzech-Leśniak K. Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(21):6593. https://doi.org/10.3390/jcm11216593
Chicago/Turabian StyleEl Mobadder, Marwan, Samir Nammour, Zuzanna Grzech-Leśniak, and Kinga Grzech-Leśniak. 2022. "Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 21: 6593. https://doi.org/10.3390/jcm11216593
APA StyleEl Mobadder, M., Nammour, S., Grzech-Leśniak, Z., & Grzech-Leśniak, K. (2022). Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(21), 6593. https://doi.org/10.3390/jcm11216593







