The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Evaluation of Biochemical Parameters in Sera and Blood Cell Number
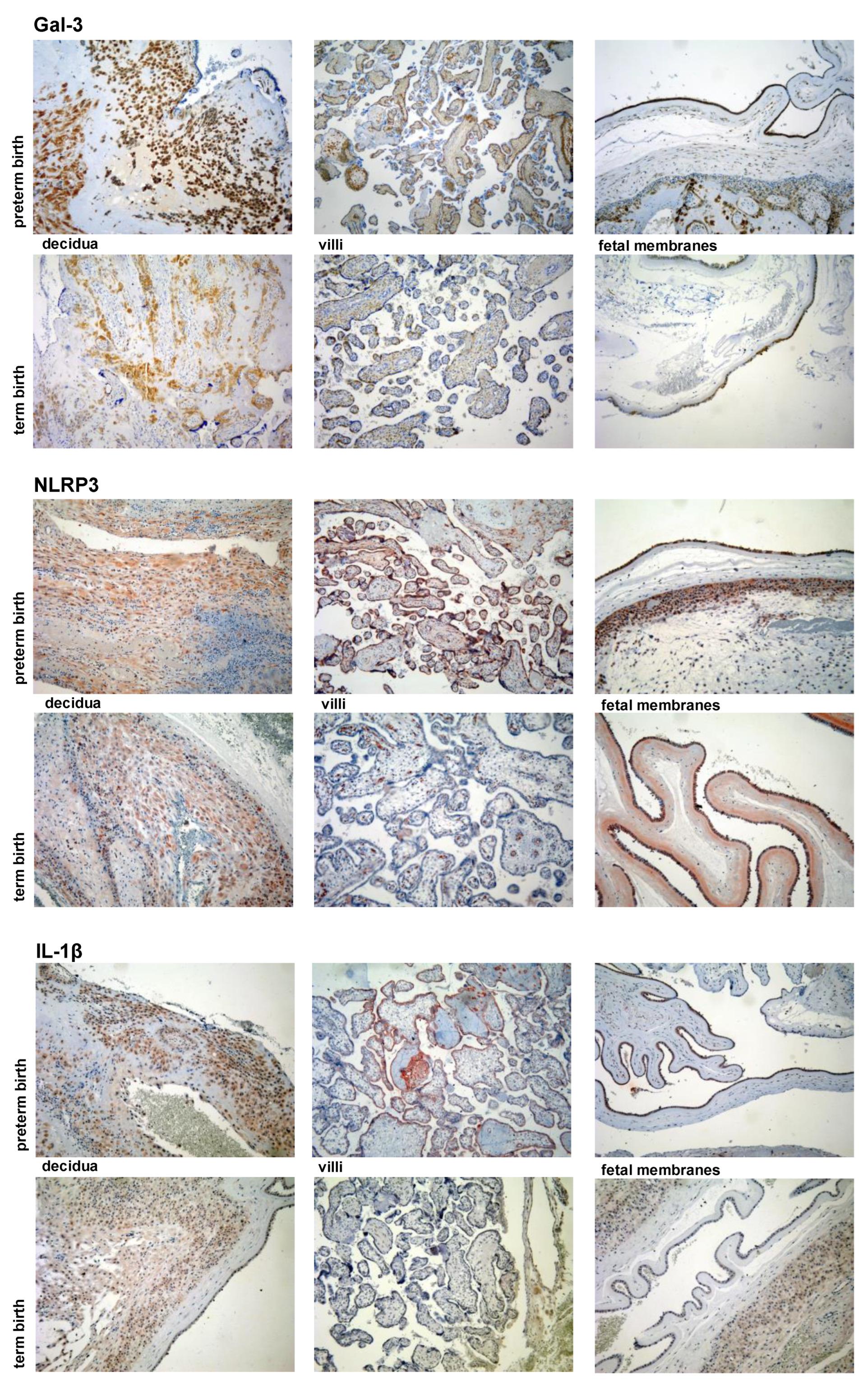
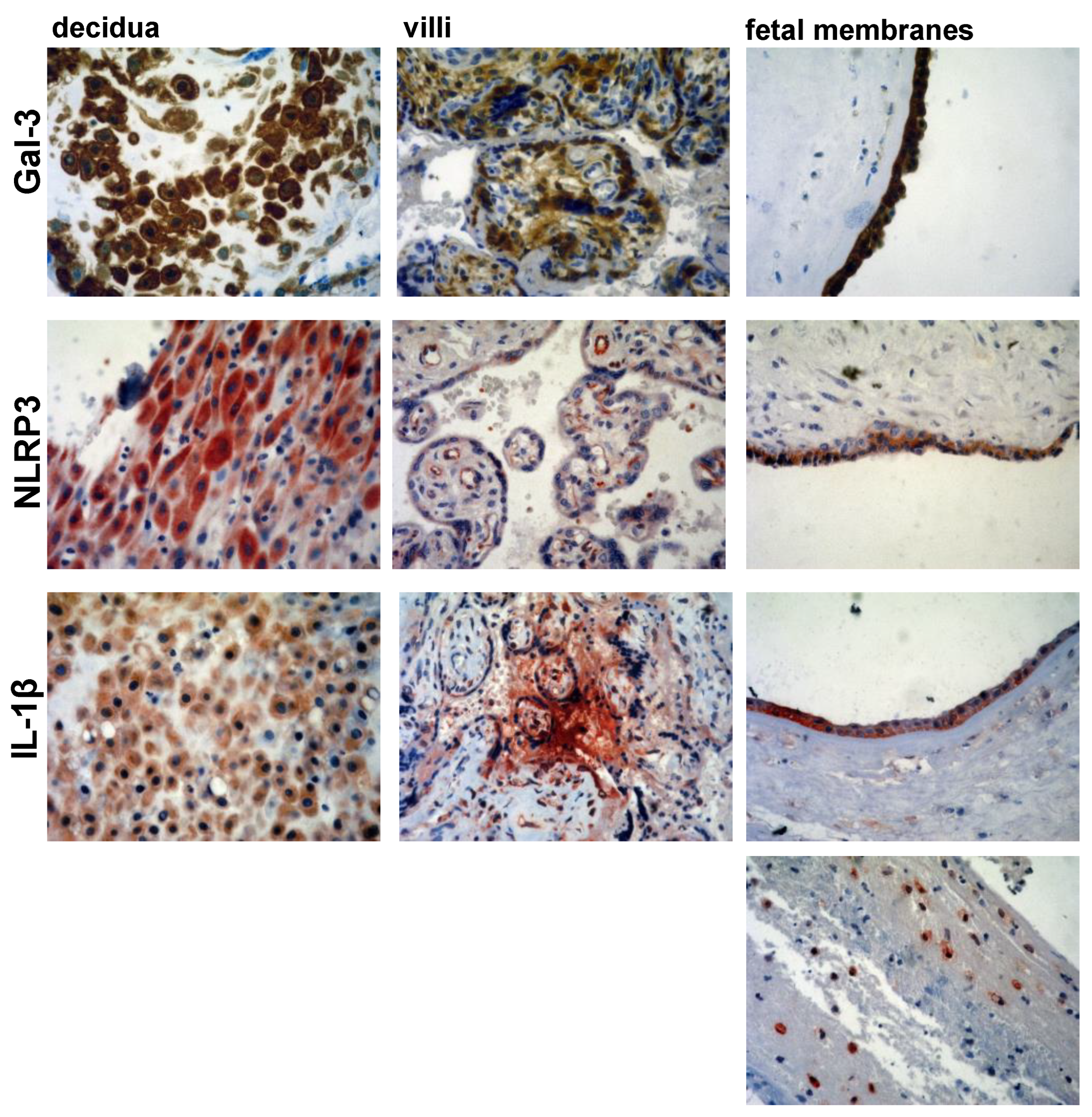
2.4. Immunohistochemistry
2.5. Immunohistochemistry Scoring
2.6. Statistical Analysis
3. Results
3.1. Maternal and Fetal Inflammatory Blood Parameters Are Increased in PTB Subject
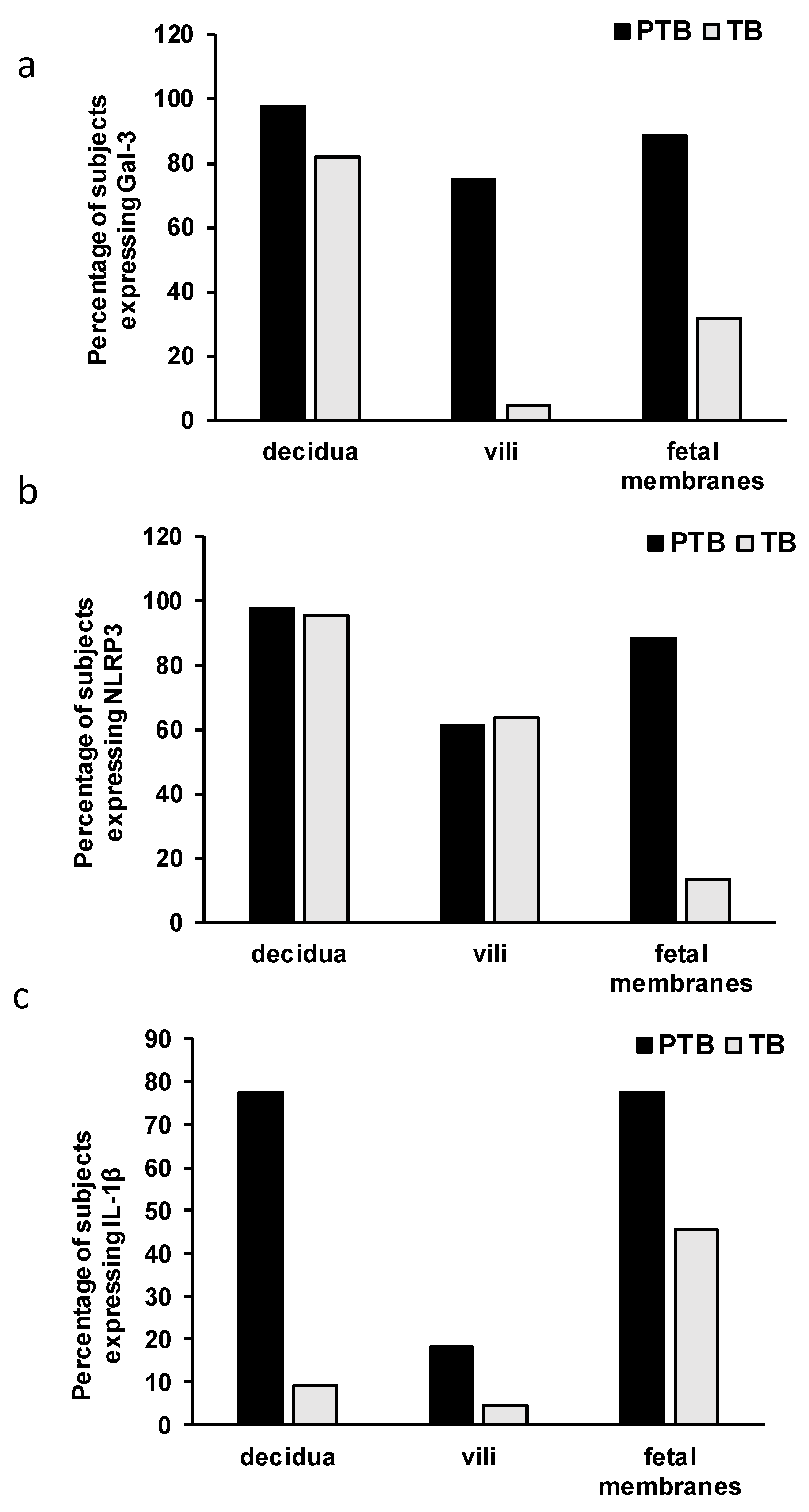
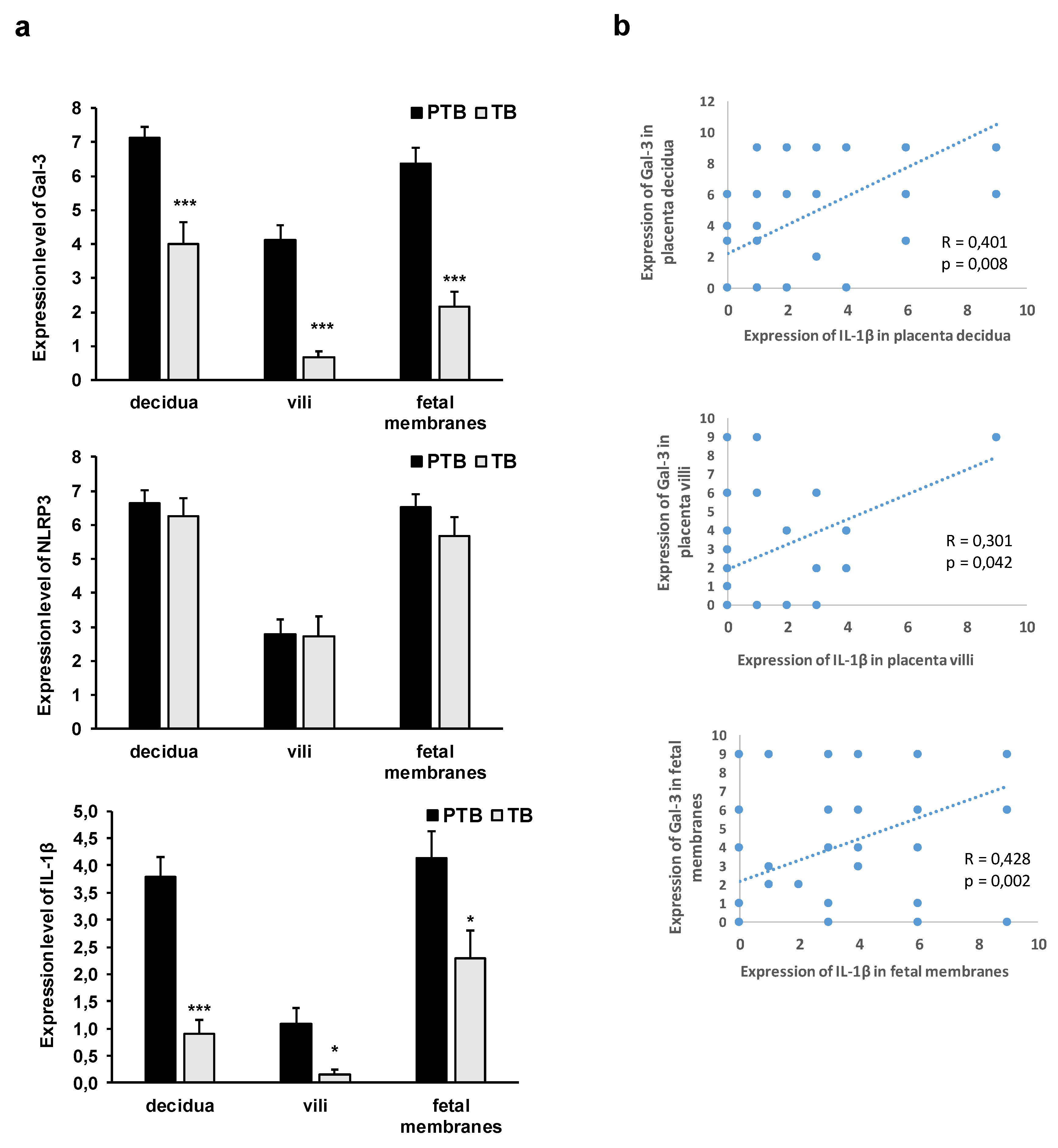
3.2. Expression Levels of Galectin-3 and IL-1β and Percentages of Positive Fetal Membranes and Placental Disc Samples Are Higher in the PTB Group
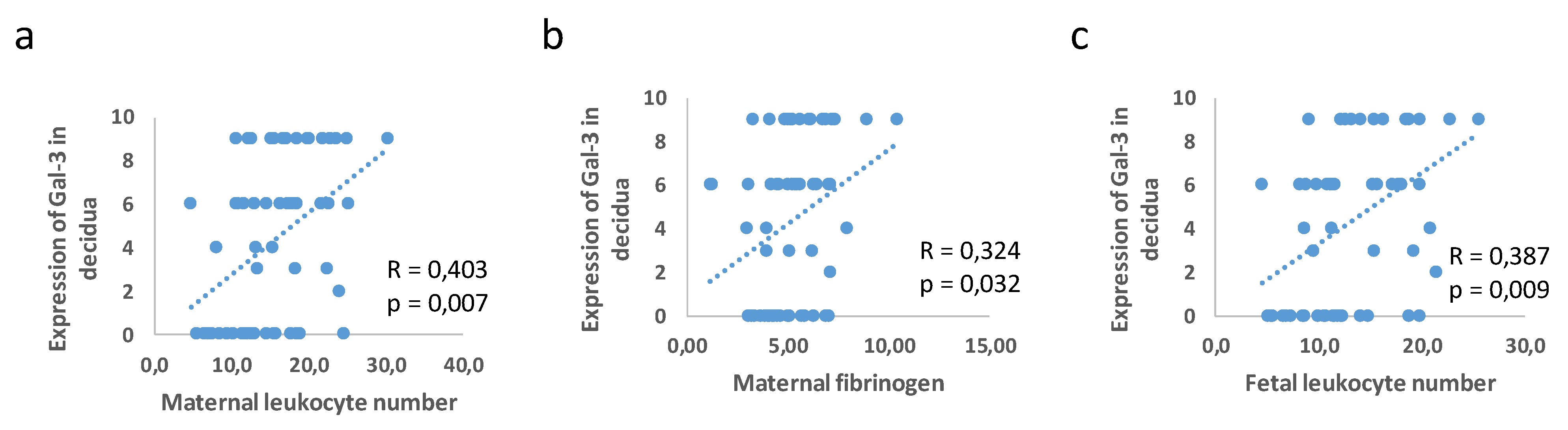
3.3. Correlation between Placental and Fetal Membranes Expression of Gal-3 and IL-1β and Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef]
- Luu, T.M.; Rehman Mian, M.O.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [CrossRef]
- Romero, R.; Gómez, R.; Chaiworapongsa, T.; Conoscenti, G.; Kim, J.C.; Kim, Y.M. The role of infection in preterm labour and delivery. Paediatr. Perinat. Epidemiol. 2001, 2, 41–56. [Google Scholar] [CrossRef]
- Gomez, R.; Romero, R.; Edwin, S.S.; David, C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect. Dis. Clin. N. Am. 1997, 11, 135–176. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 2014, 71, 330–358. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns with a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef]
- Czikk, M.J.; McCarthy, F.P.; Murphy, K.E. Chorioamnionitis: From pathogenesis to treatment. Clin. Microbiol. Infect. 2011, 17, 1304–1311. [Google Scholar] [CrossRef]
- Newton, E.R. Chorioamnionitis and intraamniotic infection. Clin. Obstet. Gynecol. 1993, 36, 795–808. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.È.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Familari, M.; Nääv, Å.; Erlandsson, L.; de Iongh, R.U.; Isaxon, C.; Strandberg, B.; Lundh, T.; Hansson, S.R.; Malmqvist, E. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. PLoS ONE 2019, 14, e0218799. [Google Scholar] [CrossRef]
- Menon, R.; Fortunato, S.J.; Yu, J.; Milne, G.L.; Sanchez, S.; Drobek, C.O.; Lappas, M.; Taylor, R.N. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta 2011, 32, 317–322. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.A.; Nien, J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006, 11, 317–326. [Google Scholar] [CrossRef]
- Saji, F.; Samejima, Y.; Kamiura, S.; Sawai, K.; Shimoya, K.; Kimura, T. Cytokine production in chorioamnionitis. J. Reprod. Immunol. 2000, 47, 185–196. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Edwin, S.; Romero, R.J. Prostaglandin biosynthesis by human decidual cells: Effects of inflammatory mediators. Prostaglandins Leukot. Essent. Fat. Acids 1990, 41, 35–38. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Quiniou, C.; Lubell, W.D.; Olson, D.M.; Girard, S.; Chemtob, S. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev. 2016, 28, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Elliott, C.L.; Loudon, J.A.; Brown, N.; Slater, D.M.; Bennett, P.R.; Sullivan, M.H. IL-1beta and IL-8 in human fetal membranes: Changes with gestational age, labor, and culture conditions. Am. J. Reprod. Immunol. 2001, 46, 260–267. [Google Scholar] [CrossRef]
- Romero, R.; Brody, D.T.; Oyarzun, E.; Mazor, M.; Wu, Y.K.; Hobbins, J.C.; Durum, S.K. Infection and labor. III. Interleukin-1: A signal for the onset of parturition. Am. J. Obstet. Gynecol. 1989, 160 Pt 1, 1117–1123. [Google Scholar] [CrossRef]
- Puchner, K.; Iavazzo, C.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Kouskouni, E.; Economou, E.; Malamitsi-Puchner, A.; Creatsas, G. Mid-trimester amniotic fluid interleukins (IL-1β, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo 2011, 25, 141–148. [Google Scholar]
- Vitoratos, N.; Mastorakos, G.; Kountouris, A.; Papadias, K.; Creatsas, G. Positive association of serum interleukin-1beta and CRH levels in women with pre-term labor. J. Endocrinol. Investig. 2007, 30, 35–40. [Google Scholar] [CrossRef]
- Himes, K.P.; Handley, D.; Chu, T.; Burke, B.; Bunce, K.; Simhan, H.N.; Peters, D.G. Comprehensive analysis of the transcriptional response of human decidual cells to lipopolysaccharide stimulation. J. Reprod. Immunol. 2012, 93, 17–27. [Google Scholar] [CrossRef]
- Pavlov, O.; Pavlova, O.; Ailamazyan, E.; Selkov, S. Characterization of cytokine production by human term placenta macrophages in vitro. Am. J. Reprod. Immunol. 2008, 60, 556–567. [Google Scholar] [CrossRef]
- Zaragoza, D.B.; Wilson, R.R.; Mitchell, B.F.; Olson, D.M. The interleukin 1beta-induced expression of human prostaglandin F2alpha receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFkappaB. Biol. Reprod. 2006, 75, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Chevillard, G.; Derjuga, A.; Devost, D.; Zingg, H.H.; Blank, V. Identification of interleukin-1beta regulated genes in uterine smooth muscle cells. Reproduction 2007, 134, 811–822. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.T.; Liew, F.Y.; Lukic, M.L. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, E.P.; Al Rabesi, Z.; Shahin, A.; Al Shamsi, M.; Arsenijevic, N.; Hsu, D.K.; Liu, F.T.; Lukic, M.L. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009, 130, 83–88. [Google Scholar] [CrossRef]
- Pejnovic, N.N.; Pantic, J.M.; Jovanovic, I.P.; Radosavljevic, G.D.; Milovanovic, M.Z.; Nikolic, I.G.; Zdravkovic, N.S.; Djukic, A.L.; Arsenijevic, N.N.; Lukic, M.L. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes 2013, 62, 1932–1944. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, A.; Milovanovic, J.; Stojanovic, B.; Djordjevic, D.; Stanojevic, I.; Jankovic, N.; Vojvodic, D.; Arsenijevic, N.; Lukic, M.L.; Milovanovic, M. Gal-3 Deficiency Suppresses Novosphyngobium aromaticivorans Inflammasome Activation and IL-17 Driven Autoimmune Cholangitis in Mice. Front. Immunol. 2019, 10, 1309. [Google Scholar] [CrossRef]
- Arsenijevic, A.; Milovanovic, M.; Milovanovic, J.; Stojanovic, B.; Zdravkovic, N.; Leung, P.S.; Liu, F.T.; Gershwin, M.E.; Lukic, M.L. Deletion of Galectin-3 Enhances Xenobiotic Induced Murine Primary Biliary Cholangitis by Facilitating Apoptosis of BECs and Release of Autoantigens. Sci. Rep. 2016, 6, 23348. [Google Scholar] [CrossRef]
- Arsenijevic, A.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, D.; Arsenijevic, N.; Milovanovic, M. Galectin-3 in Inflammasome Activation and Primary Biliary Cholangitis Development. Int. J. Mol. Sci. 2020, 21, 5097. [Google Scholar] [CrossRef]
- Simovic Markovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J. Crohns Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef]
- Sato, S.; Bhaumik, P.; St-Pierre, G.; Pelletier, I. Role of galectin-3 in the initial control of Leishmania infection. Crit. Rev. Immunol. 2014, 34, 147–175. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in innate immunity: Dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Komai-Koma, M.; Gilchrist, D.S.; Hsu, D.K.; Liu, F.T.; Springall, T.; Xu, D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008, 181, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Gittens, B.R.; Bodkin, J.V.; Nourshargh, S.; Perretti, M.; Cooper, D. Galectin-3: A Positive Regulator of Leukocyte Recruitment in the Inflamed Microcirculation. J. Immunol. 2017, 198, 4458–4469. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Gross, O.; Yazdi, A.S.; Thomas, C.J.; Masin, M.; Heinz, L.X.; Guarda, G.; Quadroni, M.; Drexler, S.K.; Tschopp, J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012, 36, 388–400. [Google Scholar] [CrossRef]
- Tian, J.; Yang, G.; Chen, H.Y.; Hsu, D.K.; Tomilov, A.; Olson, K.A.; Dehnad, A.; Fish, S.R.; Cortopassi, G.; Zhao, B.; et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016, 30, 4202–4213. [Google Scholar] [CrossRef]
- Wei, S.Q.; Fraser, W.; Luo, Z.C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: A systematic review. Obstet. Gynecol. 2010, 116 Pt 1, 393–401. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; McDonald, S.W.; Vinturache, A.E.; Xu, J.; Lee, M.W.; Briollais, L.; Lyon, A.W.; Slater, D.M.; Bocking, A.D.; et al. Maternal Whole Blood Gene Expression at 18 and 28 Weeks of Gestation Associated with Spontaneous Preterm Birth in Asymptomatic Women. PLoS ONE 2016, 11, e0155191. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; Chua, H.N.; Perkins, J.E.; Lye, S.J. Whole blood gene expression profile associated with spontaneous preterm birth in women with threatened preterm labor. PLoS ONE 2014, 9, e96901. [Google Scholar] [CrossRef]
- von Wolff, M.; Wang, X.; Gabius, H.J.; Strowitzki, T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol. Hum. Reprod. 2005, 11, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Maquoi, E.; van den Brûle, F.A.; Castronovo, V.; Foidart, J.M. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 1997, 18, 433–439. [Google Scholar] [CrossRef]
- Freitag, N.; Tirado-González, I.; Barrientos, G.; Cohen, M.; Daher, S.; Goldman-Wohl, D.; Mincheva-Nilsson, L.; John, C.M.; Jeschke, U.; Blois, S.M. The chimera-type galectin-3 is a positive modulator of trophoblast functions with dysregulated expression in gestational diabetes mellitus. Am. J. Reprod. Immunol. 2020, 84, e13311. [Google Scholar] [CrossRef] [PubMed]
- Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Vilotić, A.; Kolundžić, N.; Stefanoska, I.; Zetterberg, F.; Nilsson, U.J.; Leffler, H.; Vićovac, L. Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep. 2019, 9, 2136. [Google Scholar] [CrossRef]
- Yang, H.; Lei, C.; Zhang, W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Reprod Biomed. Online 2012, 24, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Powell, K.L.; Boehm-Sturm, P.; Koch, S.P.; Hecher, K.; Staff, A.C.; Arck, P.C.; Diemert, A.; et al. Galectin-3 deficiency in pregnancy increases the risk of fetal growth restriction (FGR) via placental insufficiency. Cell Death Dis. 2020, 11, 560. [Google Scholar] [CrossRef]
- Stefanoska, I.; Tadić, J.; Vilotić, A.; Jovanović Krivokuća, M.; Abu Rabi, T.; Vićovac, L. Histological chorioamnionitis in preterm prelabor rupture of the membranes is associated with increased expression of galectin-3 by amniotic epithelium. J. Matern. Fetal Nenatal Med. 2017, 30, 2232–2236. [Google Scholar] [CrossRef]
- Leimert, K.B.; Xu, W.; Princ, M.M.; Chemtob, S.; Olson, D.M. Inflammatory Amplification: A Central Tenet of Uterine Transition for Labor. Front. Cell Infect. Microbiol. 2021, 11, 660983. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Quiniou, C.; Palacios, J.; Hou, X.; Erfani, A.; Madaan, A.; Sanchez, M.; Leimert, K.; Boudreault, A.; Duhamel, F.; et al. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J. Immunol. 2015, 195, 3402–3415. [Google Scholar] [CrossRef]
- Ammälä, M.; Nyman, T.; Salmi, A.; Rutanen, E.M. The interleukin-1 system in gestational tissues at term: Effect of labour. Placenta 1997, 18, 717–723. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M.; Brandt, F.; Sepulveda, W.; Avila, C.; Cotton, D.B.; Dinarello, C.A. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am. J. Reprod. Immunol. 1992, 27, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M.; Tartakovsky, B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am. J. Obstet. Gynecol. 1991, 165 Pt 1, 969–971. [Google Scholar] [CrossRef]
- Kallapur, S.G.; Presicce, P.; Senthamaraikannan, P.; Alvarez, M.; Tarantal, A.F.; Miller, L.M.; Jobe, A.H.; Chougnet, C.A. Intra-amniotic IL-1β induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J. Immunol. 2013, 191, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Garry, D.J.; Baker, D.A.; Persad, M.D.; Peresleni, T.; Kocis, C.; Demishev, M. Progesterone effects on vaginal cytokines in women with a history of preterm birth. PLoS ONE 2018, 13, e0209346. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Amann, N.; Kuhn, C.; Schmoeckel, E.; Wöckel, A.; Zati Zehni, A.; Kaltofen, T.; Keckstein, S.; Mumm, J.N.; Meister, S.; et al. Interleukin-1 beta is significantly upregulated in the decidua of spontaneous and recurrent miscarriage placentas. J. Reprod. Immunol. 2021, 144, 103283. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Plazyo, O.; Unkel, R.; Leng, Y.; Than, N.G.; Chaiworapongsa, T.; Panaitescu, B.; Dong, Z.; et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod. Sci. 2017, 24, 1382–1401. [Google Scholar] [CrossRef]
- Miller, A.S.; Hidalgo, T.N.; Abrahams, V.M. Human fetal membrane IL-1β production in response to bacterial components is mediated by uric-acid induced NLRP3 inflammasome activation. J. Reprod. Immunol. 2022, 149, 103457. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Slutsky, R.; Levenson, D.; Gomez-Lopez, N. The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome. Mol. Hum. Reprod. 2020, 26, 712–726. [Google Scholar] [CrossRef]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef]
- Chen, Z.; Shan, Y.; You, X.; Gu, H.; Xu, C.; Long, J.; Ni, X. NLRP3 inflammasome is involved in uterine activation for labor at term and preterm. Reproduction 2021, 162, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Ranson, N.; Veldhuis, M.; Mitchell, B.; Fanning, S.; Cook, A.L.; Kunde, D.; Eri, R. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int. J. Mol. Sci. 2018, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum. Reprod. 1999, 14, 229–236. [Google Scholar] [CrossRef]
- Young, A.; Thomson, A.J.; Ledingham, M.; Jordan, F.; Greer, I.A.; Norman, J.E. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol. Reprod. 2002, 66, 445–449. [Google Scholar] [CrossRef]
- Romero, R.; Ceska, M.; Avila, C.; Mazor, M.; Behnke, E.; Lindley, I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am. J. Obstet. Gynecol. 1991, 165 Pt 1, 813–820. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.C.; Ruck, P.; Marx, T.; Kaiserling, E.; Oberpichler, A.; Tschesche, H.; Rath, W. Parturition at term: Parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum. Reprod. 1999, 14, 1096–1100. [Google Scholar] [CrossRef]
- Helmig, B.R.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Bujold, E.; Gomez, R.; Ohlsson, K.; Uldbjerg, N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J. Matern. Fetal Neonatal Med. 2002, 12, 237–246. [Google Scholar] [CrossRef]
- Jeon, S.B.; Yoon, H.J.; Chang, C.Y.; Koh, H.S.; Jeon, S.H.; Park, E.J. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J. Immunol. 2010, 185, 7037–7046. [Google Scholar] [CrossRef]
- Uchino, Y.; Woodward, A.M.; Mauris, J.; Peterson, K.; Verma, P.; Nilsson, U.J.; Rajaiya, J.; Argüeso, P. Galectin-3 is an amplifier of the interleukin-1β-mediated inflammatory response in corneal keratinocytes. Immunology 2018, 154, 490–499. [Google Scholar] [CrossRef] [PubMed]
- El-Azzamy, H.; Balogh, A.; Romero, R.; Xu, Y.; LaJeunesse, C.; Plazyo, O.; Xu, Z.; Price, T.G.; Dong, Z.; Tarca, A.L.; et al. Characteristic Changes in Decidual Gene Expression Signature in Spontaneous Term Parturition. J. Pathol. Transl. Med. 2017, 51, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Turhan, U.; Sezer, S.; Kaya, S.; Dağ, İ.; Tayyar, A. Maternal serum galectin-1 and galectin-3 levels in pregnancies complicated with preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2020, 33, 861–868. [Google Scholar] [CrossRef]
- Faust, K.; Freitag, N.; Barrientos, G.; Hartel, C.; Blois, S.M. Galectin-Levels Are Elevated in Infants Born Preterm Due to Amniotic Infection and Rapidly Decline in the Neonatal Period. Front. Immunol. 2021, 11, 599104. [Google Scholar] [CrossRef] [PubMed]
- Farladansky-Gershnabel, S.; Dekel, N.; Biron-Shental, T.; Shechter-Maor, G.; Amiel, A.; Weisz, A.; Benchetrit, S.; Zitman-Gal, T. Spontaneous Preterm Birth: Elevated Galectin-3 and Telomere Shortening May Reflect a Common Pathway of Enhanced Inflammation and Senescence. Reprod. Sci. 2022. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ao, M.; Furusho, H.; Chea, C.; Nagasaki, A.; Sakamoto, S.; Ando, T.; Inubushi, T.; Kozai, K.; Takata, T. Galectin-3 Plays an Important Role in Preterm Birth Caused by Dental Infection of Porphyromonas gingivalis. Sci. Rep. 2018, 8, 2867. [Google Scholar] [CrossRef]




| General Characteristics of the Patients | Control Group (Term Delivery) (n = 22) | Experimental Group (Preterm Delivery) (n = 40) |
|---|---|---|
| Age | 32.55 ± 6.22 | 29.68 ± 7.19 |
| Gestational age | 38.95 ± 1.53 | 30.20 ± 3.84 ** |
| Parameters of Inflammation | Control Group (Term Delivery) (n = 22) | Experimental Group (Preterm Delivery) (n = 40) | |
|---|---|---|---|
| From maternal blood | Leukocytes (×109/L) | 9.89 ± 3.04 | 18.46 ± 4.47 ** |
| CRP (mg/L) | 7.34 ± 3.36 | 44.74 ± 44.89 ** | |
| Fibrinogen (g/L) | 4.22 ± 0.83 | 5.70 ± 1.78 ** | |
| From fetal blood | Leukocytes (×109/L) | 8.53 ± 2.29 | 15.80 ± 3.95 ** |
| CRP (mg/L) | 6.09 ± 2.96 | 29.89 ± 30.68 ** | |
| Fibrinogen (g/L) | 3.97 ± 0.74 | 4.88 ± 1.44 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovic, N.; Milovanovic, M.; Jovic, J.J.; Ilic, M.B.; Rakic, D.; Milenkovic, V.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Arsenijevic, N.; et al. The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor. J. Clin. Med. 2022, 11, 6521. https://doi.org/10.3390/jcm11216521
Jovic N, Milovanovic M, Jovic JJ, Ilic MB, Rakic D, Milenkovic V, Stojanovic B, Milovanovic J, Arsenijevic A, Arsenijevic N, et al. The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor. Journal of Clinical Medicine. 2022; 11(21):6521. https://doi.org/10.3390/jcm11216521
Chicago/Turabian StyleJovic, Nikola, Marija Milovanovic, Jovana Joksimovic Jovic, Marija Bicanin Ilic, Dejana Rakic, Vladimir Milenkovic, Bojana Stojanovic, Jelena Milovanovic, Aleksandar Arsenijevic, Nebojsa Arsenijevic, and et al. 2022. "The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor" Journal of Clinical Medicine 11, no. 21: 6521. https://doi.org/10.3390/jcm11216521
APA StyleJovic, N., Milovanovic, M., Jovic, J. J., Ilic, M. B., Rakic, D., Milenkovic, V., Stojanovic, B., Milovanovic, J., Arsenijevic, A., Arsenijevic, N., & Varjacic, M. (2022). The Expression of IL-1β Correlates with the Expression of Galectin-3 in the Tissue at the Maternal–Fetal Interface during the Term and Preterm Labor. Journal of Clinical Medicine, 11(21), 6521. https://doi.org/10.3390/jcm11216521








