Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil
Abstract
1. Introduction
2. Materials and Methods
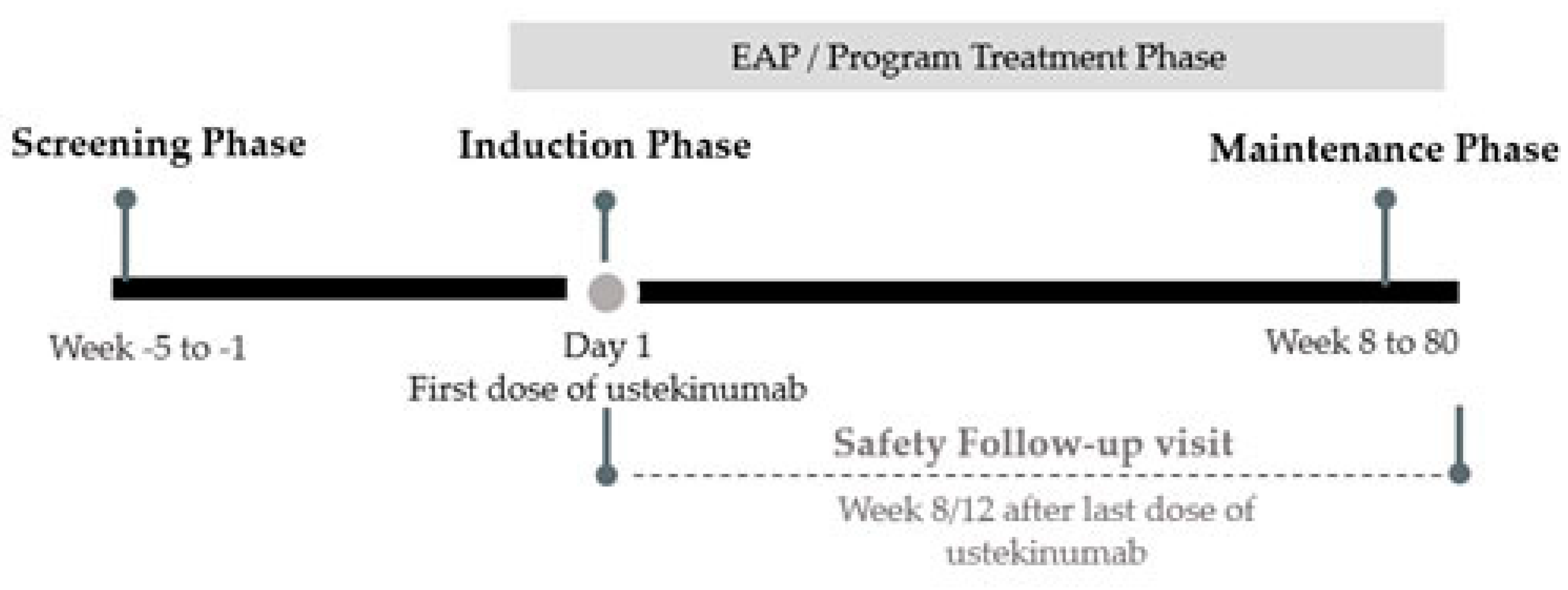
2.1. Patients
2.2. Assessments
2.3. Statistical Analysis
2.4. Ethical Considerations
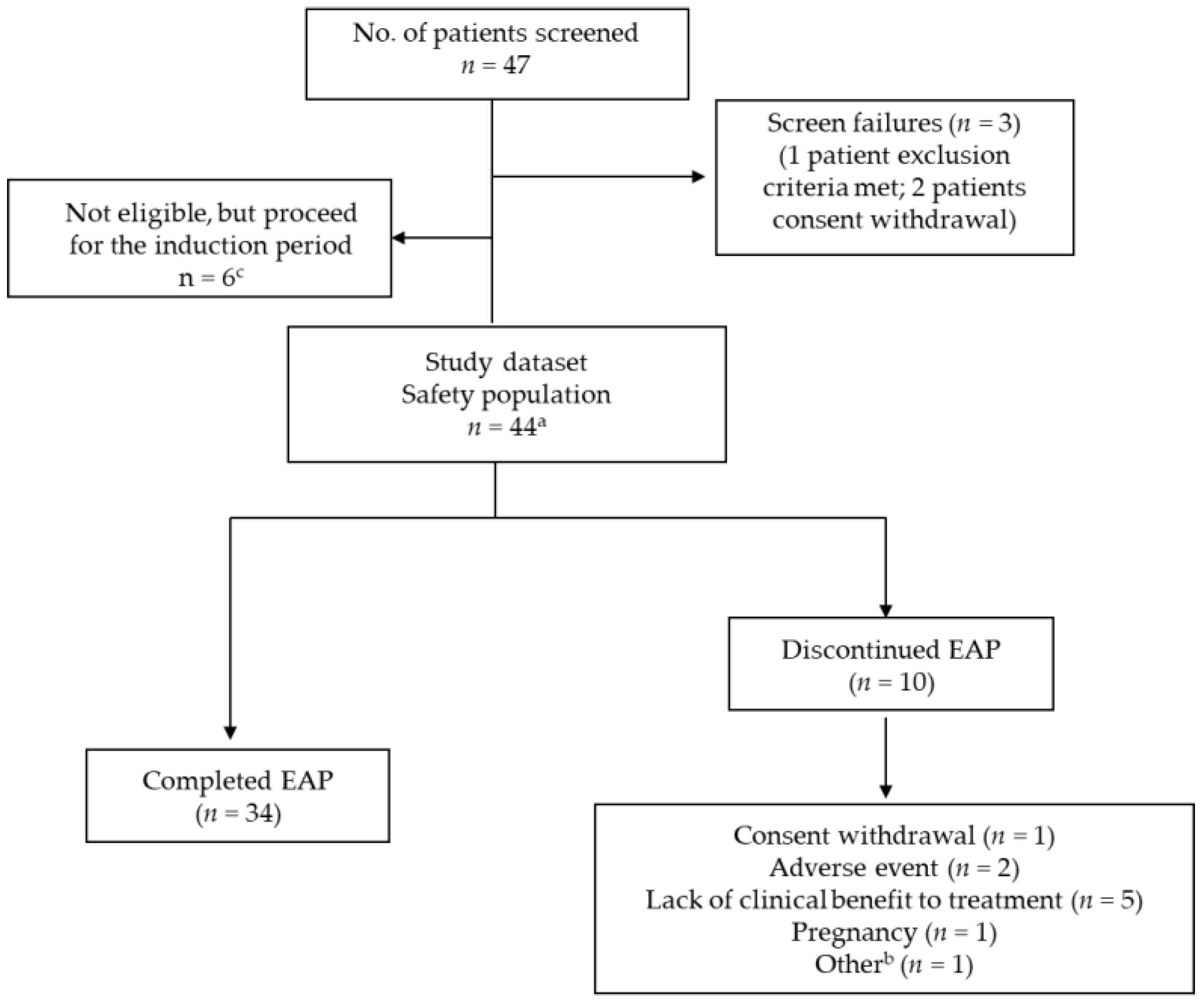
3. Results
3.1. Baseline Demographics and Disease Characteristics
3.2. Safety Outcomes
3.3. Treatment Effectiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Fortes, F.M.L.; Sorte, N.B.; Mariano, V.D.; Andrade, L.D.; Oliveira, F.A.; Santos, M.C.A.; Santos, C.I.N.d.; Passos, C.A.; Pacheco, M.P.; Surlo, V.C.; et al. Active tuberculosis in inflammatory bowel disease patients under treatment from an endemic area in Latin America. World J. Gastroenterol. 2020, 26, 6993–7004. [Google Scholar] [CrossRef] [PubMed]
- Economou, E.Z.M.; Michopoulos, S. Incidence and prevalence of Crohn’s disease and its etiological influences. Ann. Gatroentrol. 2009, 22, 158–167. [Google Scholar]
- Gasparini, R.G.; Sassaki, L.Y.; Saad-Hossne, R. Inflammatory bowel disease epidemiology in São Paulo State, Brazil. Clin. Exp. Gastroenterol. 2018, 11, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.S.; Chebli, J.M.F.; Amarante, H.; Flores, C.; Parente, J.M.L.; Ramos, O.; Fernandes, M.; Rocha, J.J.R.; Feitosa, M.R.; Feres, O.; et al. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J. Gastroenterol. 2019, 25, 5862–5882. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2016, 11, 3–25. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, B.L.; Mao, R.; Zhang, S.; He, Y.; Zeng, Z.; Ben-Horin, S.; Chen, M. Systematic review with meta-analysis: Loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J. Gastroenterol. 2017, 52, 535–554. [Google Scholar] [CrossRef]
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. mAbs 2011, 3, 535–545. [Google Scholar] [CrossRef]
- European Medicines Agency. Stelara, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/stelara#product-information-section (accessed on 5 April 2022).
- STELARA. Ustekinumab; Janssen Biotech, Inc.: Horsham, PA, USA, 2016. [Google Scholar]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Iglesias-Flores, E.; Navarro, P.; Rivero, M.; Gutiérrez, A.; Sierra-Ausin, M.; Mesonero, F.; Ferreiro-Iglesias, R.; et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol Ther. 2020, 52, 1017–1030. [Google Scholar] [CrossRef]
- Chaparro, M.; Sulleiro, S.; Bastón-Rey, I.; Rodríguez, C.; García-Tercero, I.; Ramírez, P.; García-López, S.; Rojas-Feria, M.; Gutiérrez, A.; Malavés, J.M.H.; et al. P629 Long-term effectiveness and safety of ustekinumab (UST) in patients with active Crohn’s disease (CD) in real life: Interim analysis of the SUSTAIN study. J. Crohn’s Colitis 2020, 14 (Suppl. S1), S520–S522. [Google Scholar] [CrossRef]
- Janssen Presents Results of First Head-to-Head Study of Biologic Therapies in Patients with Moderate to Severe Crohn’s Disease. 2021. Available online: https://www.jnj.com/janssen-presents-results-of-first-head-to-head-study-of-biologic-therapies-in-patients-with-moderate-to-severe-crohns-disease (accessed on 1 October 2021).
- Parra, R.S.; Feitosa, M.R.; Féres, O.; da Rocha, J.J.R.; Chebli, J.M.F.; Chebli, L.; Bertges, E.R.; Gomes, T.N.F.; Ambrogini, O., Jr.; Junior, A.J.T.A.; et al. P460 Efficacy of ustekinumab in patients with anti-TNF refractory Crohn’s disease: Data from a real-world study in Brazil. J. Crohn’s Colitis 2019, 13 (Suppl. S1), S341–S342. [Google Scholar] [CrossRef]
- Zaltman, C.; Parra, R.S.; Sassaki, L.Y.; Santana, G.O.; Ferrari, M.L.A.; Miszputen, S.J.; Amarante, H.M.B.S.; Kaiser Junior, R.L.; Flores, C.; Catapani, W.R.; et al. Real-world disease activity and sociodemographic, clinical and treatment characteristics of moderate-to-severe inflammatory bowel disease in Brazil. World J. Gastroenterol. 2021, 27, 208–223. [Google Scholar] [CrossRef]
- Eberl, A.; Hallinen, T.; Af Björkesten, C.G.; Heikkinen, M.; Hirsi, E.; Kellokumpu, M.; Koskinen, I.; Moilanen, V.; Nielsen, C.; Nuutinen, H.; et al. Ustekinumab for Crohn’s disease: A nationwide real-life cohort study from Finland (FINUSTE). Scand. J. Gastroenterol. 2019, 54, 718–725. [Google Scholar] [CrossRef]
- Kubesch, A.; Rueter, L.; Farrag, K.; Krause, T.; Stienecker, K.; Hausmann, J.; Filmann, N.; Dignass, A.; Stein, J.; Blumenstein, I. Short and Long-Term Effectiveness of Ustekinumab in Patients with Crohn’s Disease: Real-World Data from a German IBD. Cohort. J. Clin. Med. 2019, 8, 2140. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Wils, P.; Bouhnik, Y.; Michetti, P.; Flourie, B.; Brixi, H.; Bourrier, A.; Allez, M.; Duclos, B.; Serrero, M.; Buisson, A.; et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: A multicentre experience. Aliment. Pharmacol. Ther. 2018, 47, 588–595. [Google Scholar] [CrossRef]
- Ma, C.; Fedorak, R.N.; Kaplan, G.G.; Dieleman, L.A.; Devlin, S.M.; Stern, N.; Kroeker, K.I.; Seow, C.H.; Leung, Y.; Novak, K.L.; et al. Long-term Maintenance of Clinical, Endoscopic, and Radiographic Response to Ustekinumab in Moderate-to-Severe Crohn’s Disease: Real-world Experience from a Multicenter Cohort Study. Inflamm. Bowel Dis. 2017, 23, 833–839. [Google Scholar] [CrossRef]
- Ma, C.; Fedorak, R.N.; Kaplan, G.G.; Dieleman, L.A.; Devlin, S.M.; Stern, N.; Kroeker, K.I.; Seow, C.H.; Leung, Y.; Novak, K.L.; et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: Real world experience from a multicentre cohort. Aliment. Pharmacol. Ther. 2017, 45, 1232–1243. [Google Scholar] [CrossRef]
- Crosby, S.; Schuh, M.J.; Farraye, F.A. Rechallenge with Subcutaneous Ustekinumab after Hypersensitivity Reaction to Intravenous Ustekinumab. J. Crohn’s Colitis 2021, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: The CLASSIC-I trial. Gastroenterology 2006, 130, 323–333. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Stoinov, S.; Honiball, P.J.; Rutgeerts, P.; Mason, D.; Bloomfield, R.; Schreiber, S. Certolizumab pegol for the treatment of Crohn’s disease. N. Engl. J. Med. 2007, 357, 228–238. [Google Scholar] [CrossRef]
- Dubois-Camacho, K.; Ottum, P.A.; Franco-Muñoz, D.; de la Fuente, M.; Torres-Riquelme, A.; Díaz-Jiménez, D.; Olivares-Morales, M.; Astudillo, G.; Quera, R.; Hermoso, M.A.; et al. Glucocorticosteroid therapy in inflammatory bowel diseases: From clinical practice to molecular biology. World J. Gastroenterol. 2017, 23, 6628–6638. [Google Scholar] [CrossRef]
- Nic Suibhne, T.; Raftery, T.C.; McMahon, O.; Walsh, C.; O’Morain, C.; O’Sullivan, M. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J. Crohn’s Colitis 2013, 7, e241–e248. [Google Scholar] [CrossRef]
- Loftus, E.; Sloan, S.; Ramachandran, P.; Yang, Z.; Guo, C.; Gasink, C. Comparison of Rates of Active Tuberculosis Infection in the Phase 2 and 3 Clinical Trial Programs for Anti-IL12/23 and Anti-TNFS. Gastroenterology 2017, 152, S596. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.B.; Lochs, H.; Löfberg, R.; Modigliani, R.; Present, D.H.; Rutgeerts, P.; Schölmerich, J.; Stange, E.F.; et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002, 122, 512–530. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Sandborn, W.J.; Dubois, C.; Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 2010, 8, 357–363. [Google Scholar] [CrossRef]
- Ishida, N.; Miyazu, T.; Suzuki, T.; Tamura, S.; Tani, S.; Yamade, M.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Furuta, T.; et al. Evaluation of the modified Crohn’s disease activity index in patients with Crohn’s disease with enterostomy: A single-center observational study. Medicine 2021, 100, e24717. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Ma, C.; Almutairdi, A.; Panaccione, R. Clinical utility of ustekinumab in Crohn’s disease. J. Inflamm. Res. 2018, 11, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Gutiérrez, A.; Antolín, B.; Huguet, J.M.; de Francisco, R.; Merino, O.; Carpio, D.; García-López, S.; et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2019, 50, 278–288. [Google Scholar] [CrossRef]
- Verstockt, B.; Dreesen, E.; Noman, M.; Outtier, A.; van den Berghe, N.; Aerden, I.; Compernolle, G.; van Assche, G.; Gils, A.; Vermeire, S.; et al. Ustekinumab Exposure-outcome Analysis in Crohn’s Disease Only in Part Explains Limited Endoscopic Remission Rates. J. Crohn’s Colitis 2019, 13, 864–872. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 44) | |
|---|---|
| Age (years) | |
| N (%) | 44 (100) |
| Mean (standard deviation) | 39.93 (13.81) |
| Gender, n (%) | |
| N (%) | 44 (100) |
| Female | 23 (52.3) |
| Male | 21 (47.7) |
| Body mass index (kg/m2) | |
| Mean (standard deviation) | 23.71 (4.90) |
| HBI score | |
| N (%) | 41 (93.2) |
| Mean (standard deviation) | 11.54 (4.43) |
| CDAI score (points) | |
| N (%) | 44 (100) |
| Mean (standard deviation) | 287.67 (67.99) |
| C-reactive protein levels (mg/L) | |
| N | 42 (95.5) |
| Mean (standard deviation) | 17.67 (20.73) |
| Previous intestinal resections | |
| N (%) | 8 (18.2) |
| Participants (n = 44) n (%) | nAEs | |
|---|---|---|
| Adverse event, n (%) | 41 (93.2) | 249 |
| 95% CI (%) | 85.7, 100.0 | |
| Relationship with program, n (%) | ||
| Not related | - | 176 (71.0) |
| Doubtful | - | 29 (11.7) |
| Possible | - | 30 (12.1) |
| Probable | - | 7 (2.8) |
| Very Likely | - | 6 (2.4) |
| Severity, n (%) | ||
| Mild | 171 (68.7) | |
| Moderate | 69 (27.7) | |
| Severe | 9 (3.6) | |
| Action taken with program patient, n (%) | ||
| None | - | 114 (45.8) |
| Pharmacological treatment | - | 108 (43.4) |
| Non-pharmacological treatment | - | 10 (4.0) |
| Hospitalization or prolongation of existing hospitalization | - | 13 (5.2) |
| Other | - | 4 (1.6) |
| Action taken with program drug, n (%) | ||
| None | - | 232 (93.2) |
| Dose adjustment | - | 6 (2.4) |
| Temporary treatment discontinuation | - | 7 (2.8) |
| Permanent treatment discontinuation | - | 3 (1.2) |
| Other | - | 1 (0.4) |
| Serious, n (%) | ||
| Yes | - | 14 (5.6) |
| Required inpatient hospitalization or prolongation of existing hospitalization | - | 14 (100) |
| No | - | 235 (94.4) |
| Ongoing, n (%) | ||
| Yes | - | 45 (18.1) |
| Recovering/Resolving | - | 13 (28.9) |
| Not recovered/Not resolved | - | 31 (68.9) |
| Unknown | - | 1 (2.2) |
| No | - | 204 (81.9) |
| Recovered/Resolved | - | 203 (100.0) |
| SAE, n (%) | 9 (20.5) | 14 |
| 95% CI (%) | 8.5, 32.4 | |
| Gastrointestinal disorders | 4 (9.1) | 4 |
| Infections and infestations | 4 (9.1) | 5 |
| Surgical and medical procedures | 2 (4.5) | 2 |
| Cardiac disorders | 1 (2.3) | 1 |
| Injury, poisoning, and procedural complications | 1 (2.3) | 1 |
| Renal and urinary disorders | 1 (2.3) | 1 |
| Non-SAE, n (%) | 41 (93.2) | 235 |
| ADR, n (%) | 19 (44.2) | 43 |
| 95% CI (%) | 29.3, 59.0 | |
| Serious ADR, n (%) | 4 (9.3) | 6 |
| Infections and infestations | 4 (9.1) | 5 |
| Renal and urinary disorders | 1 (2.3) | 1 |
| Non-serious ADR, n (%) | 18 (41.9) | 37 |
| SAE or non-serious ADR, n (%) | 23 (53.5) | 51 |
| 95% CI (%) | 38.6, 68.4 | |
| Gastrointestinal disorders | 11 (25.0) | 17 |
| Infections and infestations | 11 (25.0) | 15 |
| Cardiac disorders | 2 (4.5) | 2 |
| Investigations | 2 (4.5) | 2 |
| Musculoskeletal and connective tissue disorders | 2 (4.5) | 2 |
| Reproductive system and breast disorders | 2 (4.5) | 2 |
| Surgical and medical procedures | 2 (4.5) | 2 |
| Hepatobiliary disorders | 1 (2.3) | 1 |
| Immune system disorders | 1 (2.3) | 1 |
| Injury, poisoning and procedural complications | 1 (2.3) | 1 |
| Nervous system disorders | 1 (2.3) | 2 |
| Psychiatric disorders | 1 (2.3) | 1 |
| Renal and urinary disorders | 1 (2.3) | 1 |
| Skin and subcutaneous tissue disorders | 1 (2.3) | 2 |
| At Baseline (n = 44) | Week 8 (n = 44) | Week 16/20 (n = 42) | Week 40/44 (n = 41) | At Week 80 (n = 34) | |
|---|---|---|---|---|---|
| Hematology | |||||
| Red blood cell count performed, n (%) | 44 (100.0) | 44 (100.0) | 42 (100.0) | 40 (97.6) | 33 (97.1) |
| Mean (SD), (106/mm3) | 4.43 (0.52) | 4.46 (0.55) | 4.51 (0.52) | 4.60 (0.54) | 4.74 (0.52) |
| Median (Q1–Q3), (106/mm3) | 4.39 (4.02−4.69) | 4.39 (4.07−4.88) | 4.49 (4.24−4.78) | 4.54 (4.26−5.05) | 4.77 (4.45−5.00) |
| Normal, n (%) | 28 (63.6) | 31 (70.5) | 31 (73.8) | 29 (72.5) | 26 (78.8) |
| Abnormal (clinically significant), n (%) | 3 (6.8) | 1 (2.3) | 2 (4.8) | 1 (2.5) | 0 (0.0) |
| White blood cell performed, n (%) | 44 (100.0) | 44 (100.0) | 42 (100.0) | 40 (97.6) | 33 (97.1) |
| Mean (SD), (103/mm3) | 8.71 (2.96) | 8.25 (2.63) | 8.37 (3.09) | 7.78 (2.81) | 7.59 (2.58) |
| Median (Q1–Q3), (103/mm3) | 8.30 (7.05−9.99) | 7.17 (6.36−10.35) | 7.61 (6.10−9.60) | 7.10 (5.83−9.26) | 6.85 (6.00−9.28) |
| Normal, n (%) | 34 (77.3) | 38 (86.4) | 36 (85.7) | 33 (82.5) | 26 (78.8) |
| Abnormal (clinically significant), n (%) | 1 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Platelet count performed, n (%) | 43 (97.7) | 44 (100.0) | 42 (100.0) | 40 (97.6) | 33 (97.1) |
| Mean (SD), (103/mm3) | 311 (123) | 294 (110) | 295 (107) | 271 (915) | 272 (871) |
| Median (Q1–Q3), (103/mm3) | 310 (219−383) | 273 (225−344) | 274 (230−344) | 266 (214−326) | 277 (200−336) |
| Normal, n (%) | 33 (76.7) | 37 (84.1) | 33 (78.6) | 34 (85.0) | 29 (87.9) |
| Abnormal (clinically significant), n (%) | 1 (2.3) | 2 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI, n (%) | 41 (93.2) | 37 (84.1) | 38 (90.4) | 36 (87.8) | 33 (97.1) |
| Mean (SD), (kg/m2) | 23.71 (4.90) | 24.61 (5.36) | 24.74 (5.19) | 25.32 (5.58) | 25.88 (5.50) |
| Median (Q1–Q3), (kg/m2) | 22.19 (20.59−25.82) | 23.47 (20.75−27.88) | 23.90 (20.90−28.84) | 24.65 (21.85−29.71) | 24.43 (22.54−28.68) |
| Categories, n (%) | |||||
| Underweight | 5 (12.2) | 4 (10.8) | 3 (7.9) | 3 (8.3) | 1 (3.0) |
| Normal weight | 24 (58.5) | 18 (48.6) | 18 (47.4) | 17 (47.2) | 17 (51.5) |
| Pre-obesity | 8 (19.5) | 8 (21.6) | 9 (23.7) | 7 (19.4) | 8 (24.2) |
| Obese | 4 (9.8) | 7 (18.9) | 8 (21.1) | 9 (25.0) | 7 (21.2) |
| CRP, n (%) | 42 (95.5) | - | 42 (100.0) | 40 (97.6) | 32 (94.1) |
| Mean (SD) (mg/L) | 17.67 (20.73) | - | 11.38 (13.40) | 15.78 (21.47) | 9.47 (12.51) |
| Normal, n (%) | 14 (33.3) | - | 16 (38.1) | 13 (32.5) | 16 (50.0) |
| Abnormal ¹, n (%) | 28 (66.7) | - | 26 (61.9) | 27 (67.5) | 16 (50.0) |
| 95% CI (%) | [23.6%; 54.4%] | [18.6%; 49.1%] | 31.9%; 68.1%] | ||
| Fecal calprotectin, n (%) | 37 (84.1) | - | 33 (78.6) | 32 (78.0) | 29 (85.3) |
| Mean (SD) (mg/L) | 1,055.66 (1141.46) | - | 762.79 (768.08) | 997.46 (1465.16) | 805.25 (1195.55) |
| Normal, n (%) | 6 (16.2) | - | 8 (24.2) | 12 (37.5) | 12 (41.4) |
| Abnormal ², n (%) | 31 (83.8) | - | 25 (75.8) | 20 (62.5) | 17 (58.6) |
| 95% CI (%) | - | [11.1%; 42.3%] | [21.1%; 56.3%] | [23.5%; 61.1%] | |
| CDAI, n (%) | 44 (100.0) | 42 (95.5) | 38 (90.5) | 38 (92.7) | 32 (94.1) |
| Mean (SD) (points) | 287.67 (67.99) | 134.03 (78.01) | 137.32 (97.65) | 99.63 (90.22) | 75.26 (70.02) |
| Clinical response 3 | - | 31 (73.8) | 28 (73.7) | 35 (92.1) | 30 (93.8) |
| 95% CI (%) | - | [56.9%; 86.6%] | [78.6%; 98.3%] | [79.2%; 99.2%] | |
| HBI, n (%) | 41 (93.2) | 44 (100.0) | 38 (90.5) | 41 (100.0) | 33 (97.1) |
| Mean (SD) (points) | 11.54 (4.43) | 4.25 (3.21) | 4.87 (4.70) | 3.61 (3.79) | 2.18 (2.48) |
| Clinical response 4 | - | - | 26 (74.3) | 35 (92.1) | 31 (100.0) |
| 95% CI (%) | - | [56.7%; 87.5%] | [78.6%; 98.3%] | [88.8%; 100.0%] | |
| Clinical remission 5, n (%) | - | 44 (100.0) | 38 (90.5) | 41 (100.0) | 33 (97.1) |
| Mean (SD) (points) | - | 33 (75.0) | 29 (76.3) | 33 (80.5) | 29 (87.9) |
| 95% CI (%) | - | [59.8%; 88.6%] | [65.1%; 91.2%] | [71.8%; 96.6%] |
| Result (n = 44) | |
|---|---|
| Colonoscopy, n (%) | 30 (68.2%) |
| Suggestive of inadequate control of activity, n (%) | 10 (37.0%) |
| Endoscopic improvement, n (%) | 17 (63.0%) |
| Electrocardiogram, n (%) | 44 (100.0%) |
| Normal | 30 (68.2%) |
| Abnormal (clinically non-significant) | 13 (29.5%) |
| Abnormal (clinically significant) | 1 (2.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebli, J.M.F.; Parra, R.S.; Flores, C.; Moraes, A.C.; Nones, R.B.; Gomes, T.N.F.; Perdomo, A.M.B.; Scapini, G.; Zaltman, C. Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil. J. Clin. Med. 2022, 11, 6481. https://doi.org/10.3390/jcm11216481
Chebli JMF, Parra RS, Flores C, Moraes AC, Nones RB, Gomes TNF, Perdomo AMB, Scapini G, Zaltman C. Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil. Journal of Clinical Medicine. 2022; 11(21):6481. https://doi.org/10.3390/jcm11216481
Chicago/Turabian StyleChebli, Julio Maria Fonseca, Rogério Serafim Parra, Cristina Flores, Antonio Carlos Moraes, Rodrigo Bremer Nones, Tarcia Nogueira Ferreira Gomes, Ana Maria Bravo Perdomo, Gustavo Scapini, and Cyrla Zaltman. 2022. "Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil" Journal of Clinical Medicine 11, no. 21: 6481. https://doi.org/10.3390/jcm11216481
APA StyleChebli, J. M. F., Parra, R. S., Flores, C., Moraes, A. C., Nones, R. B., Gomes, T. N. F., Perdomo, A. M. B., Scapini, G., & Zaltman, C. (2022). Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil. Journal of Clinical Medicine, 11(21), 6481. https://doi.org/10.3390/jcm11216481






