Perinatal Outcome and Its Association with Blood Pressure Levels in Women with Preeclampsia
Abstract
1. Background
2. Methods
- moderate hypertension: systolic blood pressure < 160 mmHg and diastolic blood pressure < 109 mmHg
- severe hypertension: systolic blood pressure 160–179 mmHg and/or diastolic blood pressure 110–119 mmHg
- hypertensive crisis: systolic blood pressure ≥ 180 mmHg and/or diastolic blood pressure ≥ 120 mmHg
- extremely preterm: < 28 weeks of gestation at delivery
- very preterm: 28 to 32 weeks of gestation at delivery
- mild or moderate/late preterm: 32 to 37 weeks of gestation at delivery.
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2015, 387, 999–1011. [Google Scholar] [CrossRef]
- Deharde, D.; Klockenbusch, W.; Schmitz, R.; Brand, M.; Köster, H.A.; de Murcia, K.O. Hydroxychloroquine as a Preventive and Therapeutic Option in Preeclampsia—A Literature Review. Geburtshilfe Frauenheilkd. 2020, 80, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Platt, M. Outcomes in preterm infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef]
- Guida, J.P.D.S.; Surita, F.G.; Parpinelli, M.A.; Costa, M.L. Preterm Preeclampsia and Timing of Delivery: A Systematic Literature Review. Rev. Bras. Ginecol. Obs. 2017, 39, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, T.P.; Zwertbroek, E.F.; Broekhuijsen, K.; Koopmans, C.; Boers, K.; Owens, M.; Thornton, J.; van Pampus, M.; Scherjon, S.A.; Wallace, K.; et al. Delivery or expectant management for prevention of adverse maternal and neonatal outcomes in hypertensive disorders of pregnancy: An individual participant data meta-analysis. Ultrasound Obstet. Gynecol. 2019, 53, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; The WHO Global Survey on Maternal and Perinatal Health Research Group; Gülmezoglu, A.; Lumbiganon, P.; Laopaiboon, M.; Carroli, G.; Fawole, B.; Ruyan, P. Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: The 2004–2008 WHO Global Survey on Maternal and Perinatal Health. BMC Med. 2010, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S. Hypertensive disorders in pregnancy. Ann. Transl. Med. 2017, 5, 266. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A Critical Review of Early-Onset and Late-Onset Preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, A.; Sibai, B.M.; Caritis, S.; MacPherson, C.; Hauth, J.; Lindheimer, M.D.; Klebanoff, M.; VanDorsten, P.; Landon, M.; Paul, R.; et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am. J. Obstet. Gynecol. 2002, 186, 66–71. [Google Scholar] [CrossRef]
- Willy, D.; Willy, K.; Köster, H.-A.; Braun, J.; Möllers, M.; Sourouni, M.; Klockenbusch, W.; Schmitz, R.; Oelmeier, K. Blood Pressure Levels and Maternal Outcome in Women with Preeclampsia—A Retrospective Study from a Large Tertiary Obstetric Centre. Geburtshilfe Frauenheilkd. 2022, 82, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.; Steyn, W.; Zeeman, G.; Brown, M. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Anonymous] Hypertensive Pregnancy Disorders: Diagnosis and Therapy. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF-Registry No. 015/018, March 2019). Available online: https://www.awmf.org/uploads/tx_szleitlinien/015-018l_S2k_Diagnostik_Therapie_hypertensiver_Schwangerschaftserkrankungen_2019-07.pdf (accessed on 13 July 2022).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e484–e594. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. News: Preterm Birth. Available online: http://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 1 December 2018).
- Moutquin, J. Classification and heterogeneity of preterm birth. BJOG: Int. J. Obstet. Gynaecol. 2003, 110, 30–33. [Google Scholar] [CrossRef]
- Anonymous. Intrauterine Growth Restriction. Guideline of the German Society of Gynecology and Obstetrics (S2k, AWMF-Registry-No.:015/080, October 2016). Available online: http://www.awmf.org/leitlinien/detail/ll/015-080.html (accessed on 13 July 2022).
- Proussaloglou, E.; Mueller, A.; Minhas, R.; Rana, S. Severe antepartum hypertension and associated peripartum morbidity among pregnant women in an urban tertiary care medical center. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2019, 19, 31–36. [Google Scholar] [CrossRef]
- O’Brien, L.; Duong, J.; Winterton, T.; Haring, A.; Kuhlmann, Z. Management of Hypertension on the Labor and Delivery Unit: Delivering Care in the Era of Protocols and Algorithms. Perm. J. 2018, 22, 17–170. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.Z.; Cherkos, E.A.; Badi, M.B.; Anteneh, K.T.; Demssie, F.W.; Abdo, A.A.; Mihret, M.S. Factors and outcomes associated with the induction of labor in referral hospitals of Amhara regional state, Ethiopia: A multicenter study. BMC Pregnancy Childbirth 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.H.; Cheng, Y.W.; Delaney, S.; Jelin, A.C.; Caughey, A.B. Is preeclampsia associated with an increased risk of cesarean delivery if labor is induced? J. Matern. Neonatal Med. 2010, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Durst, J.K.; Subramaniam, A.; Tang, Y.; Szychowski, J.; Campbell, S.B.; Biggio, J.; Harper, L.M. Mode of delivery in nulliparous women with gestational hypertension undergoing early term induction of labor. J. Matern. Neonatal Med. 2016, 30, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Dahlen, H.G.; Hennessy, A. Does induction of labour in nulliparous hypertensive women result in vaginal birth?—A descriptive study utilising birth registry data. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2018, 12, 16–22. [Google Scholar] [CrossRef]
- Alanis, M.C.; Robinson, C.J.; Hulsey, T.C.; Ebeling, M.; Johnson, D.D. Early-onset severe preeclampsia: Induction of labor vs elective cesarean delivery and neonatal outcomes. Am. J. Obstet. Gynecol. 2008, 199, 262.e1–262.e6. [Google Scholar] [CrossRef] [PubMed]
- Coviello, E.M.; Iqbal, S.N.; Grantz, K.L.; Huang, C.-C.; Landy, H.J.; Reddy, U.M. Early preterm preeclampsia outcomes by intended mode of delivery. Am. J. Obstet. Gynecol. 2018, 220, 100.e1–100.e9. [Google Scholar] [CrossRef]
- van Eerden, L.; Gaugler-Senden, I.; de Vries, R.; Zeeman, G.G.; de Groot, C.J.M.; Bolte, A.C. Mode of Delivery in Severe Preeclampsia Before 28 Weeks’ Gestation: A Systematic Review. Obstet. Gynecol. Surv. 2018, 73, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Coppage, K.H.; Polzin, W.J. Severe preeclampsia and delivery outcomes: Is immediate cesarean delivery beneficial? Am. J. Obstet. Gynecol. 2002, 186, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, C.M.; Bijlenga, D.; Groen, H.; MC Vijgen, S.; Aarnoudse, J.G.; Bekedam, D.J.; Berg, P.P.V.D.; de Boer, K.; Burggraaff, J.M.; Bloemenkamp, K.W.; et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): A multicentre, open-label randomised controlled trial. Lancet 2009, 374, 979–988. [Google Scholar] [CrossRef]
- Pretscher, J.; Weiss, C.; Dammer, U.; Stumpfe, F.; Faschingbauer, F.; Beckmann, M.W.; Kehl, S. Influence of Preeclampsia on Induction of Labor at Term: A Cohort Study. In Vivo 2020, 34, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Colvin, Z.; Feng, M.; Pan, A.; Palatnik, A. Duration of labor induction in nulliparous women with hypertensive disorders of pregnancy and maternal and neonatal outcomes. J. Matern. Neonatal Med. 2020, 35, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
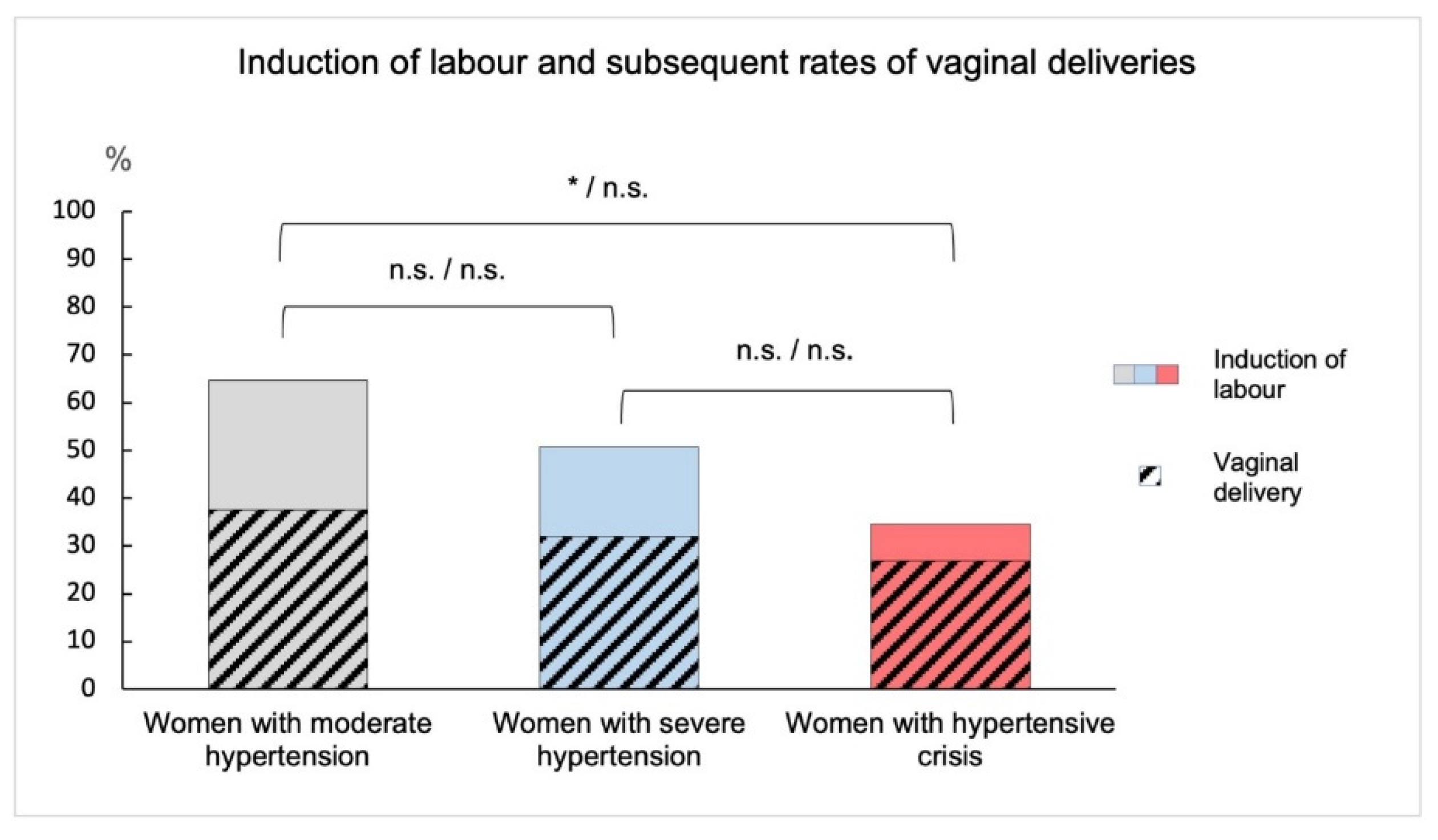
| Group 1: Moderate Hypertension (n = 48) | Group 2: Severe Hypertension (n = 69) | Group 3: Hypertensive Crisis (n = 41) | Significant Pairs among Groups | Significances | |
|---|---|---|---|---|---|
| mean age ± SD (years) | 31.9 ± 4.5 | 31.1 ± 5.3 | 32.8 ± 5.4 | none | 1 vs. 2: p = n.s. 1 vs. 3: p = n.s. 2 vs. 3: p = n.s. |
| BMI ± SD (kg/m2) | 31.1 ± 7.3 | 30.7 ± 7.4 | 32.4 ± 7.7 | none | 1 vs. 2: p = n.s. 1 vs. 3: p = n.s. 2 vs. 3: p = n.s. |
| nulliparous women | 36 (75.0%) | 49 (71.0%) | 24 (58.5%) | none | 1 vs. 2: OR = 1.06 p = n.s. 1 vs. 3: OR = 1.28 p = n.s. 2 vs. 3: OR = 1.21 p = n.s. |
| multiple pregnancies | 9 (18.6%) | 9 (13.0%) | 4 (9.8%) | none | 1 vs. 2: OR = 1.43 p = n.s. 1 vs. 3: OR = 1.90 p = n.s. 2 vs. 3: OR = 1.33 p = n.s. |
| pre-existing hypertension | 4 (8.3%) | 7 (10.1%) | 14 (34.1%) | 1 vs. 3, 2 vs. 3 | 1 vs. 2: OR = 0.82 p = n.s. 1 vs. 3: OR = 0.24 p = 0.01 2 vs. 3: OR = 0.30 p = 0.01 |
| Group 1: Moderate Hypertension (n = 48) | Group 2: Severe Hypertension (n = 69) | Group 3: Hypertensive Crisis (n = 41) | Significant Pairs among Groups | Significances | |
|---|---|---|---|---|---|
| gestational age at delivery (weeks) ± SD (days) | 36 1/7 ± 4 | 35 0/7 ± 4 | 33 3/7 ± 4 | 1 vs. 3, 2 vs. 3 | 1 vs. 2: p = n.s. 1 vs. 3: p = 0.001 2 vs. 3: p = 0.03 |
| birth weight ± SD (grams) | 2604 ± 1016 | 2279 ± 913 | 2015 ± 875 | 1 vs. 3 | 1 vs. 2: p = n.s. 1 vs. 3: p = 0.001 2 vs. 3: p = n.s. |
| number of FGR | 7 (14.6%) | 15 (21.8%) | 12 (29.3%) | none | 1 vs. 2: OR = 0.67 p = n.s. 1 vs. 3: OR = 0.50 p = n.s. 2 vs. 3: OR = 0.74 p = n.s. |
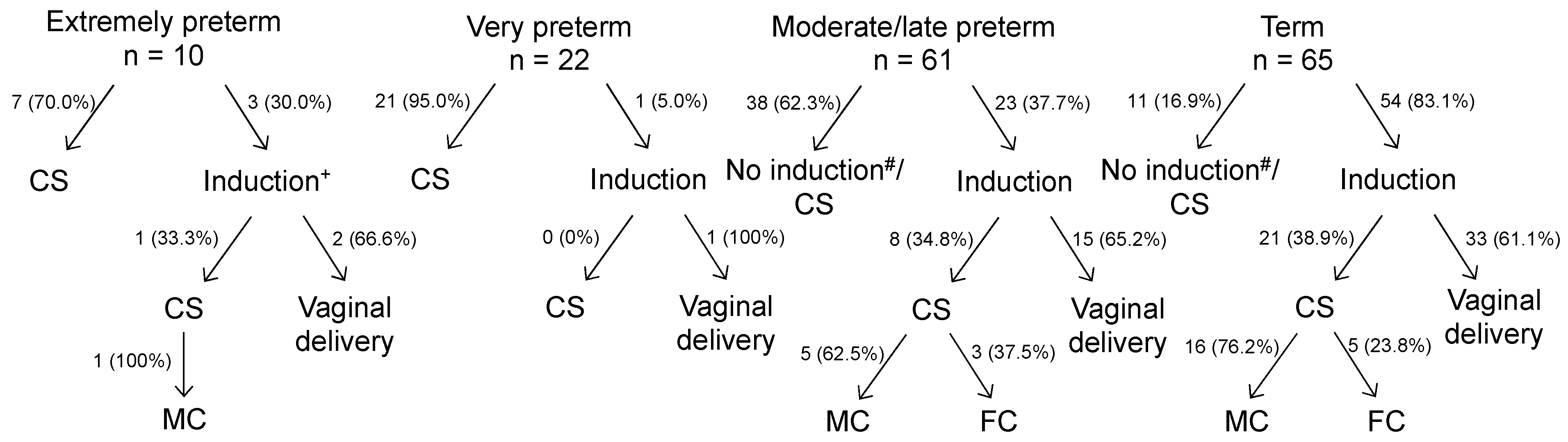
| Extremely Preterm (n = 10) | Very Preterm (n = 22) | Moderate/Late Preterm (n = 61) | Term (n = 65) | |
|---|---|---|---|---|
| group 1: moderate hypertension (n = 48) | 2 (4.2%) | 6 (12.5%) | 12 (25.0%) | 28 (58.3%) |
| group 2: severe hypertension (n = 69) | 2 (2.9%) | 11 (15.9%) | 28 (40.6%) | 28 (40.6%) |
| group 3: hypertensive crisis (n = 41) | 6 (14.6%) | 5 (12.2%) | 21 (51.2%) | 9 (22.0%) |
| Significant pairs among groups and significances | 1 vs. 3, 2 vs. 3 1 vs. 2: OR = 1.44 p = n.s. 1 vs. 3: OR 0.29 p = 0.05 2 vs. 3: OR = 0.20 p = 0.02 | None 1 vs. 2: OR = 0.79 p = n.s. 1 vs. 3: OR = 1.02 p = n.s. 2 vs. 3: OR = 1.30 p = 0.29 | 1 vs. 2, 1 vs. 3 1 vs. 2: OR = 0.62 p = 0.04 1 vs. 3: OR = 0.49 p = 0.004 2 vs. 3: OR = 0.79 p = n.s. | All 1 vs. 2: OR = 1.44 p = 0.03 1 vs. 3: OR = 2.65 p = 0.001 2 vs. 3: OR = 1.85 p = 0.02 |
| Spontaneous Vaginal Delivery | Operative Vaginal Delivery | Primary Caesarean Section | Secondary Caesarean Section | |
|---|---|---|---|---|
| group 1: moderate hypertension (n = 48) | 13 (27.1%) | 7 (14.6%) | 12 (25.0%) | 16 (33.3%) |
| group 2: severe hypertension (n = 69) | 14 (20.3%) | 9 (13.0%) | 38 (55.1%) | 8 (11.6%) |
| group 3: hypertensive crisis (n = 41) | 10 (24.4%) | 1 (2.4%) | 25 (61.0%) | 5 (12.2%) |
| Significant pairs among groups and significances | None 1 vs. 2: OR = 1.33 p = n.s. 1 vs. 3: OR = 1.11 p = n.s. 2 vs. 3: OR = 0.83 p = n.s. | 1 vs. 3, 2 vs. 3 1 vs. 2: OR = 1.12 p = n.s. 1 vs. 3: OR = 6.08 p = 0.015 2 vs. 3: OR = 5.42 p = 0.012 | 1 vs. 2, 1 vs. 3 1 vs. 2: OR = 0.45 p = 0.005 1 vs. 3: OR = 0.41 p = 0.005 2 vs. 3: OR = 0.90 p = n.s. | 1 vs. 2, 1 vs. 3 1 vs. 2: OR = 2.87 p = 0.003 1 vs. 3: OR = 2.73 p = 0.007 2 vs. 3: OR = 0.95 p = 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willy, D.; Schmitz, R.; Klockenbusch, W.; Köster, H.A.; Willy, K.; Braun, J.; Möllers, M.; Oelmeier, K. Perinatal Outcome and Its Association with Blood Pressure Levels in Women with Preeclampsia. J. Clin. Med. 2022, 11, 6334. https://doi.org/10.3390/jcm11216334
Willy D, Schmitz R, Klockenbusch W, Köster HA, Willy K, Braun J, Möllers M, Oelmeier K. Perinatal Outcome and Its Association with Blood Pressure Levels in Women with Preeclampsia. Journal of Clinical Medicine. 2022; 11(21):6334. https://doi.org/10.3390/jcm11216334
Chicago/Turabian StyleWilly, Daniela, Ralf Schmitz, Walter Klockenbusch, Helen Ann Köster, Kevin Willy, Janina Braun, Mareike Möllers, and Kathrin Oelmeier. 2022. "Perinatal Outcome and Its Association with Blood Pressure Levels in Women with Preeclampsia" Journal of Clinical Medicine 11, no. 21: 6334. https://doi.org/10.3390/jcm11216334
APA StyleWilly, D., Schmitz, R., Klockenbusch, W., Köster, H. A., Willy, K., Braun, J., Möllers, M., & Oelmeier, K. (2022). Perinatal Outcome and Its Association with Blood Pressure Levels in Women with Preeclampsia. Journal of Clinical Medicine, 11(21), 6334. https://doi.org/10.3390/jcm11216334







