Proprioceptive Cervicogenic Dizziness: A Narrative Review of Pathogenesis, Diagnosis, and Treatment
Abstract
1. Introduction
2. Pathophysiology
2.1. Cervical Proprioceptors and Proprioception
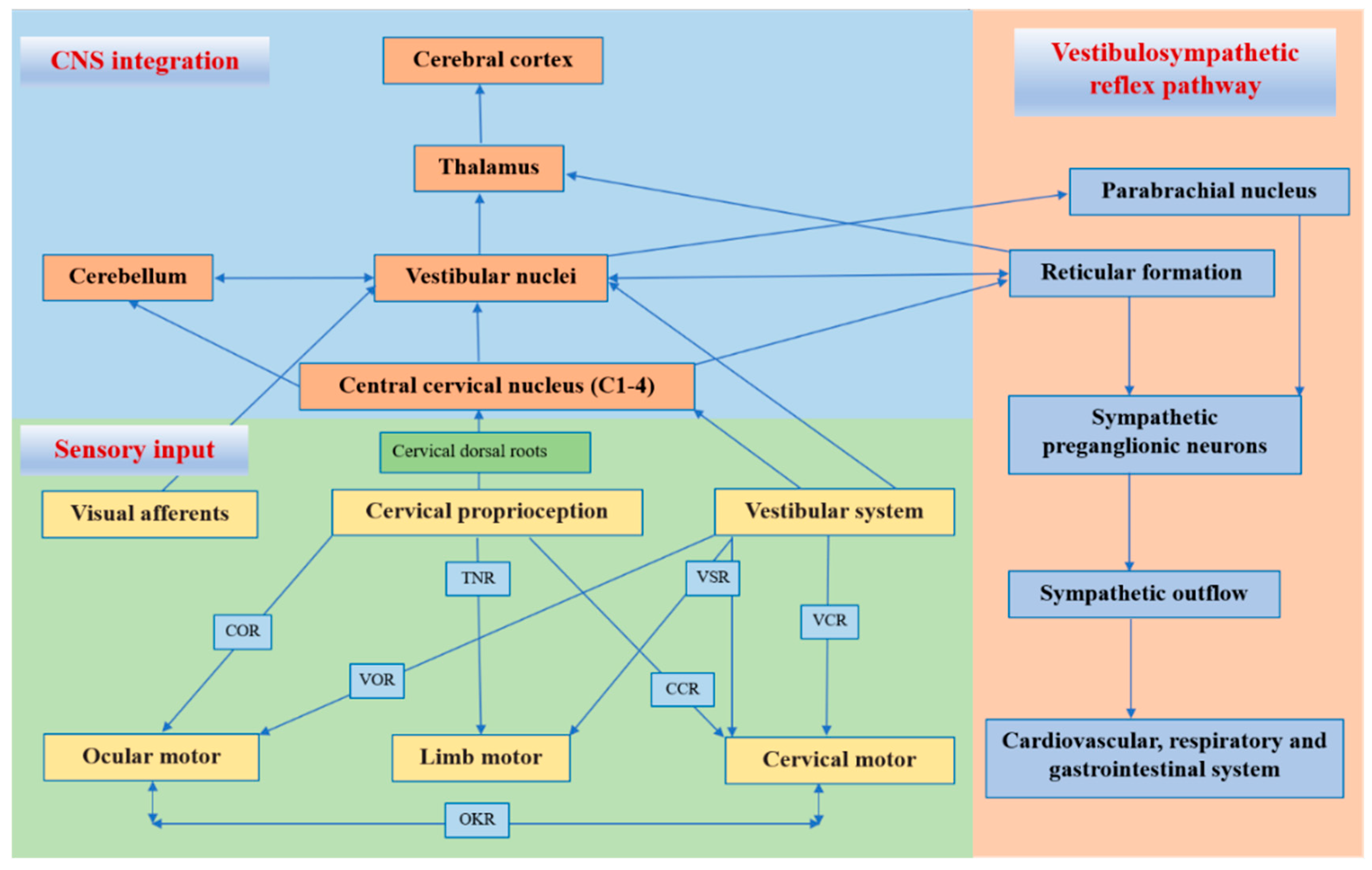
2.2. Central and Reflex Connection for Cervical Proprioceptive Signals
2.3. Altered Cervical Proprioceptive Afferent and Cervicogenic Dizziness
3. Diagnosis
3.1. Clinical Features
3.2. Diagnostic Tests
3.2.1. Cervical Joint Position Error Test
3.2.2. Seated Cervical Torsion Test
3.2.3. Smooth Pursuit Neck Torsion Test
3.2.4. Posturography
3.2.5. Vestibular Laboratory Tests
3.2.6. Diagnostic Blockade Test
3.3. Imaging Features
3.4. Diagnostic Criteria
- Diagnostic Criteria:
- A. Clinical, laboratory, and/or imaging evidence of a disorder or lesion within the cervical spine or soft tissues of the neck known to be able to cause dizziness.
- B. Temporal coincidence of the appearance or increase in both neck pain and dizziness.
- C. Evidence demonstrated by at least two of the following:
- 1. Dizziness has developed in temporal relation to the onset of the cervical disorder or appearance of the lesion.
- 2. Dizziness has significantly improved or been resolved in parallel with an improve-ment in or resolution of the cervical disorder or lesion.
- 3. at least two clinical diagnostic tests (cervical torsion test, cervical joint position error, or posturography) are positive.
- 4. Dizziness is abolished following a diagnostic blockade of a cervical structure or its nerve supply.
- D. Exclusion of other possible sources of dizziness, including the vestibular, visual, central nervous system, or psychosomatic pathologies.
- Notes:
- 1. Abnormal imaging findings of the cervical spine are common in people without dizziness; they are suggestive but do not have exact etiological evidence.
- 2. Tumors, fractures, infections, and rheumatoid arthritis of the cervical spine have not been formally validated as causes of dizziness but are accepted to fulfill criteria A in individual cases.
3.5. Differential Diagnosis
4. Treatment
4.1. Conservative Treatment
4.1.1. Pharmacological Treatment
4.1.2. Physical Therapy
4.1.3. Acupuncture and Pharmacopuncture
4.2. Surgical Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vural, M.; Karan, A.; Albayrak Gezer, İ.; Çalışkan, A.; Atar, S.; Yıldız Aydın, F.; Coşkun Benlidayı, İ.; Gökşen, A.; Koldaş Doğan, Ş.; Karacan, G.; et al. Prevalence, etiology, and biopsychosocial risk factors of cervicogenic dizziness in patients with neck pain: A multi-center, cross-sectional study. Turk. J. Phys. Med. Rehabil. 2021, 67, 399–408. [Google Scholar] [CrossRef]
- Barin, K.; Dodson, E.E. Dizziness in the elderly. Otolaryngol. Clin. North Am. 2011, 44, 437–454. [Google Scholar] [CrossRef]
- Bisdorff, A.; Von Brevern, M.; Lempert, T.; Newman-Toker, D.E. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J. Vestib. Res. 2009, 19, 1–13. [Google Scholar] [CrossRef]
- Ryan, G.M.; Cope, S. Cervical vertigo. Lancet 1955, 269, 1355–1358. [Google Scholar] [CrossRef]
- Takahashi, S. Importance of cervicogenic general dizziness. J. Rural. Med. 2018, 13, 48–56. [Google Scholar] [CrossRef]
- Li, Y.; Peng, B. Pathogenesis, diagnosis, and treatment of cervical vertigo. Pain Physician 2015, 18, E583–E595. [Google Scholar] [PubMed]
- Yacovino, D.A.; Hain, T.C. Clinical characteristics of cervicogenic-related dizziness and vertigo. Semin Neurol. 2013, 33, 244–255. [Google Scholar] [PubMed]
- Wrisley, D.M.; Sparto, P.J.; Whitney, S.L.; Furman, J.M. Cervicogenic dizziness: A review of diagnosis and treatment. J. Orthop. Sports Phys. Ther. 2000, 30, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Bronstein, A.M. Cervical vertigo. J. Neurol. Neurosurg. Psychiatry 2001, 71, 8–12. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar]
- Hillier, S.; Immink, M.; Thewlis, D. Assessing proprioception: A systematic review of possibilities. Neurorehabil. Neural. Repair 2015, 29, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, J.C.; Azim, E. Proprioception. Curr. Biol. 2018, 28, R194–R203. [Google Scholar] [CrossRef]
- Jerosch, J.; Prymka, M. Proprioception and joint stability. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kröger, S. Proprioception 2.0: Novel functions for muscle spindles. Curr. Opin. Neurol. 2018, 31, 592–598. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Kulkarni, V.; Chandy, M.J.; Babu, K.S. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol. India 2001, 49, 355–359. [Google Scholar]
- Boyd-Clark, L.C.; Briggs, C.A.; Galea, M.P. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine 2002, 27, 694–701. [Google Scholar] [CrossRef]
- Banks, R.W. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J. Anat. 2006, 208, 753–768. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The kinaesthetic senses. J. Physiol. 2009, 587, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, P.; Johansson, H.; Djupsjöbacka, M. Spinal and supraspinal effects of activity in ligament afferents. J. Electromyogr. Kinesiol. 2002, 12, 167–176. [Google Scholar] [CrossRef]
- Richmond, F.J.; Abrahams, V.C. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J. Neurophysiol. 1979, 42, 604–617. [Google Scholar] [CrossRef]
- Thunberg, J.; Hellström, F.; Sjölander, P.; Bergenheim, M.; Wenngren, B.; Johansson, H. Influences on the fusimotor-muscle spindle system from chemosensitive nerve endings in cervical facet joints in the cat: Possible implications for whiplash induced disorders. Pain 2001, 91, 15–22. [Google Scholar] [CrossRef]
- Freeman, M.A.; Wyke, B. The innervation of the knee joint. An anatomical and histological study in the cat. J. Anat. 1967, 101, 505–532. [Google Scholar] [PubMed]
- McLain, R.F. Mechanoreceptor endings in human cervical facet joints. Spine 1994, 19, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.M.; Lu, Y.; Chen, C.; Kallakuri, S. Pain generation in lumbar and cervical facet joints. J. Bone Joint Surg. Am. 2006, 88, 63–67. [Google Scholar] [PubMed]
- Yang, L.; Yang, C.; Pang, X.; Li, D.; Yang, H.; Zhang, X.; Yang, Y.; Peng, B. Mechanoreceptors in diseased cervical intervertebral disc and vertigo. Spine 2017, 42, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Yang, C.; Pang, X.; Li, D.; Wu, B.; Wu, Y.; Lu, X.; Xu, J.; Chen, X.; et al. Cervical intervertebral disc degeneration contributes to dizziness: A clinical and immunohistochemical study. World Neurosurg. 2018, 119, e686–e693. [Google Scholar] [CrossRef]
- Peng, B. Cervical vertigo: Historical reviews and advances. World Neurosurg. 2018, 109, 347–350. [Google Scholar] [CrossRef]
- Gdowski, G.T.; McCrea, R.A. Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp. Brain Res. 2000, 135, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.K. Cervical outcome measures: Testing for postural stability and balance. J. Manipulative Physiol. Ther. 2008, 31, 540–546. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Cullen, K.E. Vestibular system: The many facets of a multimodal sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.M.; Young, J.H.; Milne, A.C.; Schwarz, D.W.; Fredrickson, J.M. Vestibular-neck integration in the vestibular nuclei. Brain Res. 1975, 96, 99–102. [Google Scholar] [CrossRef]
- Mergner, T.; Hlavacka, F.; Schweigart, G. Interaction of vestibular and proprioceptive inputs. J. Vestib. Res. 1993, 3, 41–57. [Google Scholar]
- Armstrong, B.; McNair, P.; Taylor, D. Head and neck position sense. Sports Med. 2008, 38, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Gao, X.; Yaginuma, H. Spinovestibular projections in the rat, with particular reference to projections from the central cervical nucleus to the lateral vestibular nucleus. J. Comp. Neurol. 1995, 361, 334. [Google Scholar] [CrossRef] [PubMed]
- Dutia, M.B. The muscles and joints of the neck: Their specialisation and role in head movement. Prog. Neurobiol. 1991, 37, 165–178. [Google Scholar] [CrossRef]
- Hongo, T.; Kitama, T.; Yoshida, K. Integration of vestibular and neck afferent signals in the central cervical nucleus. Prog. Brain Res. 1988, 76, 155–162. [Google Scholar] [PubMed]
- Pompeiano, O. Spinovestibular relations: Anatomical and physiological aspects. Prog. Brain Res. 1972, 37, 263–296. [Google Scholar] [PubMed]
- McCall, A.A.; Miller, D.M.; Yates, B.J. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front. Neurol. 2017, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D. Vestibular nucleus projections to the parabrachial nucleus in rabbits: Implications for vestibular influences on the autonomic nervous system. Exp. Brain Res. 1996, 108, 367–381. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.T.; de Jong, J.M.; Cohen, B.; Jongkees, L.B. Ataxia and nystagmus induced by injection of local anesthetics in the Neck. Ann. Neurol. 1977, 1, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Biemond, A.; De Jong, J.M. On cervical nystagmus and related disorders. Brain 1969, 92, 437–458. [Google Scholar] [CrossRef]
- Ishikawa, K.; Matsuzaki, Z.; Yokomizo, M.; Terada, N.; Miyazaki, S.; Togawa, K. Effect of unilateral section of cervical afferent nerve upon optokinetic response and vestibular nystagmus induced by sinusoidal rotation in guinea pigs. Acta Otolaryngol. Suppl. 1998, 537, 6–10. [Google Scholar] [PubMed]
- Lennerstrand, G.; Han, Y.; Velay, J.L. Properties of eye movements induced by activation of neck muscle proprioceptors. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; McCloskey, D.I. Illusions of head and visual target displacement induced by vibration of neck muscles. Brain 1991, 114, 755–759. [Google Scholar] [CrossRef]
- Kavounoudias, A.; Gilhodes, J.C.; Roll, R.; Roll, J.P. From balance regulation to body orientation: Two goals for muscle proprioceptive information processing? Exp. Brain Res. 1999, 124, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M.; Golding, J.F.; Gresty, M.A. Visual vertigo, motion sickness, and disorientation in vehicles. Semin. Neurol. 2020, 40, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Malmström, E.M.; Karlberg, M.; Holmström, E.; Fransson, P.A.; Hansson, G.A.; Magnusson, M. Influence of prolonged unilateral cervical muscle contraction on head repositioning--decreased overshoot after a 5-min static muscle contraction task. Man. Ther. 2010, 15, 229–234. [Google Scholar] [CrossRef]
- Johansson, H.; Sojka, P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: A hypothesis. Med. Hypotheses 1991, 35, 196–203. [Google Scholar] [CrossRef]
- Hubbard, D.R.; Berkoff, G.M. Myofascial trigger points show spontaneous needle EMG activity. Spine 1993, 18, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.P.; Donga, R.; Widmer, C.G.; Stohler, C.S. The pain-adaptation model: A discussion of the relationship between chronic musculoskeletal pain and motor activity. Can. J. Physiol. Pharmacol. 1991, 69, 683–694. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, R.; Coppieters, I.; Kregel, J.; De Meulemeester, K.; Danneels, L.; Cagnie, B. Does muscle morphology change in chronic neck pain patients?—A systematic review. Man. Ther. 2016, 22, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Tedla, J.S.; Dixit, S.; Abohashrh, M. Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet. Disord. 2019, 20, 447. [Google Scholar] [CrossRef] [PubMed]
- Malmström, E.M.; Westergren, H.; Fransson, P.A.; Karlberg, M.; Magnusson, M. Experimentally induced deep cervical muscle pain distorts head on trunk orientation. Eur. J. Appl. Physiol. 2013, 113, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Fiaschi, A.; Rosso, T.; Faccioli, F.; Grosslercher, J.; Aglioti, S.M. Neuroplastic changes related to pain occur at multiple levels of the human somatosensory system: A somatosensory-evoked potentials study in patients with cervical radicular pain. J. Neurosci. 2000, 20, 9277–9283. [Google Scholar] [CrossRef]
- Elliott, J.; Jull, G.; Noteboom, J.T.; Darnell, R.; Galloway, G.; Gibbon, W.W. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: A magnetic resonance imaging analysis. Spine 2006, 31, E847–E855. [Google Scholar] [CrossRef] [PubMed]
- Passatore, M.; Roatta, S. Influence of sympathetic nervous system on sensorimotor function: Whiplash associated disorders (WAD) as a model. Eur. J. Appl. Physiol. 2006, 98, 423–449. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hooten, W.M. Advances in the diagnosis and management of neck pain. BMJ 2017, 358, j3221. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.; Peng, B. Ingrowth of nociceptive receptors into diseased cervical intervertebral disc is associated with discogenic neck pain. Pain Med. 2019, 20, 1072–1077. [Google Scholar] [CrossRef]
- Indahl, A.; Kaigle, A.; Reikerås, O.; Holm, S. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine 1995, 20, 2652–2658. [Google Scholar] [CrossRef]
- Persson, L.; Karlberg, M.; Magnusson, M. Effects of different treatments on postural performance in patients with cervical root compression. A randomized prospective study assessing the importance of the neck in postural control. J. Vestib. Res. 1996, 6, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Muratsu, H.; Doita, M.; Harada, T.; Yoshiya, S.; Kurosaka, M. Impaired joint proprioception in patients with cervical myelopathy. Spine 2005, 30, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.Y.; Xu, H.W.; Zhang, S.B.; Hu, T.; Wang, S.J.; Wu, D.S. Does the C3/4 disc play a role in cervical spondylosis with dizziness? A retrospective study. Int. Orthop. 2020, 44, 1159–1168. [Google Scholar] [CrossRef]
- Li, C.; Qi, Y.; Liu, G.; Yin, X.; Jin, Y.; Jiang, Z.; Li, P.; Kang, X.; Ye, C. Long-term clinical outcomes of percutaneous cervical nucleoplasty for cervical degenerative diseases with neck pain and cervical vertigo. World Neurosurg. 2020, 133, e205–e210. [Google Scholar] [CrossRef]
- Peng, B.; Yang, L.; Yang, C.; Pang, X.; Chen, X.; Wu, Y. The effectiveness of anterior cervical decompression and fusion for the relief of dizziness in patients with cervical spondylosis: A multicentre prospective cohort study. Bone Joint J. 2018, 100, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, D.J.; Wang, X.W.; Yuan, W.; Liang, L.; Wang, Z.C. Mid-term outcomes of anterior cervical fusion for cervical spondylosis with sympathetic symptoms. Clin. Spine Surg. 2016, 29, 255–260. [Google Scholar] [CrossRef]
- Blecher, R.; Krief, S.; Galili, T.; Biton, I.E.; Stern, T.; Assaraf, E.; Levanon, D.; Appel, E.; Anekstein, Y.; Agar, G.; et al. The proprioceptive system masterminds spinal alignment: Insight into the mechanism of scoliosis. Dev. Cell. 2017, 42, 388–399.e3. [Google Scholar] [CrossRef]
- Radovanovic, D.; Peikert, K.; Lindström, M.; Domellöf, F.P. Sympathetic innervation of human muscle spindles. J. Anat. 2015, 226, 542–548. [Google Scholar] [CrossRef]
- Reiley, A.S.; Vickory, F.M.; Funderburg, S.E.; Cesario, R.A.; Clendaniel, R.A. How to diagnose cervicogenic dizziness. Arch. Physiother. 2017, 7, 12. [Google Scholar] [CrossRef]
- Treleaven, J. Dizziness, unsteadiness, visual disturbances, and sensorimotor control in traumatic neck pain. J. Orthop. Sports Phys. Ther. 2017, 47, 492–502. [Google Scholar] [CrossRef]
- Li, S.; Chen, R.; Chen, Y.; Mo, G.; Zhang, L.; Xie, P.; Wang, Q.; Liu, B.; Dong, J.; Rong, L. Therapeutic effects and safety of percutaneous disc decompression with coblation nucleoplasty in cervical vertigo: A retrospective outcome study with 74 consecutive patients and minimum 1-year follow-up. Pain Physician 2019, 22, E205–E214. [Google Scholar] [PubMed]
- Malmström, E.M.; Karlberg, M.; Melander, A.; Magnusson, M.; Moritz, U. Cervicogenic dizziness—Musculoskeletal findings before and after treatment and long-term outcome. Disabil. Rehabil. 2007, 29, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux-Lebeau, B.; Godbout, A.; Berbiche, D.; Saliba, I. Evaluation of paraclinical tests in the diagnosis of cervicogenic dizziness. Otol. Neurotol. 2014, 35, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.K.; Peterson, C. Comparison of outcomes in neck pain patients with and without dizziness undergoing chiropractic treatment: A prospective cohort study with 6 month follow-up. Chiropr. Man. Therap. 2013, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Ischebeck, B.K.; Voogt, L.P.; van der Geest, J.N.; Janssen, M.; Frens, M.A.; Kleinrensink, G.J. Joint position sense error in people with neck pain: A systematic review. Man. Ther. 2015, 20, 736–744. [Google Scholar] [CrossRef]
- Alund, M.; Larsson, S.E.; Ledin, T.; Odkvist, L.; Möller, C. Dynamic posturography in cervical vertigo. Acta Otolaryngol. Suppl. 1991, 481, 601–602. [Google Scholar] [CrossRef]
- Alahmari, K.A.; Reddy, R.S.; Silvian, P.S.; Ahmad, I.; Kakaraparthi, V.N.; Alam, M.M. Association of age on cervical joint position error. J. Adv. Res. 2017, 8, 201–207. [Google Scholar] [CrossRef]
- Hain, T.C. Cervicogenic causes of vertigo. Curr. Opin. Neurol. 2015, 28, 69–73. [Google Scholar] [CrossRef]
- Tjell, C.; Rosenhall, U. Smooth pursuit neck torsion test: A specific test for cervical dizziness. Am. J. Otol. 1998, 19, 76–81. [Google Scholar]
- Karlberg, M.; Johansson, R.; Magnusson, M.; Fransson, P.A. Dizziness of suspected cervical origin distinguished by posturographic assessment of human postural dynamics. J. Vestib. Res. 1996, 6, 37–47. [Google Scholar] [CrossRef]
- Endo, K.; Suzuki, H.; Yamamoto, K. Consciously postural sway and cervical vertigo after whiplash injury. Spine 2008, 33, E539–E542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Grover, M.J. Cervicogenic dizziness successfully treated with upper cervical medial branch nerve radiofrequency ablation: A case report. A A Pract. 2018, 10, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Knapstad, M.K.; Nordahl, S.; Goplen, F.K. Clinical characteristics in patients with cervicogenic dizziness: A systematic review. Health Sci. Rep. 2019, 2, e134. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Sanchez, T.G.; Rocha, C.B. Diagnosis and management of somatosensory tinnitus: Review article. Clinics 2011, 66, 1089–1094. [Google Scholar] [CrossRef]
- Oh, H.; Shin, S.; Lee, E.; Chung, W.S. Chinese herbal medicine for cervicogenic dizziness: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2022, 2022, 2425851. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Diab, A.A.; Harrison, D.E. The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: A 1-year randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 57–71. [Google Scholar] [CrossRef]
- Bittar, R.; Alves, N.G.; Bertoldo, C.; Brugnera, C.; Oiticica, J. Efficacy of carbon microcoils in relieving cervicogenic dizziness. Int. Arch. Otorhinolaryngol. 2017, 21, 4–7. [Google Scholar] [CrossRef]
- Minguez-Zuazo, A.; Grande-Alonso, M.; Saiz, B.M.; La Touche, R.; Lara, S.L. Therapeutic patient education and exercise therapy in patients with cervicogenic dizziness: A prospective case series clinical study. J. Exerc. Rehabil. 2016, 12, 216–225. [Google Scholar] [CrossRef]
- Reid, S.A.; Rivett, D.A. Manual therapy treatment of cervicogenic dizziness: A systematic review. Man. Ther. 2005, 10, 4–13. [Google Scholar] [CrossRef]
- Karlberg, M.; Magnusson, M.; Malmström, E.M.; Melander, A.; Moritz, U. Postural and symptomatic improvement after physiotherapy in patients with dizziness of suspected cervical origin. Arch. Phys. Med. Rehabil. 1996, 77, 874–882. [Google Scholar] [CrossRef]
- Reid, S.A.; Rivett, D.A.; Katekar, M.G.; Callister, R. Sustained natural apophyseal glides (SNAGs) are an effective treatment for cervicogenic dizziness. Man. Ther. 2008, 13, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.A.; Rivett, D.A.; Katekar, M.G.; Callister, R. Comparison of mulligan sustained natural apophyseal glides and maitland mobilizations for treatment of cervicogenic dizziness: A randomized controlled trial. Phys. Ther. 2014, 94, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.A.; Callister, R.; Katekar, M.G.; Rivett, D.A. Effects of cervical spine manual therapy on range of motion, head repositioning, and balance in participants with cervicogenic dizziness: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2014, 95, 1603–1612. [Google Scholar] [CrossRef]
- Reid, S.A.; Callister, R.; Snodgrass, S.J.; Katekar, M.G.; Rivett, D.A. Manual therapy for cervicogenic dizziness: Long-term outcomes of a randomised trial. Man. Ther. 2015, 20, 148–156. [Google Scholar] [CrossRef]
- Yao, M.; Tang, Z.Y.; Cui, X.J.; Sun, Y.L.; Ye, X.L.; Wang, P.; Zhong, W.H.; Zhang, R.C.; Li, H.Y.; Hu, Z.J.; et al. Shi-style cervical mobilizations versus massage for cervical vertigo: A multicenter, randomized, controlled clinical trial. J. Altern. Complement. Med. 2020, 26, 58–66. [Google Scholar] [CrossRef]
- Carrasco-Uribarren, A.; Pardos-Aguilella, P.; Pérez-Guillén, S.; López-de-Celis, C.; Rodríguez-Sanz, J.; Cabanillas-Barea, S. Combination of two manipulative techniques for the treatment of cervicogenic dizziness: A randomized controlled trial. Life 2022, 12, 1023. [Google Scholar] [CrossRef]
- Carrasco-Uribarren, A.; Rodríguez-Sanz, J.; López-de-Celis, C.; Fanlo-Mazas, P.; Cabanillas-Barea, S. An upper cervical spine treatment protocol for cervicogenic dizziness: A randomized controlled trial. Physiother. Theory Pract. 2021; 1–10, ahead of print. [Google Scholar]
- Carrasco-Uribarren, A.; Rodriguez-Sanz, J.; López-de-Celis, C.; Pérez-Guillen, S.; Tricás-Moreno, J.M.; Cabanillas-Barea, S. Short-term effects of the traction-manipulation protocol in dizziness intensity and disability in cervicogenic dizziness: A randomized controlled trial. Disabil. Rehabil. 2022, 44, 3601–3609. [Google Scholar] [CrossRef]
- Micarelli, A.; Viziano, A.; Granito, I.; Carlino, P.; Micarelli, R.X.; Augimeri, I.; Alessandrini, M. Postural and clinical outcomes of sustained natural apophyseal glides treatment in cervicogenic dizziness patients: A randomised controlled trial. Clin. Rehabil. 2021, 35, 1566–1576. [Google Scholar] [CrossRef]
- Lystad, R.P.; Bell, G.; Bonnevie-Svendsen, M.; Carter, C.V. Manual therapy with and without vestibular rehabilitation for cervicogenic dizziness: A systematic review. Chiropr. Man. Therap. 2011, 19, 21. [Google Scholar] [CrossRef]
- De Vestel, C.; Vereeck, L.; Reid, S.A.; Van Rompaey, V.; Lemmens, J.; De Hertogh, W. Systematic review and meta-analysis of the therapeutic management of patients with cervicogenic dizziness. J. Man. Manip. Ther. 2022, 30, 273–283. [Google Scholar] [CrossRef]
- Hou, Z.; Xu, S.; Li, Q.; Cai, L.; Wu, W.; Yu, H.; Chen, H. The Efficacy of acupuncture for the treatment of cervical vertigo: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2017, 2017, 7597363. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, S.H. Pharmacopuncture for cervicogenic dizziness. J. Pharmacopunct. 2018, 21, 241–248. [Google Scholar] [CrossRef]
- Liu, T.H.; Liu, Y.Q.; Peng, B.G. Cervical intervertebral disc degeneration and dizziness. World J. Clin. Cases. 2021, 9, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Guo, B.; Zhang, J.; Han, Z.; Zhang, T.; Bai, Q.; Zeng, Y. Mid-term efficacy of percutaneous laser disc decompression for treatment of cervical vertigo. Eur. J. Orthop. Surg. Traumatol. 2014, 24, S153–S158. [Google Scholar] [CrossRef]
- Yin, H.D.; Zhang, X.M.; Huang, M.G.; Chen, W.; Song, Y.; Du, Q.J.; Wu, Y.N.; Yang, R.B. Curative effect and mechanism of radiofrequency ablation nucleoplasty in the treatment of cervical vertigo. Br. J. Radiol. 2017, 90, 20150772. [Google Scholar] [CrossRef]
- He, L.L.; Lai, R.J.; Leff, J.; Yuan, R.; Yue, J.N.; Ni, J.X.; Yang, L.Q. Cervicogenic dizziness alleviation after coblation discoplasty: A retrospective study. Ann. Med. 2021, 53, 639–646. [Google Scholar] [CrossRef]
| Category | Description |
|---|---|
| Vertigo | A sense of spinning experienced even when someone is perfectly still. |
| Disequilibrium | A loss or lack of equilibrium or stability. |
| Presyncope | Feeling of losing consciousness or blacking out. |
| Lightheadedness | Feeling a little woozy or faint. |
| Classification | Description |
|---|---|
| Dizziness | The sensation of disturbed or impaired spatial orientation without a hallucinatory or distorted sense of motion. |
| Vertigo | The sensation of self-motion when no self-motion is occurring or the sensation of distorted self-motion during an otherwise normal head movement. |
| Test | Mechanism | Purpose | Measurement (Unit) | Method | Positive Test |
|---|---|---|---|---|---|
| Cervical joint position error | Improper cervicocollic reflex inhibition | Measure joint position sense | Neutral head position or target error (degree or centimeter) | Participants sit in chairs and face the target on a wall 90 cm away. They are blindfolded there and a specific laser pointer is placed on top of their heads. Participants are asked to move their head away from the target when the laser pointer is right in the center of the target. After returning to the center, the error is evaluated between the starting position and the final position. | The joint position error in one position is above 4.5 degrees |
| Seated cervical torsion test | Impaired cervicoocular reflex | Measure cervicoocular reflex | Ratio of eye to target motion in neutral and torsion positions | The participants are seated on a stool or chair and eye movements are recorded when ocular fixation is inhibited. Participants turn trunk 90 degrees to right, keeping their heads still. Then, return to the center, turn the trunk 90 degrees to the left, and return to the center. Hold each position for 30 s, and the observer stabilizes the head in all positions. | Nystagmus > 2 degrees persecond at any of the four positions |
| Smooth pursuit neck torsion test | Impaired cervicoocular reflex | Measure cervicoocular reflex | Ratio of eye to target motion in neutral and torsion positions | Participants focus their eyes on a moving target and keep their head still, in a neutral position, and rotate their torso relative to the head (torsion). Record the speed of eye movement while tracking the target in the vertical or horizontal plane. The average gain is calculated as a parameter defining smooth pursuits. The difference between the average gain of the neutral position of the head and left and right torsion of the head on the body is calculated to determine the difference. | Nystagmus > 2 degrees persecond in left or right neck torsion (excluding a spontaneous nystagmus) |
| Posturography | Quantitative test of the vestibulospinal reflex. | Measure postural control stability in upright stance in either static or dynamic conditions | Sway area (cm2) or total sway path (mm) | Records were taken while standing on a power platform with both eyes open and closed. | Disturbed postural control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, L.; Dai, C.; Peng, B. Proprioceptive Cervicogenic Dizziness: A Narrative Review of Pathogenesis, Diagnosis, and Treatment. J. Clin. Med. 2022, 11, 6293. https://doi.org/10.3390/jcm11216293
Li Y, Yang L, Dai C, Peng B. Proprioceptive Cervicogenic Dizziness: A Narrative Review of Pathogenesis, Diagnosis, and Treatment. Journal of Clinical Medicine. 2022; 11(21):6293. https://doi.org/10.3390/jcm11216293
Chicago/Turabian StyleLi, Yongchao, Liang Yang, Chen Dai, and Baogan Peng. 2022. "Proprioceptive Cervicogenic Dizziness: A Narrative Review of Pathogenesis, Diagnosis, and Treatment" Journal of Clinical Medicine 11, no. 21: 6293. https://doi.org/10.3390/jcm11216293
APA StyleLi, Y., Yang, L., Dai, C., & Peng, B. (2022). Proprioceptive Cervicogenic Dizziness: A Narrative Review of Pathogenesis, Diagnosis, and Treatment. Journal of Clinical Medicine, 11(21), 6293. https://doi.org/10.3390/jcm11216293






