Primary Aldosteronism Masked by Accessory Renal Arteries: A Case Report
Abstract
1. Introduction
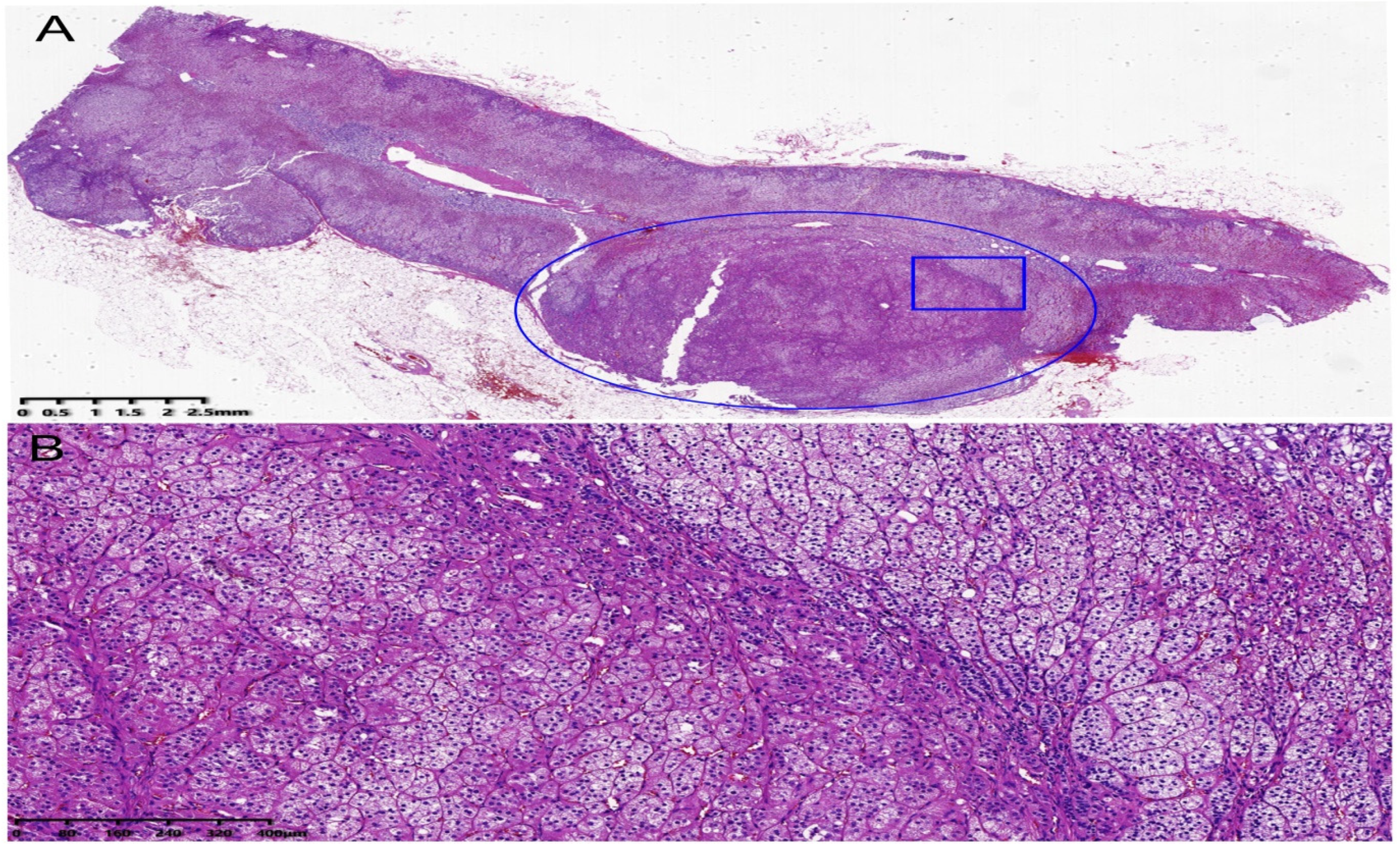
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, J.; Hu, J.; Song, Y.; He, W.; Luo, T.; Cheng, Q.; Ma, L.; Luo, R.; Fuller, P.J.; et al. Primary Aldosteronism in Patients in China With Recently Detected Hypertension. J. Am. Coll. Cardiol. 2020, 75, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Stowasser, M.; Sharman, J.; Leano, R.; Gordon, R.D.; Ward, G.; Cowley, D.; Marwick, T.H. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J. Clin. Endocrinol. Metab. 2005, 90, 5070–5076. [Google Scholar] [CrossRef]
- Unwin, R.J.; Luft, F.C.; Shirley, D.G. Pathophysiology and management of hypokalemia: A clinical perspective. Nat. Rev. Nephrol. 2011, 7, 75–84. [Google Scholar] [CrossRef]
- Jędrusik, P.; Symonides, B.; Lewandowski, J.; Gaciong, Z. The Effect of Antihypertensive Medications on Testing for Primary Aldosteronism. Front. Pharmacol. 2021, 12, 684111. [Google Scholar] [CrossRef]
- Galati, S.J. Primary aldosteronism: Challenges in diagnosis and management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 355–369. [Google Scholar] [CrossRef]
- Kuo, C.C.; Hsu, H.L.; Huang, C.Y.; Liu, K.L.; Wu, V.C.; Tsai, C.W.; Wang, W.J.; Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group). A patient with concurrent primary aldosteronism and Page kidney. Endocrine 2010, 38, 6–10. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, J.; Zhou, Y.; Dong, X.; Luo, F.; Jiang, X.; Du, X.; Zhou, X.; Meng, X. Concurrent Primary Aldosteronism and Renal Artery Stenosis: An Overlooked Condition Inducing Resistant Hypertension. Front. Cardiovasc. Med. 2022, 9, 818872. [Google Scholar] [CrossRef]
- Oelkers, W.; Diederich, S.; Bähr, V. Primary hyperaldosteronism without suppressed renin due to secondary hypertensive kidney damage. J. Clin. Endocrinol. Metab. 2000, 85, 3266–3270. [Google Scholar] [CrossRef]
- Rossi, G.P.; Auchus, R.J.; Brown, M.; Lenders, J.W.; Naruse, M.; Plouin, P.F.; Satoh, F.; Young, W.F., Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014, 63, 151–160. [Google Scholar] [CrossRef]
- van den Born, B.J.; Koopmans, R.P.; van Montfrans, G.A. The renin-angiotensin system in malignant hypertension revisited: Plasma renin activity, microangiopathic hemolysis, and renal failure in malignant hypertension. Am. J. Hypertens. 2007, 20, 900–906. [Google Scholar] [CrossRef]
- Nunes, I.; Santos, T.; Tavares, J.; Correia, L.; Coutinho, J.; Nogueira, J.; Carvalho, L.; Soares, J. Secondary hypertension due to a juxtaglomerular cell tumor. J. Am. Soc. Hypertens. 2018, 12, 637–640. [Google Scholar] [CrossRef]
- McVicar, M.; Carman, C.; Chandra, M.; Abbi, R.J.; Teichberg, S.; Kahn, E. Hypertension secondary to renin-secreting juxtaglomerular cell tumor: Case report and review of 38 cases. Pediatr. Nephrol. 1993, 7, 404–412. [Google Scholar] [CrossRef]
- Wong, L.; Hsu, T.H.; Perlroth, M.G.; Hofmann, L.V.; Haynes, C.M.; Katznelson, L. Reninoma: Case report and literature review. J. Hypertens. 2008, 26, 368–373. [Google Scholar] [CrossRef]
- Jansen, P.M.; Stowasser, M. Aldosterone-producing adenoma associated with non-suppressed renin: A case series. J. Hum. Hypertens. 2022, 36, 373–380. [Google Scholar] [CrossRef]
- Shen, J.; Lyu, L.; Wu, X.; Ji, J.; Zeng, C.; Li, S.; Zhao, Y.; Xu, J.; Lin, L.; Lu, C.; et al. Correlation between Renal Artery Anatomy and Hypertension: A Retrospective Analysis of 3000 Patients. Evid. Based Complement. Altern. Med. 2021, 2021, 9957361. [Google Scholar] [CrossRef]
- Kem, D.C.; Lyons, D.F.; Wenzl, J.; Halverstadt, D.; Yu, X. Renin-dependent hypertension caused by nonfocal stenotic aberrant renal arteries: Proof of a new syndrome. Hypertension 2005, 46, 380–385. [Google Scholar] [CrossRef]
- Funes Hernandez, M.; Bhalla, V.; Isom, R.T. Hypothesis: Accessory renal arteries may be an overlooked cause of renin-dependent hypertension. J. Hum. Hypertens. 2022, 36, 493–497. [Google Scholar] [CrossRef]
- De Bruyne, B.; Manoharan, G.; Pijls, N.H.; Verhamme, K.; Madaric, J.; Bartunek, J.; Vanderheyden, M.; Heyndrickx, G.R. Assessment of renal artery stenosis severity by pressure gradient measurements. J. Am. Coll. Cardiol. 2006, 48, 1851–1855. [Google Scholar] [CrossRef]
- Glodny, B.; Cromme, S.; Reimer, P.; Lennarz, M.; Winde, G.; Vetter, H. Hypertension associated with multiple renal arteries may be renin-dependent. J. Hypertens. 2000, 18, 1437–1444. [Google Scholar] [CrossRef]
- Kang, K.; Ma, Y.; Jia, C.; Cheng, Y.; Yang, Y.; Wang, L.; Jiang, Y.; Lu, Y. Relationship between Accessory Renal Artery and Clinical Characteristics of Middle-Aged Patients with Primary Hypertension. Int. J. Hypertens. 2020, 2020, 7109502. [Google Scholar] [CrossRef]

| Variable | Serum Potassium (mmol/L) (Reference: 3.5–5.3) | Renin (μIU/mL) | Aldosterone (ng/dL) | ARR {(ng/dL)/(μIU/mL)} (Reference: <3.7) |
|---|---|---|---|---|
| First screening test | 2.95 | |||
| Supine position | 26.61 (reference: 2.8–39.9) | 22.8 (reference: 3–23.6) | 0.86 | |
| Upright position | 118.2 (reference: 4.4–46.1) | 39.7 (reference: 3–35.3) | 0.34 | |
| Second screening test | 3.53 | |||
| Supine position | 50.56 (reference: 2.8–39.9) | 47.6 (reference: 3–23.6) | 0.94 | |
| Upright position | 50.22 (reference: 4.4–46.1) | 45.9 (reference: 3–35.3) | 0.91 | |
| 1 month after surgery | 4.81 | |||
| Upright position | 121.4 (reference: 4.4–46.1) | 11.9 (reference: 3–35.3) | 0.1 |
| Variable | Inferior Vena Cava | Right Renal Vein | Left Renal Vein |
|---|---|---|---|
| Renin (μIU/mL) | 41.80 | 72.54 | 66.95 |
| Aldosterone (ng/dL) | 53.10 | 44.10 | 43 |
| Variable | Aldosterone (ng/dL) | Cortisol (nmol/L) | Selectivity Index (SI) | Aldosterone/ Cortisol Ratio | Lateralization Index |
|---|---|---|---|---|---|
| ACTH Stimulation | |||||
| Inferior Vena Cava | 47.20 | 654 | |||
| Right Adrenal Vein | 1050 | 32856 | 50.2 | 0.0319 | 4.78 |
| Left Adrenal Vein | 4960 | 32411 | 49.5 | 0.1530 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Yang, X.; Wang, S.; Chen, X.; Liu, K. Primary Aldosteronism Masked by Accessory Renal Arteries: A Case Report. J. Clin. Med. 2022, 11, 6276. https://doi.org/10.3390/jcm11216276
Yang C, Yang X, Wang S, Chen X, Liu K. Primary Aldosteronism Masked by Accessory Renal Arteries: A Case Report. Journal of Clinical Medicine. 2022; 11(21):6276. https://doi.org/10.3390/jcm11216276
Chicago/Turabian StyleYang, Changqiang, Xiangyu Yang, Si Wang, Xiaoping Chen, and Kai Liu. 2022. "Primary Aldosteronism Masked by Accessory Renal Arteries: A Case Report" Journal of Clinical Medicine 11, no. 21: 6276. https://doi.org/10.3390/jcm11216276
APA StyleYang, C., Yang, X., Wang, S., Chen, X., & Liu, K. (2022). Primary Aldosteronism Masked by Accessory Renal Arteries: A Case Report. Journal of Clinical Medicine, 11(21), 6276. https://doi.org/10.3390/jcm11216276





