Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature
Abstract
1. Introduction
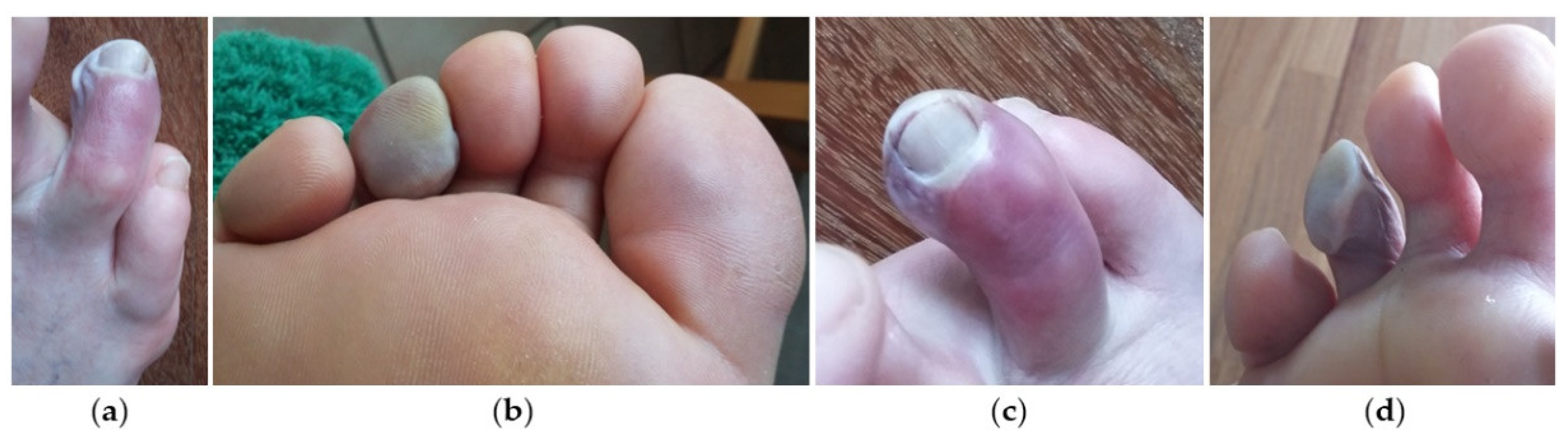
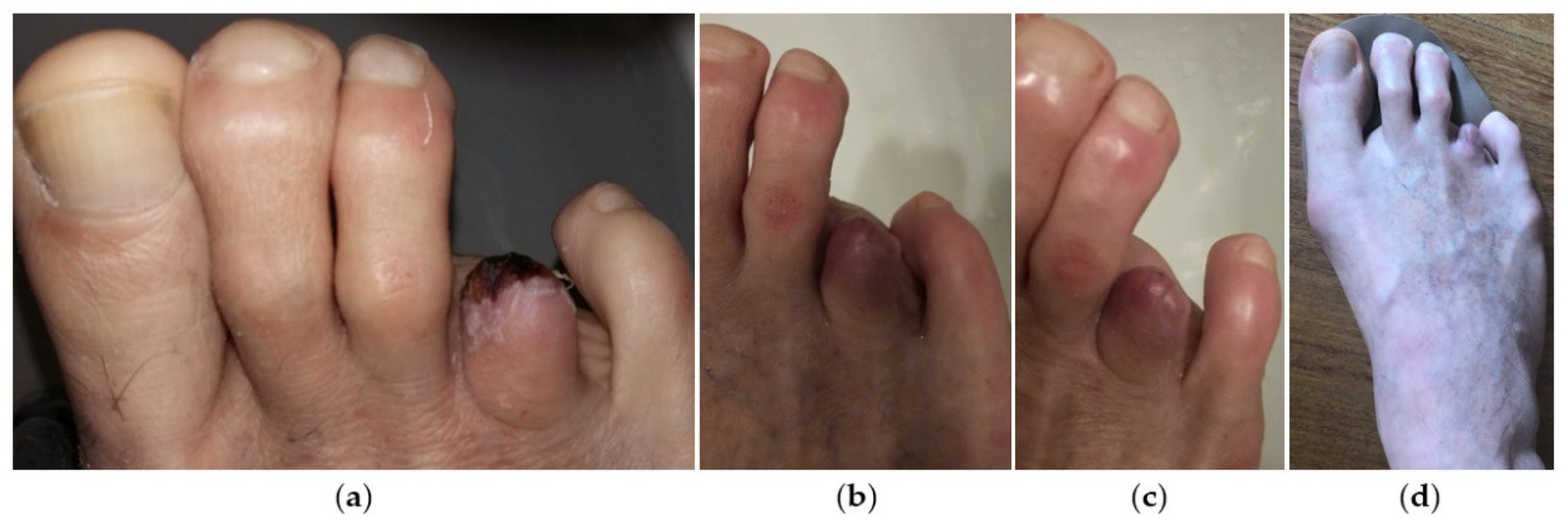
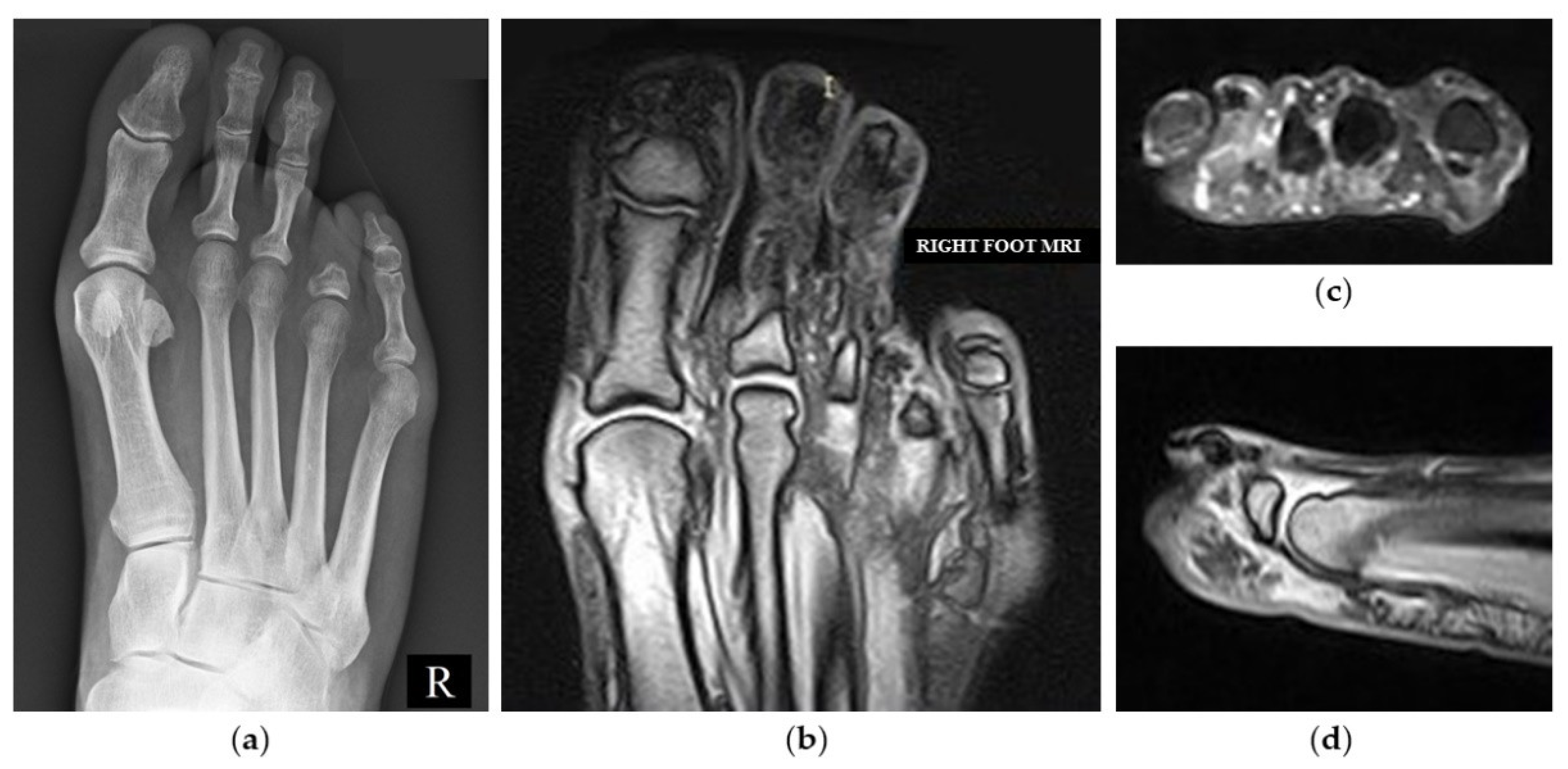
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, K.K. Morton’s Interdigital Neuroma: A Clinical Review of Its Etiology, Treatment, and Results. J. Foot Ankle Surg. 1996, 35, 112–119. [Google Scholar] [CrossRef]
- Civinini, F. Su d’un Nervoso Gangliare Rigonfiamento alla Pianta del Piede. Lettera Anatomica al Dr. Salomone Lampronti; Tip. Bracali: Pistoia, Italy, 1835. [Google Scholar]
- Morton, T.G. A Peculiar and Painful Affection of the Fourth Metatarso-Phalangeal Articulation. Am. J. Med. Sci. 1876, 71, 37–45. [Google Scholar] [CrossRef]
- Matthews, B.G.; Hurn, S.E.; Harding, M.P.; Henry, R.A.; Ware, R.S. The Effectiveness of Non-Surgical Interventions for Common Plantar Digital Compressive Neuropathy (Morton’s Neuroma): A Systematic Review and Meta-Analysis. J. Foot Ankle Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, N.; Lampridis, V.; Sakellariou, A. Morton’s Interdigital Neuroma: Instructional Review. EFORT Open Rev. 2019, 4, 14–24. [Google Scholar] [CrossRef]
- Hassouna, H.; Singh, D. Morton’s metatarsalgia: Pathogenesis, aetiology and current management. Acta Orthop. Belg. 2005, 71, 646–655. [Google Scholar]
- Stecco, C.; Fantoni, I.; Macchi, V.; Del Borrello, M.; Porzionato, A.; Biz, C.; De Caro, R. The role of fasciae in Civinini-Morton’s syndrome. J. Anat. 2015, 227, 654–664. [Google Scholar] [CrossRef]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; Caro, R.; Stecco, C. Fascia and Soft Tissues Innervation in the Human Hip and Their Possible Role in Post-Surgical Pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Pirri, C.; Neuhuber, W.; Tiengo, C.; Biz, C.; De Caro, R.; Schleip, R.; Stecco, C. Innervation of human superficial fascia. Front. Neuroanat. 2022, 16, 981426. [Google Scholar] [CrossRef]
- Stecco, A.; Pirri, C.; Stecco, C. Fascial entrapment neuropathy. Clin. Anat. 2019, 32, 883–890. [Google Scholar] [CrossRef]
- Stecco, C.; Pirri, C.; Fede, C.; Fan, C.; Giordani, F.; Stecco, L.; Foti, C.; De Caro, R. Dermatome and fasciatome. Clin. Anat. 2019, 32, 896–902. [Google Scholar] [CrossRef]
- Rajput, K.; Reddy, S.; Shankar, H. Painful neuromas. Clin. J. Pain 2012, 28, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Pastides, P.; El-Sallakh, S.; Charalambides, C. Morton’s neuroma: A clinical versus radiological diagnosis. Foot Ankle Surg. 2012, 18, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mannan, K. The diagnosis and management of Morton’s neuroma: A literature review. Foot Ankle Spec. 2013, 6, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Fazal, M.A.; Khan, I.; Thomas, C. Ultrasonography and magnetic resonance imaging in the diagnosis of Morton’s neuroma. J. Am. Podiatr. Med. Assoc. 2012, 102, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Bignotti, B.; Signori, A.; Sormani, M.P.; Molfetta, L.; Martinoli, C.; Tagliafico, A. Ultrasound versus Magnetic Resonance Imaging for Morton Neuroma: Systematic Review and Meta-Analysis. Eur. Radiol. 2015, 25, 2254–2262. [Google Scholar] [CrossRef]
- Di Caprio, F.; Meringolo, R.; Shehab Eddine, M.; Ponziani, L. Morton’s interdigital neuroma of the foot: A literature review. Foot Ankle Surg. 2018, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.; Aujla, R.S.; Divall, P.; Bhatia, M. Non-surgical treatments for Morton’s neuroma: A systematic review. Foot Ankle Surg. 2020, 26, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Stecco, C.; Fantoni, I.; Aprile, G.; Giacomini, S.; Pirri, C.; Ruggieri, P. Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7952. [Google Scholar] [CrossRef]
- Elghazy, M.A.; Whitelaw, K.C.; Waryasz, G.R.; Guss, D.; Johnson, A.H.; DiGiovanni, C.W. Isolated Intermetatarsal Ligament Release as Primary Operative Management for Morton’s Neuroma: Short-term Results. Foot Ankle Spec. 2022, 15, 338–345. [Google Scholar] [CrossRef]
- Thomson, C.E.; Beggs, I.; Martin, D.J.; McMillan, D.; Edwards, R.T.; Russell, D.; Yeo, S.T.; Russell, I.T.; Gibson, J.N. Methylprednisolone injections for the treatment of Morton neuroma: A patient-blinded randomized trial. J. Bone Joint Surg. Am. 2013, 95, 790–798. [Google Scholar] [CrossRef]
- Lizano-Díez, X.; Ginés-Cespedosa, A.; Alentorn-Geli, E.; Pérez-Prieto, D.; González-Lucena, G.; Gamba, C.; De Zabala, S.; Solano-López, A.; Rigol-Ramón, P. Corticosteroid Injection for the Treatment of Morton’s Neuroma: A Prospective, Double-Blinded, Randomized, Placebo-Controlled Trial. Foot Ankle Int. 2017, 38, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Kretzmann, H.; Price, J.; Brantingham, J.; Globe, G. A single-blinded randomized placebo-controlled clinical trial of manipulation and mobilization in the treatment of Morton’s neuroma. J. Am. Chiropr. Assoc. 2007, 44, 9–18. [Google Scholar]
- Cashley, D.G.; Cochrane, L. Manipulation in the Treatment of Plantar Digital Neuralgia: A Retrospective Study of 38 Cases. J. Chiropr. Med. 2015, 14, 90–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sault, J.D.; Morris, M.V.; Jayaseelan, D.J.; Emerson-Kavchak, A.J. Manual therapy in the management of a patient with a symptomatic Morton’s Neuroma: A case report. Man. Ther. 2016, 21, 307–310. [Google Scholar] [CrossRef]
- Pérez-Domínguez, B.; Casaña-Granell, J. The effects of a combined physical therapy approach on Morton’s Neuroma. An N-of-1 Case Report. Foot 2020, 44, 101684. [Google Scholar] [CrossRef]
- Bucknall, V.; Rutherford, D.; MacDonald, D.; Shalaby, H.; McKinley, J.; Breusch, S.J. Outcomes following excision of Morton’s interdigital neuroma: A prospective study. Bone Joint J. 2016, 98-B, 1376–1381. [Google Scholar] [CrossRef]
- Bauer, T.; Gaumetou, E.; Klouche, S.; Hardy, P.; Maffulli, N. Metatarsalgia and Morton’s Disease: Comparison of Outcomes Between Open Procedure and Neurectomy Versus Percutaneous Metatarsal Osteotomies and Ligament Release with a Minimum of 2 Years of Follow-Up. J. Foot Ankle Surg. 2015, 54, 373–377. [Google Scholar] [CrossRef]
- Valisena, S.; Petri, G.J.; Ferrero, A. Treatment of Morton’s neuroma: A systematic review. Foot Ankle Surg. 2018, 24, 271–281. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, J.B.; Young, K.W.; Park, Y.U.; Kim, J.S.; Jegal, H. Long-term results of neurectomy in the treatment of Morton’s neuroma: More than 10 years’ follow-up. Foot Ankle Spec. 2011, 4, 349–353. [Google Scholar] [CrossRef]
- Rengachary, S.S.; Watanabe, I.S.; Singer, P.; Bopp, W.J. Effect of glycerol on peripheral nerve: An experimental study. Neurosurgery 1983, 13, 681–688. [Google Scholar] [CrossRef]
- Dockery, G.L. The treatment of intermetatarsal neuromas with 4% alcohol sclerosing injections. J. Foot Ankle Surg. 1999, 38, 403–408. [Google Scholar] [CrossRef]
- Fanucci, E.; Masala, S.; Fabiano, S.; Perugia, D.; Squillaci, E.; Varrucciu, V.; Simonetti, G. Treatment of intermetatarsal Morton’s neuroma with alcohol injection under US guide: 10-month follow-up. Eur. Radiol. 2004, 14, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, N.; Seybold, J.D.; Jankauskas, L.; Erschbamer, M. Alcohol sclerosing therapy is not an effective treatment for interdigital neuroma. Foot Ankle Int. 2011, 32, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, C.; Vulcano, E.; Novario, R.; Varotto, D.; Montoli, C.; Volpe, A. Ultrasound-guided alcohol injection for Morton’s neuroma. Foot Ankle Int. 2015, 36, 55–59. [Google Scholar] [CrossRef]
- Hughes, R.J.; Ali, K.; Jones, H.; Kendall, S.; Connell, D.A. Treatment of Morton’s neuroma with alcohol injection under sonographic guidance: Follow-up of 101 cases. Am. J. Roentgenol. 2007, 188, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Perini, L.; Perini, C.; Tagliapietra, M.; Varotto, D.; Valcarenghi, A.; Postorino, A.; Volpe, A. Percutaneous alcohol injection under sonographic guidance in Morton’s neuroma: Follow-up in 220 treated lesions. Radiol. Med. 2016, 121, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Ortu, S.; Fiori, E.; Bagnoli, I.; Valente, A.; Pisanu, F.; Caggiari, G.; Doria, C.; Milano, L. Complications of alcohol injections for Morton’s neuroma. JOTR 2022, 29, 22104917221116392. [Google Scholar] [CrossRef]
- Musson, R.; Sawhney, J.; Lamb, L.; Wilkinson, A.; Obaid, H. Ultrasound guided alcohol ablation of Morton’s neuroma. Foot Ankle Int. 2012, 33, 196–201. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef]
- Flow Diagram—Case Reports Following the CARE Guidelines; CARE Group. Available online: https://www.equator-network.org/wp-content/uploads/2013/09/CAREFlowDiagram-updated-2019.pdf (accessed on 6 October 2022).
- Bourke, G.; Owen, J.; Machet, D. Histological comparison of the third interdigital nerve in patients with Morton’s metatarsalgia and control patients. Aust. N. Z. J. Surg. 1994, 64, 421–424. [Google Scholar] [CrossRef]
- Samaila, E.; Colò, G.; Rava, A.; Negri, S.; Valentini, R.; Felli, L.; Magnan, B. Effectiveness of corticosteroid injections in Civinini-Morton’s Syndrome: A systematic review. Foot Ankle Surg. 2021, 27, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mozena, J.D.; Clifford, J.T. Efficacy of chemical neurolysis for the treatment of interdigital nerve compression of the foot: A retrospective study. J. Am. Podiatr. Med. Assoc. 2007, 97, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Samaila, E.M.; Ambrosini, C.; Negri, S.; Maluta, T.; Valentini, R.; Magnan, B. Can percutaneous alcoholization of Morton’s neuroma with phenol by electrostimulation guidance be an alternative to surgical excision? Long-term results. Foot Ankle Surg. 2020, 26, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Diamond, E.; Schmidt, W.K.; Kelly, M.; Allen, R.; Houghton, W.; Brady, K.L.; Campbell, J.N. A randomized, double-blind, placebo-controlled trial of injected capsaicin for pain in Morton’s neuroma. Pain 2016, 157, 1297–1304. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.I.; Hong, W.H.; Suh, J.S.; Hur, J.W. Corticosteroid Injection for Morton’s Interdigital Neuroma: A Systematic Review. Clin. Orthop. Surg. 2021, 13, 266–277. [Google Scholar] [CrossRef]
- Auersperg, V.; Trieb, K. Extracorporeal shock wave therapy: An update. EFORT Open Rev. 2020, 5, 584–592. [Google Scholar] [CrossRef]
- Gimber, L.H.; Melville, D.M.; Bocian, D.A.; Krupinski, E.A.; Guidice, M.P.; Taljanovic, M.S. Ultrasound Evaluation of Morton Neuroma Before and After Laser Therapy. AJR Am. J. Roentgenol. 2017, 208, 380–385. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Tsoumakidou, G.; Caudrelier, J.; Thenint, M.A.; Rao, P.; Koch, G.; Gangi, A. Percutaneous MR-Guided Cryoablation of Morton’s Neuroma: Rationale and Technical Details After the First 20 Patients. CardioVasc. Interv. Radiol. 2016, 39, 1491–1498. [Google Scholar] [CrossRef]
- Deniz, S.; Purtuloglu, T.; Tekindur, S.; Cansız, K.H.; Yetim, M.; Kılıckaya, O.; Senkal, S.; Bilgic, S.; Atim, A.; Kurt, E. Ultrasound-guided pulsed radio frequency treatment in Morton’s neuroma. J. Am. Podiatr. Med. Assoc. 2015, 105, 302–306. [Google Scholar] [CrossRef]
- Gurdezi, S.; White, T.; Ramesh, P. Alcohol injection for Morton’s neuroma: A five-year follow-up. Foot Ankle Int. 2013, 34, 1064–1067. [Google Scholar] [CrossRef]
- Pace, A.; Scammell, B.; Dhar, S. The outcome of Morton’s neurectomy in the treatment of metatarsalgia. Int. Orthop. 2010, 34, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Morrison, G.; Coda, A. Sclerosing alcohol injections for the management of intermetatarsal neuromas: A systematic review. Foot 2018, 35, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Koyyalagunta, D.; Engle, M.P.; Yu, J.; Feng, L.; Novy, D.M. The Effectiveness of Alcohol Versus Phenol Based Splanchnic Nerve Neurolysis for the Treatment of Intra-Abdominal Cancer Pain. Pain Physician 2016, 19, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Mazoch, M.J.; Cheema, G.A.; Suva, L.J.; Thomas, R.L. Effects of alcohol injection in rat sciatic nerve as a model for Morton’s neuroma treatment. Foot Ankle Int. 2014, 35, 1187–1191. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Koltsakis, E.; Kakkos, G.A.; Karantanas, A.H. Ultrasound-guided treatment of Morton’s neuroma. J. Ultrason. 2021, 21, 134–138. [Google Scholar] [CrossRef]

| Non-Surgical Invasive Techniques | Authors (Publication Year and Type) | Number of Complications (/) In Each Patient Cohort | Local Complications | Systemic Complications |
|---|---|---|---|---|
| Fanucci et al. (2004) [33] Case Series | 6/40 | Transitory plantar pain due to leakage of fluid | - | |
| Alcohol Injection (USGAI) | Hughes et al. (2007) [36] Case Series | 18/101 17/101 1/101 | Plantar pain Forefoot marrow edema | - |
| Mozena & Clifford (2007) [44] Case Series | 3/42 | Pain and Erythema around the injection area | - | |
| Musson et al. (2012) [39] Case Series | 1/75 | Pain Swelling | Allergic reaction | |
| Perini et al. (2016) [37] Case Series | 178/220 2/220 176/220 | Symptoms worsening Hypo/Anaesthesia of the innervated area | - | |
| Samaila et al. (2020) [45] Case Series | 1/115 | Osteonecrosis of the third metatarsal head | - | |
| Ortu et al. (2022) [38] Case Series | 3/200 | Skin necrosis | - | |
| Capsaicin Injection | Campbell et al. (2016) [46] Case Series | 30/30 4/30 3/30 | Severe Post-Injection Pain | Nausea Headache |
| Corticosteroid Injection | Choi et al. (2021) [47] Review | 10/294 3/51 2/216 | Skin Depigmentation Skin Atrophy Plantar Fat Pad Atrophy | - |
| ESWT | Auersperg et al. (2020) [48] Review | - | Redness Superficial Hematoma | - |
| Laser Therapy | Gimber et al. (2017) [49] Case series | - | Perilesional Scar | - |
| Percutaneous Cryoablation | Cazzato et al. (2016) [50] Case Series | 1/20 | Local Cellulitis around cryo-probe entry point | - |
| RFA | Deniz et al. (2015) [51] Case Series | 2/20 | Superficial Cellulitis and moderate Hematoma | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biz, C.; Bonvicini, B.; Sciarretta, G.; Pendin, M.; Cecchetto, G.; Ruggieri, P. Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature. J. Clin. Med. 2022, 11, 6263. https://doi.org/10.3390/jcm11216263
Biz C, Bonvicini B, Sciarretta G, Pendin M, Cecchetto G, Ruggieri P. Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature. Journal of Clinical Medicine. 2022; 11(21):6263. https://doi.org/10.3390/jcm11216263
Chicago/Turabian StyleBiz, Carlo, Barbara Bonvicini, Giovanni Sciarretta, Mattia Pendin, Giovanni Cecchetto, and Pietro Ruggieri. 2022. "Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature" Journal of Clinical Medicine 11, no. 21: 6263. https://doi.org/10.3390/jcm11216263
APA StyleBiz, C., Bonvicini, B., Sciarretta, G., Pendin, M., Cecchetto, G., & Ruggieri, P. (2022). Digital Ischemia after Ultrasound-Guided Alcohol Injection for Morton’s Syndrome: Case Report and Review of the Literature. Journal of Clinical Medicine, 11(21), 6263. https://doi.org/10.3390/jcm11216263








