Efficacy of Neurostimulations for Upper Extremity Function Recovery after Stroke: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection and Data Collection
2.5. Outcomes
2.6. Statistical Analysis
2.7. Risk of Bias
3. Results
3.1. Search Strategies and Study Characteristics
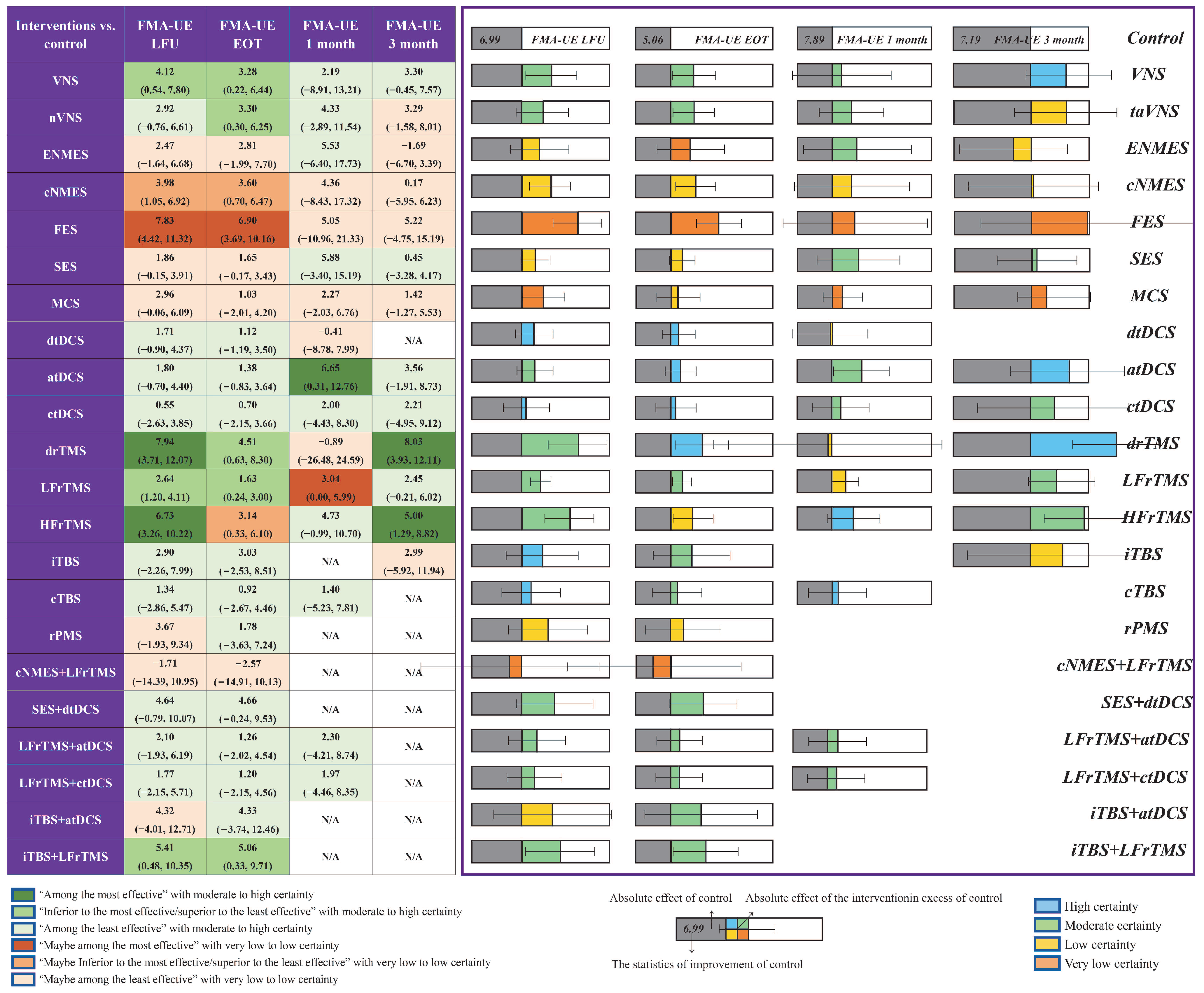
3.2. FMA-UE
3.3. Safety
3.4. ARAT and BBT
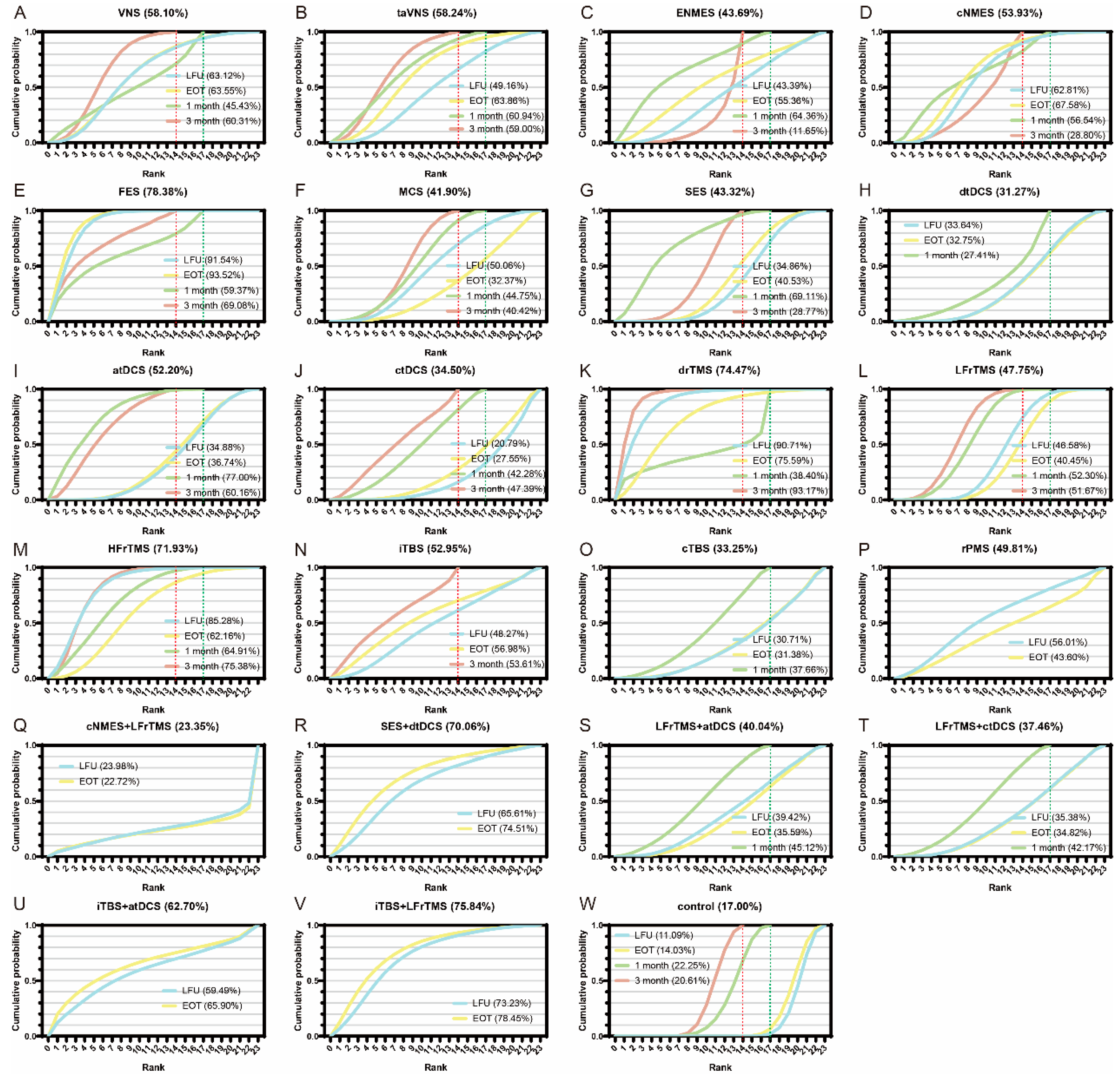
3.5. Network Meta-Regression and Sensitivity Analysis
3.6. Network Heterogeneity and Consistency
3.7. Risks of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Runchey, S.; McGee, S. Does this patient have a hemorrhagic stroke? Clinical findings distinguishing hemorrhagic stroke from ischemic stroke. JAMA 2010, 303, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Luft, A.R.; McCombe-Waller, S.; Whitall, J.; Forrester, L.W.; Macko, R.; Sorkin, J.D.; Schulz, J.B.; Goldberg, A.P.; Hanley, D.F. Repetitive bilateral arm training and motor cortex activation in chronic stroke: A randomised controlled trial. JAMA 2004, 292, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lim, S.H.; Kim, K.H.; Kim, K.J.; Kim, Y.R.; Chang, W.N.; Yeom, J.W.; Kim, Y.D.; Hwang, B.Y. Six-month functional recovery of stroke patients: A multi-time-point study. Int. J. Rehabil. Res. 2015, 38, 173–180. [Google Scholar] [CrossRef]
- Raffin, E.; Hummel, F.C. Restoring Motor Functions After Stroke: Multiple Approaches and Opportunities. Neuroscientist 2018, 24, 400–416. [Google Scholar] [CrossRef]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2011, 26, 291–313. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, CD001920. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Engineer, N.D.; Kimberley, T.J.; Prudente, C.N.; Dawson, J.; Tarver, W.B.; Hays, S.A. Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke. Front. Neurosci. 2019, 13, 280. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar] [PubMed]
- Barker, A.; Jalinous, R.; Freeston, I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 325, 1106–1107. [Google Scholar] [CrossRef]
- Farmer, S.E.; Durairaj, V.; Swain, I.; Pandyan, A.D. Assistive technologies: Can they contribute to rehabilitation of the upper limb after stroke? Arch. Phys. Med. Rehabil. 2014, 95, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst. Rev. 2013, CD009645. [Google Scholar]
- Hao, Z.; Wang, D.; Zeng, Y.; Liu, M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst. Rev. 2013, CD008862. [Google Scholar]
- Adeyemo, B.O.; Simis, M.; Macea, D.D.; Fregni, F. Systematic Review of Parameters of Stimulation, Clinical Trial Design Characteristics, and Motor Outcomes in Non-Invasive Brain Stimulation in Stroke. Front. Psychiatry 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Florez, I.D.; Izcovich, A.; Santesso, N.; Hazlewood, G.; Alhazanni, W.; Yepes-Nuñez, J.J.; Tomlinson, G.; Schünemann, H.J.; Guyatt, G.H. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 2020, 371, m3900. [Google Scholar] [CrossRef]
- Gladstone, D.; Danells, C.J.; Black, S. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.-J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiology Heal. 2019, 41, e2019013. [Google Scholar] [CrossRef] [PubMed]
- Groves, D.A.; Brown, V.J. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 2005, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.X.; Sun, F.H.; Xie, Y.J.; Ou, X.; Yang, S.B. The effect of VNS on the rehabilitation of stroke: A meta-analysis of randomised controlled studies. J. Clin. Neurosci. 2020, 81, 421–425. [Google Scholar]
- Xie, Y.L.; Wang, S.; Wu, Q.; Chen, X. Vagus nerve stimulation for upper limb motor impairment after ischemic stroke: A meta-analysis. Medicine 2021, 100, e27871. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, X.; Ma, J.; Jia, G. Effect of Combined Vagus Nerve Stimulation on Recovery of Upper Extremity Function in Patients with Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106390. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef]
- Bornheim, S.; Croisier, J.L.; Maquet, P.; Kaux, J.F. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients—A randomised, triple blind, sham-controlled study. Brain Stimul. 2020, 13, 329–336. [Google Scholar] [CrossRef]
- Elsner, B.; Kwakkel, G.; Kugler, J.; Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: A network meta-analysis of randomised controlled trials. J. Neuroeng. Rehabil. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Cheng, C.H.; Liao, K.K.; Lee, I.H.; Lin, Y.Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke 2012, 43, 1849–1857. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Cash, R.F.; Luo, X.; Luo, H.; Lu, X.; Xu, F.; Zang, Y.-F.; Fitzgerald, P.B.; Fitzgibbon, B.M. High-frequency rTMS over the dorsolateral prefrontal cortex on chronic and provoked pain: A systematic review and meta-analysis. Brain Stimul. 2021, 14, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Barber, P.A.; Smith, M.-C. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke 2017, 48, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.; Byblow, W. Predicting and accelerating motor recovery after stroke. Curr. Opin. Neurol. 2014, 27, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; Kwakkel, G.; van Wegen, E.E.; Ket, J.C.; Heymans, M.W. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke 2011, 42, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Huang, Y.-Z.; Bai, Y.-M.; Tsai, S.-J.; Su, T.-P.; Cheng, C.-M. Critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Hum. Brain Mapp. 2018, 40, 2001–2009. [Google Scholar] [CrossRef]
- Dawson, J.; Pierce, D.; Dixit, A.; Kimberley, T.J.; Robertson, M.; Tarver, B.; Hilmi, O.; McLean, J.; Forbes, K.; Kilgard, M.P.; et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation after Ischemic Stroke. Stroke 2016, 47, 143–150. [Google Scholar] [CrossRef]
- Capone, F.; Miccinilli, S.; Pellegrino, G.; Zollo, L.; Simonetti, D.; Bressi, F.; Florio, L.; Ranieri, F.; Falato, E.; Di Santo, A.; et al. Transcutaneous Vagus Nerve Stimulation Combined with Robotic Rehabilitation Improves Upper Limb Function after Stroke. Neural Plast. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Kimberley, T.J.; Pierce, D.; Prudente, C.N.; Francisco, G.E.; Yozbatiran, N.; Smith, P.; Tarver, B.; Engineer, N.D.; Dickie, D.A.; Kline, D.K.; et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018, 49, 2789–2792. [Google Scholar] [CrossRef]
- Wu, D.; Ma, J.; Zhang, L.; Wang, S.; Tan, B.; Jia, G. Effect and Safety of Transcutaneous Auricular Vagus Nerve Stimulation on Recovery of Upper Limb Motor Function in Subacute Ischemic Stroke Patients: A Randomized Pilot Study. Neural Plast. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Dawson, J.; Liu, C.Y.; E Francisco, G.; Cramer, S.C.; Wolf, S.L.; Dixit, A.; Alexander, J.; Ali, R.; Brown, B.L.; Feng, W.; et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. Lancet 2021, 397, 1545–1553. [Google Scholar] [CrossRef]
- Amasyali, S.Y.; Yaliman, A. Comparison of the effects of mirror therapy and electromyography-triggered neuromuscular stimulation on hand functions in stroke patients: A pilot study. Int. J. Rehabil. Res. 2016, 39, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.D.; Page, S.J.; Delahanty, M.; Knutson, J.S.; Gunzler, D.D.; Sheffler, L.R.; Michael, D. Upper-Limb Recovery After Stroke: A Randomized Controlled Trial Comparing EMG-Triggered, Cyclic, and Sensory Electrical Stimulation. Neurorehabilit. Neural Repair 2016, 30, 978–987. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, Y.; Jung, K.; Chung, Y. The effects of electromyography-triggered electrical stimulation on shoulder subluxation, muscle activation, pain, and function in persons with stroke: A pilot study. NeuroRehabilitation 2017, 40, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, A.; Topuz, O.; Alkan, H.; Ozgen, M.; Sarsan, A.; Yildiz, N.; Ardic, F. Comparison of the effectiveness of active and passive neuromuscular electrical stimulation of hemiplegic upper extremities: A randomized, controlled trial. Int. J. Rehabil. Res. 2013, 36, 315–322. [Google Scholar] [CrossRef]
- Hemmen, B.; Seelen, H. Effects of movement imagery and electromyography-triggered feedback on arm—Hand function in stroke patients in the subacute phase. Clin. Rehabil. 2007, 21, 587–594. [Google Scholar] [CrossRef]
- de Kroon, J.R.; Ijzerman, M. Electrical stimulation of the upper extremity in stroke: Cyclic versus EMG-triggered stimulation. Clin. Rehabil. 2008, 22, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-L.; Chen, Y.-L.; Chen, C.-C.; Li-Ling, C.; Wong, A.M.-K.; Hsu, A.-L.; Chang, Y.-J. Effect of EMG-triggered neuromuscular electrical stimulation with bilateral arm training on hemiplegic shoulder pain and arm function after stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2017, 14, 122. [Google Scholar] [CrossRef]
- McCabe, J.; Monkiewicz, M.; Holcomb, J.; Pundik, S.; Daly, J.J. Comparison of Robotics, Functional Electrical Stimulation, and Motor Learning Methods for Treatment of Persistent Upper Extremity Dysfunction After Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 981–990. [Google Scholar] [CrossRef]
- Shimodozono, M.; Noma, T.; Matsumoto, S.; Miyata, R.; Etoh, S.; Kawahira, K. Repetitive facilitative exercise under continuous electrical stimulation for severe arm impairment after sub-acute stroke: A randomized controlled pilot study. Brain Inj. 2013, 28, 203–210. [Google Scholar] [CrossRef]
- Shindo, K.; Fujiwara, T.; Hara, J.; Oba, H.; Hotta, F.; Tsuji, T.; Hase, K.; Liu, M. Effectiveness of Hybrid Assistive Neuromuscular Dynamic Stimulation Therapy in Patients With Subacute Stroke: A randomized controlled pilot trial. Neurorehabilit. Neural Repair 2011, 25, 830–837. [Google Scholar] [CrossRef]
- Shen, Y.; Yin, Z.; Fan, Y.; Chen, V.; Dai, W.; Yi, W.; Li, Y.; Zhang, W.; Zhang, Y.; Bian, R.; et al. Comparison of the Effects of Contralaterally Controlled Functional Electrical Stimulation and Neuromuscular Electrical Stimulation on Upper Extremity Functions in Patients with Stroke. CNS Neurol. Disord. - Drug Targets 2015, 14, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, Y.; Huang, J.; Wang, H.; Bao, X.; Bi, Z.; Chen, X.; Gao, Y.; Lã¼, X.; Wang, Z. Electromyographic bridge for promoting the recovery of hand movements in subacute stroke patients: A randomized controlled trial. J. Rehabil. Med. 2017, 49, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mao, M.; Cao, Y.; Lu, X. Contralaterally controlled functional electrical stimulation improves wrist dorsiflexion and upper limb function in patients with early-phase stroke: A randomized controlled trial. J. Rehabil. Med. 2019, 51, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Harley, M.Y.; Hisel, T.Z.; Hogan, S.D.; Maloney, M.M.; Chae, J. Contralaterally Controlled Functional Electrical Stimulation for Upper Extremity Hemiplegia. Neurorehabilit. Neural Repair 2011, 26, 239–246. [Google Scholar] [CrossRef]
- Knutson, J.S.; Gunzler, D.D.; Wilson, R.D.; Chae, J. Contralaterally Controlled Functional Electrical Stimulation Improves Hand Dexterity in Chronic Hemiparesis: A Randomized Trial. Stroke 2016, 47, 2596–2602. [Google Scholar] [CrossRef]
- Knutson, J.S.; Makowski, N.S.; Harley, M.Y.; Hisel, T.Z.; Gunzler, D.D.; Wilson, R.D.; Chae, J. Adding Contralaterally Controlled Electrical Stimulation of the Triceps to Contralaterally Controlled Functional Electrical Stimulation of the Finger Extensors Reduces Upper Limb Impairment and Improves Reachable Workspace but not Dexterity: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Lutsep, H.L.; Weinand, M.; Cramer, S.C. Motor Cortex Stimulation for the Enhancement of Recovery from Stroke: A Prospective, Multicenter Safety Study. Neurosurgery 2006, 58, 464–473. [Google Scholar] [CrossRef]
- Levy, R.; Ruland, S.; Weinand, M.; Lowry, D.; Dafer, R.; Bakay, R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008, 108, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Harvey, R.L.; Kissela, B.M.; Winstein, C.J.; Lutsep, H.L.; Parrish, T.B.; Cramer, S.C.; Venkatesan, L. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabilit. Neural Repair 2015, 30, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Harvey, R.L.; Stoykov, M.E.; Ruland, S.; Weinand, M.; Lowry, D.; Levy, R. Cortical Stimulation for Upper Limb Recovery Following Ischemic Stroke: A Small Phase II Pilot Study of a Fully Implanted Stimulator. Top. Stroke Rehabil. 2008, 15, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ghaziani, E.; Couppé, C.; Siersma, V.; Søndergaard, M.; Christensen, H.; Magnusson, S.P. Electrical Somatosensory Stimulation in Early Rehabilitation of Arm Paresis After Stroke: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2018, 32, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.K.; Sorinola, I.O.; Roberts-Lewis, S.F.; Wolfe, C.D.; Wellwood, I.; Newham, D.J. The Effect of Combined Somatosensory Stimulation and Task-Specific Training on Upper Limb Function in Chronic Stroke: A double-blind randomized controlled trial. Neurorehabilit. Neural Repair 2014, 29, 143–152. [Google Scholar] [CrossRef]
- Carrico, C.; Chelette, K.C., 2nd; Westgate, P.M.; Salmon-Powell, E.; Nichols, L.; Sawaki, L. Randomized Trial of Peripheral Nerve Stimulation to Enhance Modified Constraint-Induced Therapy After Stroke. Am. J. Phys. Med. Rehabil. 2016, 95, 397–406. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Moriwaki, M.; Sakamoto, T.; Domen, K. Improvement of Upper Extremity Deficit after Constraint-Induced Movement Therapy Combined with and without Preconditioning Stimulation Using Dual-hemisphere Transcranial Direct Current Stimulation and Peripheral Neuromuscular Stimulation in Chronic Stroke Patients: A Pilot Randomized Controlled Trial. Front. Neurol. 2017, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-L.H.; Yang, W.-W.; Kao, C.-L.; Tsai, M.-W.; Wei, S.-H.; Fregni, F.; Chen, V.C.-F.; Chou, L.-W. Effects of 8-week sensory electrical stimulation combined with motor training on EEG-EMG coherence and motor function in individuals with stroke. Sci. Rep. 2018, 8, 9217. [Google Scholar] [CrossRef] [PubMed]
- Alwhaibi, R.M.; Mahmoud, N.; Zakaria, H.M.; Ragab, W.M.; Al Awaji, N.; Elzanaty, M.Y.; Elserougy, H.R. Therapeutic Efficacy of Transcutaneous Electrical Nerve Stimulation Acupoints on Motor and Neural Recovery of the Affected Upper Extremity in Chronic Stroke: A Sham-Controlled Randomized Clinical Trial. Healthcare 2021, 9, 614. [Google Scholar] [CrossRef]
- Jung, K.; Jung, J.; In, T.; Kim, T.; Cho, H.-Y. The influence of Task-Related Training combined with Transcutaneous Electrical Nerve Stimulation on paretic upper limb muscle activation in patients with chronic stroke. NeuroRehabilitation 2017, 40, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, O.V.; Kilicoglu, M.S.; Rezvani, A.; Kucukakkas, O.; Eren, F.; Aydin, T. How does cross-education affects muscles of paretic upper extremity in subacute stroke survivors? Neurol. Sci. 2020, 41, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.D.; Dijkstra, P.U.; Gerritsen, J.; Geurts, A.C.; Postema, K. Combined arm stretch positioning and neuromuscular electrical stimulation during rehabilitation does not improve range of motion, shoulder pain or function in patients after stroke: A randomised trial. J. Physiother. 2013, 59, 245–254. [Google Scholar] [CrossRef]
- Alisar, D.C.; Ozen, S.; Sozay, S. Effects of Bihemispheric Transcranial Direct Current Stimulation on Upper Extremity Function in Stroke Patients: A randomized Double-Blind Sham-Controlled Study. J. Stroke Cerebrovasc. Dis. 2019, 29, 104454. [Google Scholar] [CrossRef]
- Chen, S.-C.; Yang, L.-Y.; Adeel, M.; Lai, C.-H.; Peng, C.-W. Transcranial electrostimulation with special waveforms enhances upper-limb motor function in patients with chronic stroke: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Beaulieu, L.-D.; Blanchette, A.K.; Mercier, C.; Bernard-Larocque, V.; Milot, M.-H. Efficacy, safety, and tolerability of bilateral transcranial direct current stimulation combined to a resistance training program in chronic stroke survivors: A double-blind, randomized, placebo-controlled pilot study. Restor. Neurol. Neurosci. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhao, L.; Teo, W.P.; Chen, C.; Ng, Y.S.; Chew, E. Facilitating Effects of Transcranial Direct Current Stimulation on Motor Imagery Brain-Computer Interface With Robotic Feedback for Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, S79–S87. [Google Scholar] [CrossRef] [PubMed]
- Allman, C.; Amadi, U.; Winkler, A.M.; Wilkins, L.; Filippini, N.; Kischka, U.; Stagg, C.J.; Johansen-Berg, H. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci. Transl. Med. 2016, 8, 330re1. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Z.; Bai, Z.; Fong, K.N. Timing-dependent interaction effects of tDCS with mirror therapy on upper extremity motor recovery in patients with chronic stroke: A randomized controlled pilot study. J. Neurol. Sci. 2019, 405, 116436. [Google Scholar] [CrossRef]
- Hesse, S.; Waldner, A.; Mehrholz, J.; Tomelleri, C.; Pohl, M.; Werner, C. Combined Transcranial Direct Current Stimulation and Robot-Assisted Arm Training in Subacute Stroke Patients: An exploratory, randomized multicenter trial. Neurorehabilit. Neural Repair 2011, 25, 838–846. [Google Scholar] [CrossRef]
- Lindenberg, R.; Renga, V.; Zhu, L.; Nair, D.G.; Schlaug, G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010, 75, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-W.; Chiang, W.-C.; Lin, K.-C.; Wu, C.-Y.; Liu, C.-T.; Hsieh, Y.-W.; Lin, Y.-C.; Chen, C.-L. Timing-dependent effects of transcranial direct current stimulation with mirror therapy on daily function and motor control in chronic stroke: A randomized controlled pilot study. J. Neuroeng. Rehabil. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Chun, M.H. Combination Transcranial Direct Current Stimulation and Virtual Reality Therapy for Upper Extremity Training in Patients With Subacute Stroke. Arch. Phys. Med. Rehabil. 2014, 95, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Long, X.-M.; Xu, Y.; Cai, X.-Y.; Ye, M. Effects of repetitive transcranial magnetic stimulation combined with transcranial direct current stimulation on motor function and cortex excitability in subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2021, 35, 718–727. [Google Scholar] [CrossRef]
- Fusco, A.; Assenza, F.; Iosa, M.; Izzo, S.; Altavilla, R.; Paolucci, S.; Vernieri, F. The Ineffective Role of Cathodal tDCS in Enhancing the Functional Motor Outcomes in Early Phase of Stroke Rehabilitation: An Experimental Trial. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Cortes, M.; Rykman-Peltz, A.; Chang, J.; Elder, J.; Thickbroom, G.; Mariman, J.J.; Gerber, L.M.; Oromendia, C.; I Krebs, H.; et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restor. Neurol. Neurosci. 2019, 37, 167–180. [Google Scholar] [CrossRef]
- Kim, S.H. Effects of Dual Transcranial Direct Current Stimulation and Modified Constraint-Induced Movement Therapy to Improve Upper-Limb Function after Stroke: A Double-Blinded, Pilot Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2021, 30, 105928. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Tran, V.-D.; Dario, P.; Posteraro, F. Effects of Transcranial Direct Current Stimulation (tDCS) Combined With Wrist Robot-Assisted Rehabilitation on Motor Recovery in Subacute Stroke Patients: A Randomized Controlled Trial. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Cui, L.; Wang, J.; Feng, W.; Bao, Y.; Xie, Q. Effects of transcranial direct current stimulation with virtual reality on upper limb function in patients with ischemic stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Viana, R.; Laurentino, G.; Souza, R.; Fonseca, J.; Filho, E.S.; Dias, S.; Teixeira-Salmela, L.; Monte-Silva, K. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: A pilot randomized controlled trial. NeuroRehabilitation 2014, 34, 437–446. [Google Scholar] [CrossRef]
- Triccas, L.T.; Burridge, J.; Hughes, A.; Verheyden, G.; Desikan, M.; Rothwell, J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation 2015, 37, 181–191. [Google Scholar] [CrossRef]
- Shaheiwola, N.; Zhang, B.; Jia, J.; Zhang, D. Using tDCS as an Add-On Treatment Prior to FES Therapy in Improving Upper Limb Function in Severe Chronic Stroke Patients: A Randomized Controlled Study. Front. Hum. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.P.; Cimolin, V.; Schifino, G.P.; Rech, K.D.; Marchese, R.R.; Pagnussat, A.S. Bi-cephalic transcranial direct current stimulation combined with functional electrical stimulation for upper-limb stroke rehabilitation: A double-blind randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 63, 4–11. [Google Scholar] [CrossRef]
- Rocha, S.; Silva, E.; Foerster, Á.; Wiesiolek, C.; Chagas, A.P.; Machado, G.; Baltar, A.; Silva, K.M. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: A double-blind randomized controlled trial. Disabil. Rehabil. 2015, 38, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.L.; Lindberg, P.; Khan, A.; Ruschkowski, S.; Nitsche, M.A.; Borg, J. Transcranial direct current stimulation combined with visuo-motor training as treatment for chronic stroke patients. Restor. Neurol. Neurosci. 2017, 35, 307–317. [Google Scholar] [CrossRef]
- Gottlieb, A.; Boltzmann, M.; Schmidt, S.B.; Gutenbrunner, C.; Krauss, J.K.; Stangel, M.; Höglinger, G.U.; Wallesch, C.-W.; Rollnik, J.D. Treatment of upper limb spasticity with inhibitory repetitive transcranial magnetic stimulation: A randomized placebo-controlled trial. NeuroRehabilitation 2021, 49, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.; McCane, C.D.; Lee, J.; John, B.; Nguyen, L.; Butler, K.; Gadhia, R.; Misra, V.; Volpi, J.J.; Verma, A.; et al. Multifocal transcranial stimulation in chronic ischemic stroke: A phase 1/2a randomized trial. J. Stroke Cerebrovasc. Dis. 2020, 29, 104816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chen, C.-L.; Huang, Y.-Z.; Chen, H.-C.; Chen, C.-Y.; Wu, C.-Y.; Lin, K.-C. Augmented efficacy of intermittent theta burst stimulation on the virtual reality-based cycling training for upper limb function in patients with stroke: A double-blinded, randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, W.; Bang, O.; Kim, S.; Park, Y.; Lee, P. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J. Rehabil. Med. 2010, 42, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Cui, Y.; Xu, G.; Liu, L.; Zhang, X.; Jiang, K.; Li, Z. Efficacy of functional magnetic stimulation in improving upper extremity function after stroke: A randomized, single-blind, controlled study. J. Int. Med Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Obayashia, S.; Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 2020, 46, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Krewer, C.; Hartl, S.; Müller, F.; Koenig, E. Effects of Repetitive Peripheral Magnetic Stimulation on Upper-Limb Spasticity and Impairment in Patients With Spastic Hemiparesis: A Randomized, Double-Blind, Sham-Controlled Study. Arch. Phys. Med. Rehabil. 2014, 95, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Huang, Y.-Z.; Chen, C.-Y.; Chen, C.-L.; Chen, H.-C.; Wu, C.-Y.; Lin, K.-C.; Chang, T.-L. Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: A pilot randomized controlled trial. BMC Neurol. 2019, 19, 69. [Google Scholar] [CrossRef]
- Du, J.; Tian, L.; Liu, W.; Hu, J.; Xu, G.; Ma, M.; Fan, X.; Ye, R.; Jiang, Y.; Yin, Q.; et al. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: A randomized controlled trial. Eur. J. Neurol. 2016, 23, 1666–1672. [Google Scholar] [CrossRef]
- Miu, K.Y.D.; Kok, C.; Leung, S.S.; Chan, E.Y.L.; Wong, E. Comparison of Repetitive Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation on Upper Limb Recovery Among Patients With Recent Stroke. Ann. Rehabil. Med. 2020, 44, 428–437. [Google Scholar] [CrossRef]
- Guan, Y.-Z.; Li, J.; Zhang, X.-W.; Wu, S.; Du, H.; Cui, L.-Y.; Zhang, W.-H. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: A one-year longitudinal randomized trial. CNS Neurosci. Ther. 2017, 23, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Galvão, S.C.B.; dos Santos, R.B.C.; dos Santos, P.B.; Cabral, M.E.; Monte-Silva, K. Efficacy of Coupling Repetitive Transcranial Magnetic Stimulation and Physical Therapy to Reduce Upper-Limb Spasticity in Patients With Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2014, 95, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-F.; Huang, Y.-Z.; Lin, Y.-Y.; Tang, C.-W.; Liao, K.-K.; Lee, P.-L.; Tsai, Y.-A.; Cheng, H.-L.; Cheng, H.; Chern, C.-M.; et al. Intermittent theta burst stimulation over ipsilesional primary motor cortex of subacute ischemic stroke patients: A pilot study. Brain Stimul. 2013, 6, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L.; Edwards, D.; Dunning, K.; Fregni, F.; Stein, J.; Laine, J.; Rogers, L.M.; Vox, F.; Durand-Sanchez, A.; Bockbrader, M.; et al. Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 2018, 49, 2138–2146. [Google Scholar] [CrossRef]
- Kuzu, Ö.; Adiguzel, E.; Kesikburun, S.; Yaşar, E.; Yılmaz, B. The Effect of Sham Controlled Continuous Theta Burst Stimulation and Low Frequency Repetitive Transcranial Magnetic Stimulation on Upper Extremity Spasticity and Functional Recovery in Chronic Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2021, 30, 105795. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Han, J.-Y.; Song, M.-K.; Park, G.-C.; Lee, J.-S. Synergistic Effects of Scalp Acupuncture and Repetitive Transcranial Magnetic Stimulation on Cerebral Infarction: A Randomized Controlled Pilot Trial. Brain Sci. 2020, 10, 87. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kwon, B.S.; Gil Seo, H.; Park, J.; Paik, N.-J. Low-Frequency Repetitive Transcranial Magnetic Stimulation Over Contralesional Motor Cortex for Motor Recovery in Subacute Ischemic Stroke: A Randomized Sham-Controlled Trial. Neurorehabilit. Neural Repair 2020, 34, 856–867. [Google Scholar] [CrossRef]
- Matsuura, A.; Onoda, K.; Oguro, H.; Yamaguchi, S. Magnetic stimulation and movement-related cortical activity for acute stroke with hemiparesis. Eur. J. Neurol. 2015, 22, 1526–1532. [Google Scholar] [CrossRef]
- Long, H.; Wang, H.; Zhao, C.; Duan, Q.; Feng, F.; Hui, N.; Mao, L.; Liu, H.; Mou, X.; Yuan, H. Effects of combining high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. Restor. Neurol. Neurosci. 2018, 36, 21–30. [Google Scholar] [CrossRef]
- Rose, D.K.; Patten, C.; McGuirk, T.E.; Lu, X.; Triggs, W.J. Does Inhibitory Repetitive Transcranial Magnetic Stimulation Augment Functional Task Practice to Improve Arm Recovery in Chronic Stroke? Stroke Res. Treat. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.B.; Morales-Quezada, L.; Piza, P.V.D.T.; Zeng, D.; Vélez, F.G.S.; Ferreira, I.S.; Lucena, P.; Duarte, D.; Lopes, F.; El-Hagrassy, M.M.; et al. Combining Fluoxetine and rTMS in Poststroke Motor Recovery: A Placebo-Controlled Double-Blind Randomized Phase 2 Clinical Trial. Neurorehabilit. Neural Repair 2019, 33, 643–655. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, D.; Hai, H.; Zhao, Y.-Y.; Ma, Y.-W. Efficacy of coupling intermittent theta-burst stimulation and 1 Hz repetitive transcranial magnetic stimulation to enhance upper limb motor recovery in subacute stroke patients: A randomized controlled trial. Restor. Neurol. Neurosci. 2020, 38, 109–118. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Liao, W.-J.; Xia, W.-G. Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: A double-blind randomized controlled trail. J. Huazhong Univ. Sci. Technol. 2015, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kudo, Y.; Sugawara, E.; Nakamizo, T.; Amari, K.; Takahashi, K.; Tanaka, O.; Endo, M.; Hayakawa, Y.; Johkura, K. Comparative study of ipsilesional and contralesional repetitive transcranial magnetic stimulations for acute infarction. J. Neurol. Sci. 2018, 384, 10–14. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Zhao, Y.-Y.; Hai, H.; Ma, Y.-W. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul. 2020, 13, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Türe, S.; Aşkın, A.; Yardimci, E.U.; Demirdal, S.U.; Incesu, T.K.; Tosun, O.; Kocyigit, H.; Akhan, G.; Gelal, F.M. Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: A preliminary study. Top. Stroke Rehabil. 2017, 24, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.-H.; Wang, C.-P.; Chou, C.-L.; Chen, Y.-C.; Chang, Y.-C.; Tsai, P.-Y. Efficacy of Coupling Inhibitory and Facilitatory Repetitive Transcranial Magnetic Stimulation to Enhance Motor Recovery in Hemiplegic Stroke Patients. Stroke 2013, 44, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Vishnu, V.; Kumar, N.; Sreenivas, V.; Rajeswari, M.; Bhatia, R.; Sharma, R.; Srivastava, M.P. Efficacy of Low-Frequency Repetitive Transcranial Magnetic Stimulation in Ischemic Stroke: A Double-Blind Randomized Controlled Trial. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100039. [Google Scholar] [CrossRef] [PubMed]
- Seniów, J.; Bilik, M.; Leśniak, M.; Waldowski, K.; Iwański, S.; Czlonkowska, A. Transcranial Magnetic Stimulation Combined With Physiotherapy in Rehabilitation of Poststroke Hemiparesis: A randomized, double-blind, placebo-controlled study. Neurorehabilit. Neural Repair 2012, 26, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Abo, M.; Kakuda, W.; Momosaki, R.; Harashima, H.; Kojima, M.; Watanabe, S.; Sato, T.; Yokoi, A.; Umemori, T.; Sasanuma, J. Randomized, Multicenter, Comparative Study of NEURO versus CIMT in Poststroke Patients with Upper Limb Hemiparesis: The NEURO-VERIFY Study. Int. J. Stroke 2013, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.-L.; Tong, R.K.-Y.; Chung, K.Y.-K. Bilateral Upper Limb Training With Functional Electric Stimulation in Patients With Chronic Stroke. Neurorehabilit. Neural Repair 2008, 23, 357–365. [Google Scholar] [CrossRef] [PubMed]
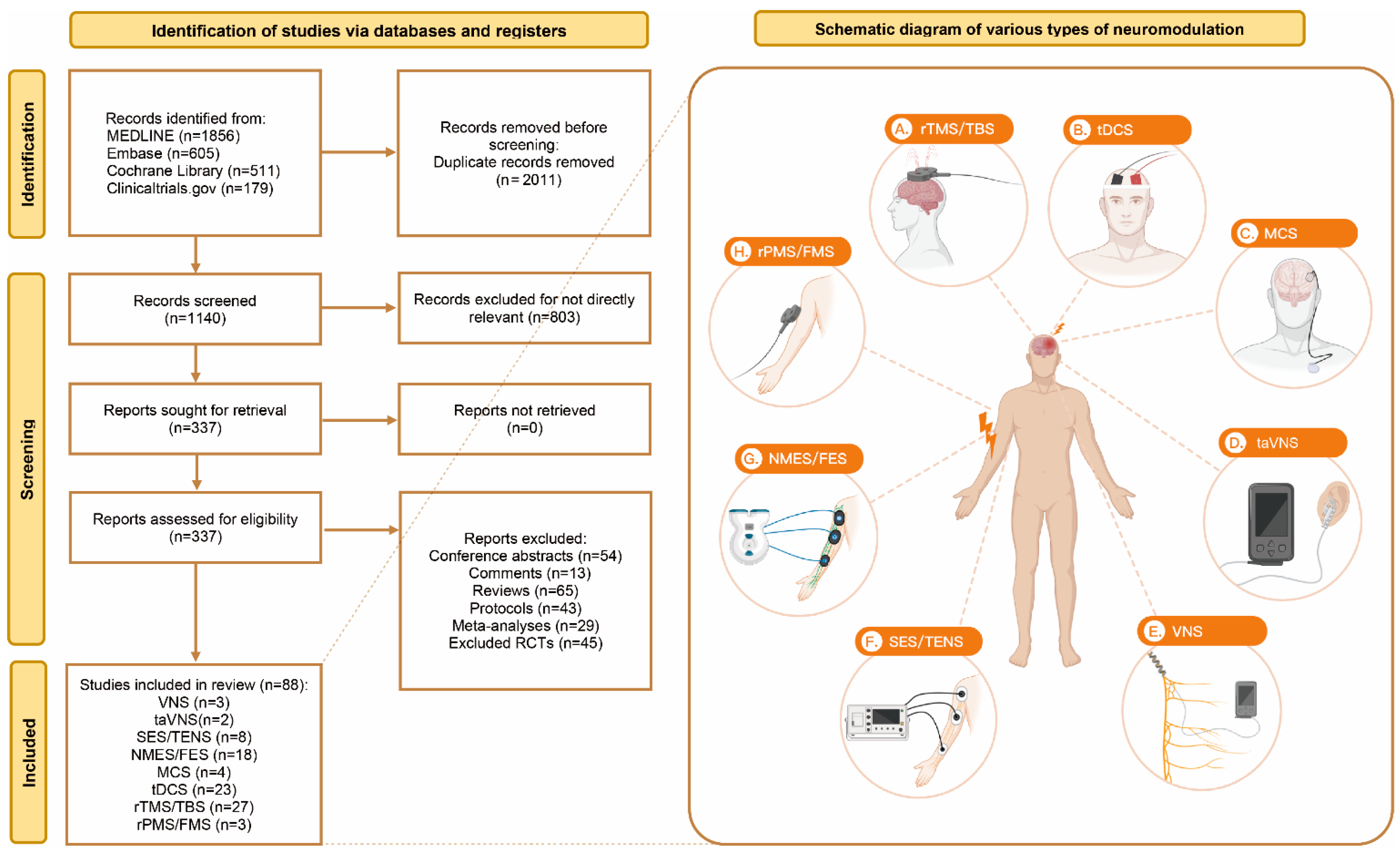
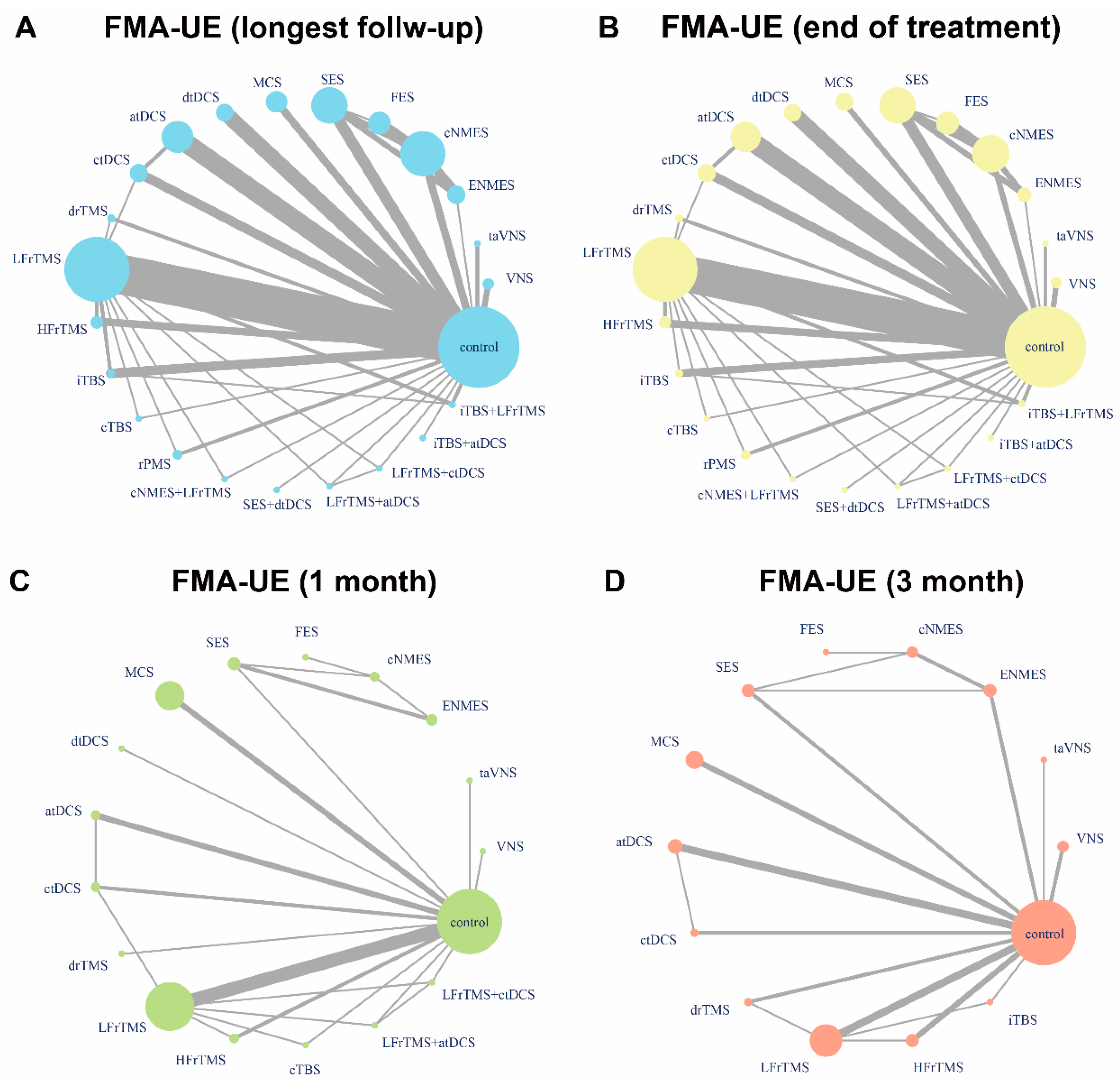

| Neuromodulation. | Subtype | Definition | Sample Size | Age, Mean ± SD | Female, n (%) | Years Since Stroke, Mean ± SD | FMA-UE, Mean ± SD | Hemiplegic Paralysis, Left/Right |
|---|---|---|---|---|---|---|---|---|
| 1. VNS (vagus nerve stimulation) | 1.1 VNS | A device is implanted into patient body to stimulate the cervical branch of vagus nerve directly through a simple surgery. | 70 | 58.99 ± 10.88 | 25 (35.71%) | 2.75 ± 2.13 | 34.57 ± 8.53 | 41/29 |
| 1.2 taVNS | A noninvasive technique stimulates the other branch of the vagus nerve in body surface, like external auditory channel at the inner side of the vagus. | 17 | 60.06 ± 13.29 | 8 (47.06%) | 3.27 ± 6.54 | 19.47 ± 7.20 | 4/6 | |
| 2. MCS (Motor cortex stimulation) | - | An invasive electrical stimulation which places the electrode at epidural area around the associated site of motor cortex activation through a craniotomy. | 122 | 55.86 ± 11.17 | 52 (42.62%) | 4.95 ± 5.49 | 36.91 ± 6.83 | 49/73 |
| 3. NMES/FES (Neuromuscular electrical stimulation /Functional electrical stimulation) | 3.1 cNMES | This stimulation is provided by electrically activating hemiplegia muscle at a set frequency while the intensity at or above motor threshold. During the entire process, patient is generally a passive participant. | 278 | 58.73 ± 12.48 | 107 (38.49%) | 0.46 ± 1.53 | 27.29 ± 13.88 | 123/123 |
| 3.2 ENMES | Patient is actively involved in the training and the electrical stimulation is provided when EMG signals generated by motion exceed a pre-set threshold. | 113 | 57.47 ± 12.29 | 39 (34.51%) | 1.04 ± 2.39 | 34.02 ± 15.52 | 57/55 | |
| 3.3 FES | It refers that tetanic muscle contractions of hemiplegia limb are induced to assist or reinstate some kinds of goal-directed movement, while patients or therapists could control the timing or intensity of stimulation. | 156 | 55.8 ± 14.05 | 48 (30.77%) | 0.70 ± 1.23 | 25.53 ± 11.39 | 72/63 | |
| 4. SES/TENS (Somatosensory electrical stimulation/Transcutaneous nerve electrical stimulation) | - | An intervention involves low intensity electrical stimulation of peripheral nerves, which merely reaches the sensory threshold and below the motor threshold. | 224 | 60.98 ± 13.95 | 102 (45.54%) | 1.32 ± 2.01 | 30.47 ± 20.54 | 99/105 |
| 5. rTMS/TBS (Repetitive transcranial magnetic stimulation /Theta burst stimulation) | 5.1 LFrTMS | A non-invasive magnetic stimulation modulates cortical excitability in stroke, and low-frequency rTMS (≤1 Hz) decreases the cortical excitability of the primary motor cortex of unaffected limb. | 586 | 59.97 ± 12.84 | 206 (35.15%) | 0.90 ± 2.33 | 34.69 ± 16.00 | 246/262 |
| 5.2 HFrTMS | Similarly, high-frequency rTMS (≥5 Hz) facilitates the cortical excitability of the hemiplegic limb. | 77 | 57.84 ± 9.13 | 24 (31.17%) | 0.03 ± 0.04 | 28.24 ± 15.06 | 38/39 | |
| 5.3 drTMS | LF-rTMS applies to the unaffected side while HF-rTMS to the hemiplegic side for synergistic effect. | 35 | 55.90 ± 8.89 | 5 (14.29%) | 0.05 ± 0.01 | 38.14 ± 18.98 | 11/10 | |
| 5.4 iTBS | A variant of rTMS modulated ipsilesional primary motor cortex intermittently with a specific pattern of stimulation sequences in a shorter time. | 49 | 59.7 ± 12.07 | 15 (30.61%) | 0.45 ± 0.32 | 32.27 ± 16.55 | 17/20 | |
| 5.5 cTBS | Continuous theta burst stimulation brings down the excitability of the contralateral primary motor cortex for the rehabilitation of stoke. | 7 | 61.3 ± 9.8 | 1 (14.29%) | 1.21 ± 0.13 | 19.4 ± 14.2 | 2/5 | |
| 6. rPMS/FMS (Repetitive peripheral magnetic stimulation /Functional magnetic stimulation) | - | A magnetic technology stimulates deep regions of muscles evoking muscle contraction with nearly no pain. | 60 | 55.78 ± 13.02 | 19 (31.67%) | 0.44 ± 1.19 | 27.55 ± 16.64 | 27/23 |
| 7. tDCS (Transcranial direct current stimulation) | 7.1 atDCS | A body surface direct current stimulation places the anode slice on the motor cortex area of the affected side and facilitates the depolarization of neurons. | 176 | 62.80 ± 11.70 | 70 (39.78%) | 1.37 ± 1.99 | 28.65 ± 17.67 | 70/72 |
| 7.2 ctDCS | On the contrary, cathodal tDCS is mounted on the the scalp surface of not damaged brain hemisphere, reducing the neuronal firing. | 109 | 63.48 ± 10.13 | 45 (41.28%) | 0.34 ± 0.93 | 22.45 ± 20.24 | 57/52 | |
| 7.3 dtDCS | The anode slice of tDCS device is mounted on the ipsilesional side while the cathode on the contralateral side at the same time to improve the rehabilitation of extremity function after stroke. | 108 | 59.00 ± 11.26 | 37 (34.26%) | 1.91 ± 1.54 | 36.79 ± 17.37 | 52/56 |
| Outcomes | No. of Trials Contributing to the Meta-Analysis | No. of Participants Contributing to the Meta-Analysis | Effect Size | Heterogeneity | GRADE | |||
|---|---|---|---|---|---|---|---|---|
| MD (95% CI) | p Value | I2 (%) | χ² | p Value | ||||
| ||||||||
| VNS | 3 | 145 | 3.49 (1.56, 5.41) | 0.0004 | 0 | 1.12 | 0.57 | ⊕⊕⊕○ Moderate * |
| taVNS | 2 | 33 | 2.95 (0.90, 5.00) | 0.005 | 0 | 0.09 | 0.77 | ⊕⊕⊕○ Moderate * |
| ENMES | 1 | 15 | 2.16 (−14.62, 18.94) | 0.80 | N/A | N/A | N/A | ⊕⊕○○ Low *, ## |
| cNMES | 4 | 111 | 4.30 (−0.38, 8.97) | 0.07 | 45 | 5.41 | 0.14 | ⊕⊕○○ Low *, $ |
| SES | 6 | 252 | 1.73 (0.73, 2.73) | 0.0007 | 23 | 6.47 | 0.26 | ⊕⊕○○ Low *, # |
| MCS | 4 | 208 | 2.63 (0.32, 4.95) | 0.03 | 28 | 4.19 | 0.24 | ⊕○○○ Very low **, # |
| dtDCS | 8 | 205 | 1.48 (−0.09, 3.05) | 0.06 | 0 | 2.86 | 0.90 | ⊕⊕⊕⊕ High |
| atDCS | 10 | 361 | 1.64 (−1.50, 4.77) | 0.31 | 45 | 16.44 | 0.06 | ⊕⊕⊕○ Moderate $ |
| ctDCS | 5 | 172 | 1.78 (−1.72, 5.29) | 0.32 | 0 | 0.22 | 0.99 | ⊕⊕⊕⊕ High |
| drTMS | 2 | 70 | 6.28 (−2.12, 14.68) | 0.14 | 22 | 1.28 | 0.26 | ⊕⊕⊕○ Moderate # |
| LFrTMS | 21 | 948 | 2.99 (1.34, 4.63) | 0.0004 | 65 | 56.91 | <0.0001 | ⊕⊕⊕○ Moderate $ |
| HFrTMS | 4 | 146 | 7.11 (4.40, 9.82) | <0.00001 | 0 | 1.49 | 0.68 | ⊕⊕⊕○ Moderate * |
| iTBS | 5 | 98 | 3.10 (−1.90, 8.10) | 0.22 | 0 | 1.93 | 0.75 | ⊕⊕⊕⊕ High |
| cTBS | 1 | 13 | 2.97 (1.26, 4.68) | 0.0007 | N/A | N/A | N/A | ⊕⊕⊕⊕ High |
| rPMS | 2 | 82 | 1.66 (−4.15, 7.47) | 0.58 | 0 | 0.51 | 0.47 | ⊕⊕○○ Low *, # |
| cNMES+LFrTMS | 1 | 16 | 8.00 (−7.84, 23.84) | 0.32 | N/A | N/A | N/A | ⊕○○○ Very low *, ## |
| SES+dtDCS | 1 | 19 | 4.64 (1.30, 7.98) | 0.006 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| LFrTMS+atDCS | 1 | 30 | 1.20 (−0.33, 2.73) | 0.12 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| LFrTMS+ctDCS | 1 | 30 | 0.87 (−0.27, 2.01) | 0.13 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| iTBS+atDCS | 1 | 24 | 4.33 (−2.93, 11.59) | 0.24 | N/A | N/A | N/A | ⊕⊕○○ Low *, # |
| iTBS+LFrTMS | 2 | 47 | 4.84 (−0.22, 9.89) | 0.06 | 0 | 0.86 | 0.35 | ⊕⊕⊕○ Moderate # |
| ||||||||
| VNS | 3 | 145 | 2.83 (1.37, 4.30) | 0.0002 | 0 | 1.26 | 0.53 | ⊕⊕⊕○ Moderate * |
| taVNS | 2 | 33 | 3.54 (2.31, 4.77) | <0.00001 | 0 | 0.42 | 0.52 | ⊕⊕⊕○ Moderate * |
| ENMES | 1 | 15 | 3.96 (−13.51, 21.43) | 0.66 | N/A | N/A | N/A | ⊕○○○ Very low *, ## |
| cNMES | 3 | 65 | 4.28 (−1.74, 10.30) | 0.16 | 63 | 5.37 | 0.07 | ⊕○○○ Very low *, #, $ |
| SES | 6 | 252 | 1.70 (0.33, 3.07) | 0.01 | 45 | 9.02 | 0.11 | ⊕⊕○○ Low #, $ |
| MCS | 3 | 184 | 0.44 (−1.04, 1.93) | 0.56 | 0 | 1.55 | 0.46 | ⊕⊕○○ Low ** |
| dtDCS | 8 | 205 | 0.95 (−0.53, 2.42) | 0.21 | 0 | 5.83 | 0.56 | ⊕⊕⊕⊕ High |
| atDCS | 10 | 361 | 0.80 (−1.10, 2.70) | 0.41 | 10 | 9.98 | 0.35 | ⊕⊕⊕⊕ High |
| ctDCS | 5 | 172 | 1.89 (−1.25, 4.81) | 0.25 | 0 | 0.83 | 0.93 | ⊕⊕⊕⊕ High |
| drTMS | 2 | 70 | 5.47 (3.25, 7.69) | <0.00001 | 0 | 0.34 | 0.56 | ⊕⊕⊕⊕ High |
| LFrTMS | 19 | 823 | 1.83 (0.69, 2.96) | 0.002 | 26 | 24.35 | 0.14 | ⊕⊕⊕○ Moderate # |
| HFrTMS | 4 | 146 | 3.21 (0.17, 6.25) | 0.04 | 34 | 4.56 | 0.21 | ⊕⊕○○ Low *, # |
| iTBS | 4 | 84 | 2.62 (−3.06, 8.29) | 0.37 | 0 | 1.62 | 0.65 | ⊕⊕⊕○ Moderate # |
| cTBS | 1 | 13 | 2.12 (0.40, 3.84) | 0.02 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate # |
| rPMS | 2 | 82 | −0.23 (−6.82, 6.37) | 0.95 | 11 | 1.12 | 0.29 | ⊕⊕○○ Low *, # |
| cNMES+LFrTMS | 1 | 16 | 8.00 (−7.84, 23.84) | 0.32 | N/A | N/A | N/A | ⊕○○○ Very low *, ## |
| SES+dtDCS | 1 | 19 | 4.64 (1.30, 7.98) | 0.006 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| LFrTMS+atDCS | 1 | 30 | 0.80 (0.00 1.60) | 0.05 | N/A | N/A | N/A | ⊕⊕○○ Low *, # |
| LFrTMS+ctDCS | 1 | 30 | 0.74 (−0.32, 1.80) | 0.17 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| iTBS+atDCS | 1 | 24 | 4.33 (−2.93, 11.59) | 0.24 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate # |
| iTBS+LFrTMS | 2 | 47 | 4.84 (−0.22, 9.89) | 0.06 | 0 | 0.86 | 0.35 | ⊕⊕⊕○ Moderate # |
| ||||||||
| VNS | 1 | 17 | 2.23 (−6.41, 10.87) | 0.61 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate # |
| taVNS | 1 | 21 | 4.34 (2.95, 5.73) | <0.00001 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| SES | 1 | 19 | 5.92 (−0.17, 12.01) | 0.06 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate # |
| MCS | 3 | 200 | 2.03 (−0.47, 4.54) | 0.11 | 58 | 4.71 | 0.09 | ⊕○○○ Very low **, $ |
| dtDCS | 1 | 19 | −0.40 (−5.02, 4.22) | 0.87 | N/A | N/A | N/A | ⊕⊕○○ Low *, # |
| atDCS | 3 | 88 | 6.37 (1.00, 11.73) | 0.02 | 0 | 0.98 | 0.61 | ⊕⊕⊕○ Moderate # |
| ctDCS | 2 | 28 | 1.61 (−6.64, 9.86) | 0.70 | 0 | 0.09 | 0.76 | ⊕⊕⊕○ Moderate # |
| drTMS | 1 | 29 | −0.82(−25.33, 23.69) | 0.95 | N/A | N/A | N/A | ⊕⊕○○ Low ## |
| LFrTMS | 7 | 493 | 3.28 (0.01, 6.55) | 0.05 | 85 | 41.15 | <0.00001 | ⊕⊕○○ Low #, $ |
| HFrTMS | 2 | 88 | 4.33 (1.49, 7.16) | 0.003 | 7 | 1.08 | 0.30 | ⊕⊕⊕⊕ High |
| cTBS | 1 | 13 | 2.97 (1.26, 4.68) | 0.0007 | N/A | N/A | N/A | ⊕⊕⊕⊕ High |
| LFrTMS+atDCS | 1 | 30 | 1.20 (−0.33,2.73) | 0.12 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| LFrTMS+ctDCS | 1 | 30 | 0.87 (−0.27, 2.01) | 0.13 | N/A | N/A | N/A | ⊕⊕⊕○ Moderate * |
| ||||||||
| VNS | 2 | 125 | 3.14 (1.08, 5.21) | 0.003 | 0 | 0.32 | 0.57 | ⊕⊕⊕⊕ High |
| taVNS | 1 | 21 | 3.22 (0.48, 5.96) | 0.02 | N/A | N/A | N/A | ⊕⊕○○ Low *, # |
| ENMES | 2 | 42 | −2.61 (−8.19, 2.98) | 0.36 | 0 | 0.35 | 0.55 | ⊕⊕○○ Low *, # |
| SES | 2 | 45 | 0.65 (−2.15, 3.44) | 0.65 | 0 | 0.04 | 0.83 | ⊕⊕⊕○ Moderate * |
| MCS | 3 | 184 | 2.01 (−1.42, 5.45) | 0.25 | 47 | 3.81 | 0.15 | ⊕○○○ Very low **, & |
| atDCS | 4 | 161 | 3.54 (−1.43, 8.51) | 0.16 | 0 | 1.92 | 0.59 | ⊕⊕⊕⊕ High |
| ctDCS | 2 | 78 | 1.12 (−5.66, 7.91) | 0.75 | 0 | 0.04 | 0.84 | ⊕⊕⊕○ Moderate # |
| drTMS | 2 | 70 | 7.48 (3.35, 11.61) | 0.0004 | 4 | 1.04 | 0.31 | ⊕⊕⊕⊕ High |
| LFrTMS | 4 | 299 | 3.76 (−0.57, 8.09) | 0.09 | 68 | 9.52 | 0.02 | ⊕⊕⊕○ Moderate & |
| HFrTMS | 3 | 116 | 5.39 (2.44, 8.34) | 0.0003 | 0 | 1.04 | 0.59 | ⊕⊕⊕○ Moderate * |
| iTBS | 1 | 14 | 4.99 (−3.33, 13.31) | 0.24 | N/A | N/A | N/A | ⊕⊕○○ Low *, # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Yan, Z.; Meng, J.; Wang, W.; Chen, S.; Wu, X.; Gu, F.; Tao, X.; Wu, W.; Chen, Z.; et al. Efficacy of Neurostimulations for Upper Extremity Function Recovery after Stroke: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 6162. https://doi.org/10.3390/jcm11206162
Xue T, Yan Z, Meng J, Wang W, Chen S, Wu X, Gu F, Tao X, Wu W, Chen Z, et al. Efficacy of Neurostimulations for Upper Extremity Function Recovery after Stroke: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(20):6162. https://doi.org/10.3390/jcm11206162
Chicago/Turabian StyleXue, Tao, Zeya Yan, Jiahao Meng, Wei Wang, Shujun Chen, Xin Wu, Feng Gu, Xinyu Tao, Wenxue Wu, Zhouqing Chen, and et al. 2022. "Efficacy of Neurostimulations for Upper Extremity Function Recovery after Stroke: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 20: 6162. https://doi.org/10.3390/jcm11206162
APA StyleXue, T., Yan, Z., Meng, J., Wang, W., Chen, S., Wu, X., Gu, F., Tao, X., Wu, W., Chen, Z., Bai, Y., Wang, Z., & Zhang, J. (2022). Efficacy of Neurostimulations for Upper Extremity Function Recovery after Stroke: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 11(20), 6162. https://doi.org/10.3390/jcm11206162






