Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia
Abstract
1. Introduction
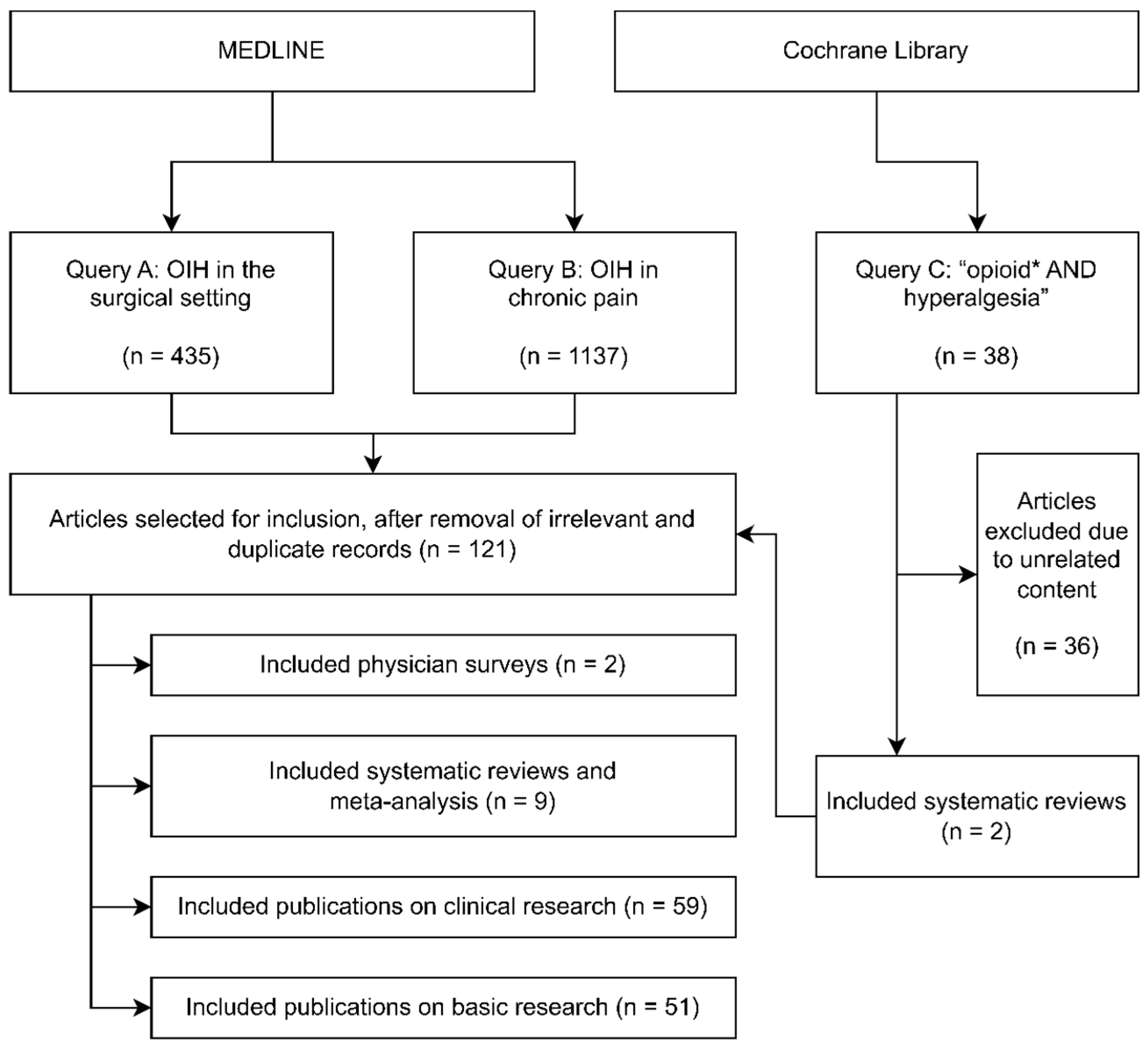
2. Materials and Methods
3. Results
3.1. OIH in Chronic Pain
3.1.1. Opioid Prescription and Epidemiology
3.1.2. Clinical Studies
| Study Reference | Subjects | Study | Tested Substances | Outcome Comparison with Respective Controls | OIH or Tolerance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | Pain Conditions (N) | Design | Test Group | Opioid Analgesia | Non-opioid Adjuvant | Control Group | Seen? | Reversible w/Adjuvant? | ||
| [36] | 41 | Fibromyalgia (n = 10) | RCT | “10 mg Morphine or 0.2 mg/mL Naloxone” | - | Placebo | MPT = (similarly ↑) | No. | - | |
| Rheumatoid Arthritis (n = 11) | MPT = (similarly ↑) | No. | - | |||||||
| [45] | 18 | Fibromyalgia (n = 18) | Intra-group analysis of MOR expression through fMRI | Patients with lower MOR expression | None (naïve) | - | Patients with higher MOR expression | PEA ↓ in antinociceptive nuclei and nucleus accumbens | No. | - |
| [38] | 10 | Fibromyalgia (n = 10) | RCT | None (naïve) | 4.5 mg naltrexone | Placebo (no naltrexone) | MPT ↓ RPI ↓ | No. | - | |
| [29] | 300 | Fibromyalgia (n = 37) Opioid addiction (n = 69) Non-specified pain (n = 148) | Observational: cold pressor test after past morphine exposure | High lifetime-opioid exposure | Variable (lifetime estimate of morphine-equivalent exposure) | - | Healthy controls (n = 46);(Low lifetime-opioid exposure) | MPT ↑ RPI ↑ | Yes. | Yes. |
| Added low-dose naltrexone | No added low-dose naltrexone | MPT ↓ RPI ↓ | ||||||||
| [13] | 76 | OIH (n = 55) Fibromyalgia (n = 21) | Open-label case series | Buprenorphine (single dose) | Added low-dose naltrexone | No added low-dose naltrexone | MPT ↓ | Yes. | Yes. | |
| [28] | 7 | chronic neuropathic pain (n = 7) | prospective observational study. | Planned opioid tapering | Variable (≥120 mg morphine equivalents/day) | - | The same patients, before opioid taper was applied | MPT ↓ RPI ↓ | Yes. | - |
| [46] | 103 | Low back pain (n = 103) | RCT | sustained-release morphine | Morphine or Remifentanil | - | The same patients, before analgesia was applied | RPI ↓ | Yes (OIH excluded). | - |
| [47] | 21 | Low back pain (n = 21) | prospective observational study. | 10 kHz spinal cord stimulation | none | - | Standard (opioid-including) practice | TOC ↓ RPI ↓ | ? | - |
| [37] | 98 | Low back pain (n = 70) | RCT | Planned opioid tapering | Morphine? | - | Healthy subjects (n = 28) | TOC ↑ RPI ↑ MPT ↑ | Yes | - |
3.2. OIH in Post-Operative Pain
3.2.1. Trends in Opioid Analgesia in Surgery
3.2.2. Review of Clinical Evidence
| Study Reference | Subjects | Tested Substances | Outcome Comparison with Respective Controls | OIH or Tolerance | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Surgery | Intraoperative Opioid | Postoperative Opioid | Non-Opioid Adjuvant | Control | Seen? | Reversible w/Adjuvant? | ||
| [62] * | 90 | Laparoendoscopic single-site urologic | Remifentanil | Morphine | Single dose Pregabalin (300 mg) | No adjuvant pregabalin | TFD ↓ AMA ↓ RPI ↓ MPT ↓ | Yes * | Yes |
| [63] | 64 | Major lung surgery | Remifentanil | Buprenorphine (25 μg h−1) | - | Postoperative morphine | TFD ↑ AMA ↓ | Yes * | - |
| [73] | 36 | Minimally invasive lumbar decompression | None (opioid-free regimen) | Morphine | - | Standard practice: opioid-containing anesthesia | TOC ↓ RPI = LOS ↓ | No | - |
| [74] | 62 | Posterior spinal fusion for adolescent idiopathic scoliosis (n = 37) | Remifentanil | Morphine | - | Intraoperative fentanyl, standard protocol (n = 25) | TOC ↓ RPI ↓ LOS = | Unlikely | - |
| [64] | 40 | Elective cardiac surgery | Remifentanil-controlled target dose (<dose) | Morphine | - | Remifentanil-continuous infusion (>dose) | AMA ↓ MPT ↓ RPI = TOC = | Yes | - |
| [65] | 48 | Thyroidectomy | Remifentanil | Morphine | Dexmedetomidine | No adjuvant dexmedetomidine | RPI ↓ MPT ↓ AMA ↓ TOC ↓ | Yes * | Yes |
| [66] | 40 | Laparoscopic gynecologic surgery | Remifentanil | Morphine | Ketamine | No adjuvant ketamine | RPI ↓ TOC ↓ | Yes * | Yes |
| [67] | 47 | Total thyroidectomy | Remifentanil | Morphine | Acetazolamide | No adjuvant acetazolamide | AMA = RPI = TOC = MPT = (↓ in 2 groups) | Yes * | No |
| [71] | 56 | Laparoscopic sleeve gastrectomy | Fentanyl, high dose (>dose) | Morphine | - | Intraoperative fentanyl, standard protocol (<dose) | TFD ↑ | Yes | - |
| [68] | 75 | Laparoscopic gynecologic surgery | Remifentanil | Morphine | Ketamine | No adjuvant ketamine | MPT ↓ TOC ↓ RPI ↓ | Yes * | Yes |
| [70] | 60 | Laparoscopic cholecystectomy | Remifentanil | Fentanyl | 2 groups: (A) ketamine and (B) esmolol | No adjuvants (neither A nor B) | Similar in groups A & B: TOC ↓ RPI ↓ | Yes * | Yes (in both groups) |
| [69] | 54 | Laparoscopic cholecystectomy | Remifentanil | Morphine | Ketamine (preoperative) | No adjuvant ketamine | RPI ↓ | Yes | Yes |
| Study Reference | Substance Used | Outcomes | Outcome Analysis |
|---|---|---|---|
| [10] | Intra-surgical remifentanil. | RPI ↑ | Mean differences (on a 100 cm visual analogue scale) for high intra-operative doses vs reference group: 9.4 cm (95% CI: 4.4, 14.5) at 1 h; 7.1 cm (95% CI: 2.8, 11.3) at 4 h; 3 cm (95% CI: 0.4, 5.6) at 24 h. |
| TOC ↑ | Higher postoperative morphine use after 24 h (Standardize mean difference: 0.7 (95% CI: 0.37, 1.02) | ||
| [39] | Peri-surgical ketamine. | TOC ↓ | Reduction of postoperative opioid consumption: 8 mg morphine equivalents (95% CI 6 to 9); 19% from 42 mg consumed by participants given placebo-over 24 h 13 mg lower (95% CI 10 to 15); 19% from 67 mg with placebo-over 48 h |
| TFD ↑ | Increased the time for the first postoperative analgesic request by 54 min (95% CI 37 to 71 min) vs. 39 min with placebo | ||
| RPI ↓ (at rest) | Reduction of pain at rest: by 5/100 mm on a visual analogue scale (95% CI 4 to 7; 19% lower from 26/100 mm with placebo-at 24 h by 5/100 mm (95% CI 3 to 7; 22% lower from 23/100 mm-at 48 h | ||
| RPI ↓ (during movement) | Reduced pain during movement: by 6/100 mm, 14% lower from 42/100 mm-at 24 h by 6/100 mm, 16% lower from 37 mm-at 48 h | ||
| AMA ↓ | Reduced the area of postoperative hyperalgesia by 7 cm² (95% CI −11.9 to −2.2), compared with placebo | ||
| [50] | Opioid-free anesthesia. | RPI = (at rest) | Pain scores at rest at two postoperative hours were equivalent in the opioid-inclusive and opioid-free groups with a mean difference of 0.2 (95%CI: −0.2 to 0.5) |
| LOS = | Similar length of stay in the recovery area, the mean difference 0.6 min (95%CI: −8.2 to 9.3) | ||
| [61] | Peri-surgical ketamine in chronic opioid users. | RPI ↓ (during movement) | Low-quality evidence that ketamine may slightly reduce postoperative pain during movement after 24 h (mean difference: −0.79; 95% CI: −1.22 to −0.36) |
| TOC ↓ | Reduction of cumulative mean opioid consumption: by 97.3 mg (95%CI: −164.8 to −29.7) after 24 h by 186.4 mg (95%CI: −347.6 to −25.2) after 48 h |
3.3. Neurobiological Research on OIH in Acute and Chronic Non-Cancer Pain and Correlations between Bench and Bed-Side Findings
3.3.1. Neuroinflammation
3.3.2. Cellular and Molecular Alterations Involved in OIH
3.4. OIH in Cancer Pain and Challenges in the Duality between Suffering (“Pathos”) and Pathology
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Roeckel, L.A.; Le Coz, G.M.; Gavériaux-Ruff, C.; Simonin, F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016, 338, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Angst, M.S.; Clark, J.D. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. Opioid hyperalgesia. Curr. Opin. Support. Palliat. Care 2010, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guichard, L.; Hirve, A.; Demiri, M.; Martinez, V. Opioid-induced Hyperalgesia in Patients With Chronic Pain: A Systematic Review of Published Cases. Clin. J. Pain 2021, 38, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kum, E.; Buckley, N.; de Leon-Casasola, O.; Lema, M.; Busse, J.W. Attitudes Towards and Management of Opioid-induced Hyperalgesia: A Survey of Chronic Pain Practitioners. Clin. J. Pain 2020, 36, 359–364. [Google Scholar] [CrossRef]
- Vargas-Schaffer, G.; Paquet, S.; Neron, A.; Cogan, J. Opioid Induced Hyperalgesia, a Research Phenomenon or a Clinical Reality? Results of a Canadian Survey. J. Pers. Med. 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Suzan, E.; Pud, D. Opioid-Induced Hyperalgesia (OIH): A Real Clinical Problem or Just an Experimental Phenomenon? J. Pain Symptom Manag. 2015, 49, 632–636. [Google Scholar] [CrossRef]
- Angst, M.S.; Koppert, W.; Pahl, I.; Clark, D.J.; Schmelz, M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain 2003, 106, 49–57. [Google Scholar] [CrossRef]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef]
- Higgins, C.; Smith, B.H.; Matthews, K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: A systematic review and meta-analysis. Br. J. Anaesth. 2019, 122, e114–e126. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Araldi, D.; Bogen, O.; Green, P.G.; Levine, J.D. Systemic Morphine Produces Dose-dependent Nociceptor-mediated Biphasic Changes in Nociceptive Threshold and Neuroplasticity. Neuroscience 2019, 398, 64–75. [Google Scholar] [CrossRef]
- Jackson, D.; Singh, S.; Zhang-James, Y.; Faraone, S.; Johnson, B. The Effects of Low Dose Naltrexone on Opioid Induced Hyperalgesia and Fibromyalgia. Front. Psychiatry 2021, 12, 593842. [Google Scholar] [CrossRef] [PubMed]
- Toljan, K.; Vrooman, B. Low-Dose Naltrexone (LDN)-Review of Therapeutic Utilization. Med. Sci. 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Crain, S.M.; Shen, K.F. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc. Natl. Acad. Sci. USA 1995, 92, 10540–10544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Frankfurt, M.; Burns, L.H. High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence. PLoS ONE 2008, 3, e1554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballantyne, J.C. Opioids for the Treatment of Chronic Pain: Mistakes Made, Lessons Learned, and Future Directions. Anesth. Analg. 2017, 125, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Grelz, H.; Fischer, M.R.; Priouzifard, M.; Midlöv, P.; Ringqvist, Å. Prevalence of long-term opioid therapy in a chronic non-cancer pain population attending a university-based tertiary pain clinic in Sweden. A cross-sectional study. J. Rehabil. Med. 2022, 54. [Google Scholar] [CrossRef] [PubMed]
- van Amsterdam, J.; Pierce, M.; van den Brink, W. Is Europe Facing an Emerging Opioid Crisis Comparable to the U.S.? Ther. Drug Monit. 2021, 43, 42–51. [Google Scholar] [CrossRef]
- Castro-Lopes, J. Impact of chronic pain on primary care across Europe. J. Pain Palliat. Care Pharm. 2014, 28, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonça, L.; Dias, C.C.; Castro-Lopes, J.M. A population-based study on chronic pain and the use of opioids in Portugal. Pain 2013, 154, 2844–2852. [Google Scholar] [CrossRef] [PubMed]
- Veiga, D.R.; Monteiro-Soares, M.; Mendonça, L.; Sampaio, R.; Castro-Lopes, J.M.; Azevedo, L.F. Effectiveness of Opioids for Chronic Noncancer Pain: A Two-Year Multicenter, Prospective Cohort Study with Propensity Score Matching. J. Pain 2019, 20, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Veiga, D.R.; Mendonça, L.; Sampaio, R.; Castro-Lopes, J.M.; Azevedo, L.F. A Two-Year Prospective Multicenter Study of Opioid Therapy for Chronic Noncancer Pain: Prescription Trends and Predictors. Pain Med. 2019, 20, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharm. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef]
- Noble, M.; Treadwell, J.R.; Tregear, S.J.; Coates, V.H.; Wiffen, P.J.; Akafomo, C.; Schoelles, K.M. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010, 2010, Cd006605. [Google Scholar] [CrossRef]
- Fishman, S.M. Opioid side effects, addiction, and anti-inflammatory medications. J. Pain Palliat. Care Pharmacother. 2005, 19, 51–55. [Google Scholar] [CrossRef]
- O’Brien, T.; Christrup, L.L.; Drewes, A.M.; Fallon, M.T.; Kress, H.G.; McQuay, H.J.; Mikus, G.; Morlion, B.J.; Perez-Cajaraville, J.; Pogatzki-Zahn, E.; et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur. J. Pain 2017, 21, 3–19. [Google Scholar] [CrossRef]
- Compton, P.; Halabicky, O.M.; Aryal, S.; Badiola, I. Opioid Taper is Associated with Improved Experimental Pain Tolerance in Patients with Chronic Pain: An Observational Study. Pain Ther. 2022, 11, 303–313. [Google Scholar] [CrossRef]
- Oaks, Z.; Stage, A.; Middleton, B.; Faraone, S.; Johnson, B. Clinical utility of the cold pressor test: Evaluation of pain patients, treatment of opioid-induced hyperalgesia and fibromyalgia with low dose naltrexone. Discov. Med. 2018, 26, 197–206. [Google Scholar]
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef]
- Sumpton, J.E.; Moulin, D.E. Fibromyalgia. Handb. Clin. Neurol. 2014, 119, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Von Korff, M.; Duhrkoop, D. Opioids for low back pain. Bmj 2015, 350, g6380. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, M.; Maldonado, R.; Baños, J.E. Why mu-opioid agonists have less analgesic efficacy in neuropathic pain? Eur. J. Pain 2019, 23, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M. Opioid resistance in chronic daily headache: A synthesis of ideas from the bench and bedside. Curr. Pain Headache Rep. 2003, 7, 67–75. [Google Scholar] [CrossRef]
- Da Silva, A.N.; Lake, A.E., 3rd. Clinical aspects of medication overuse headaches. Headache 2014, 54, 211–217. [Google Scholar] [CrossRef]
- Hermans, L.; Nijs, J.; Calders, P.; De Clerck, L.; Moorkens, G.; Hans, G.; Grosemans, S.; Roman De Mettelinge, T.; Tuynman, J.; Meeus, M. Influence of Morphine and Naloxone on Pain Modulation in Rheumatoid Arthritis, Chronic Fatigue Syndrome/Fibromyalgia, and Controls: A Double-Blind, Randomized, Placebo-Controlled, Cross-Over Study. Pain Prcatice 2018, 18, 418–430. [Google Scholar] [CrossRef]
- Wang, H.; Akbar, M.; Weinsheimer, N.; Gantz, S.; Schiltenwolf, M. Longitudinal observation of changes in pain sensitivity during opioid tapering in patients with chronic low-back pain. Pain Med. 2011, 12, 1720–1726. [Google Scholar] [CrossRef]
- Younger, J.; Mackey, S. Fibromyalgia symptoms are reduced by low-dose naltrexone: A pilot study. Pain Med. 2009, 10, 663–672. [Google Scholar] [CrossRef]
- Brinck, E.C.V.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2018, 12, Cd012033. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Wang, H.; Xie, K.; Shu, R.; Zhang, L.; Hu, N.; Yu, Y.; Wang, G. Inhibition of DOR prevents remifentanil induced postoperative hyperalgesia through regulating the trafficking and function of spinal NMDA receptors in vivo and in vitro. Brain Res. Bull. 2015, 110, 30–39. [Google Scholar] [CrossRef]
- Rieb, L.M.; Norman, W.V.; Martin, R.E.; Berkowitz, J.; Wood, E.; Milloy, M.J.; McNeil, R. Linking opioid-induced hyperalgesia and withdrawal-associated injury site pain: A case report. Pain Rep. 2018, 3, e648. [Google Scholar] [CrossRef] [PubMed]
- Varastehmoradi, B.; Wegener, G.; Sanchez, C.; Smith, K.L. Opioid system modulation of cognitive affective bias: Implications for the treatment of mood disorders. Behav. Pharm. 2020, 31, 122–135. [Google Scholar] [CrossRef]
- Navratilova, E.; Ji, G.; Phelps, C.; Qu, C.; Hein, M.; Yakhnitsa, V.; Neugebauer, V.; Porreca, F. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain 2019, 160, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Tavares, I.; Costa-Pereira, J.T.; Martins, I. Monoaminergic and Opioidergic Modulation of Brainstem Circuits: New Insights Into the Clinical Challenges of Pain Treatment? Front. Pain Res. 2021, 2, 696515. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Harper, D.E.; Harte, S.E.; Wang, H.; Ichesco, E.; Hampson, J.P.; Zubieta, J.K.; Clauw, D.J.; Harris, R.E. Endogenous opioidergic dysregulation of pain in fibromyalgia: A PET and fMRI study. Pain 2016, 157, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.F.; D’Arcy, N.; Brady, C.; Zamora, A.K.; Young, C.A.; Kim, J.E.; Clemenson, A.M.; Angst, M.S.; Clark, D.J. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: A double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain 2012, 153, 1583–1592. [Google Scholar] [CrossRef]
- Baranidharan, G.; Feltbower, R.; Bretherton, B.; Crowther, T.; Cooper, L.; Castino, P.; Radford, H. One-Year Results of Prospective Research Study Using 10 kHz Spinal Cord Stimulation in Persistent Nonoperated Low Back Pain of Neuropathic Origin: Maiden Back Study. Neuromodulation 2021, 24, 479–487. [Google Scholar] [CrossRef]
- Biskup, M.; Dzioba, A.; Sowerby, L.J.; Monteiro, E.; Strychowsky, J. Opioid prescribing practices following elective surgery in Otolaryngology-Head & Neck Surgery. J. Otolaryngol.-Head Neck Surg. 2019, 48, 29. [Google Scholar] [CrossRef]
- Clotz, M.A.; Nahata, M.C. Clinical uses of fentanyl, sufentanil, and alfentanil. Clin. Pharm. 1991, 10, 581–593. [Google Scholar] [CrossRef]
- Frauenknecht, J.; Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: A systematic review and meta-analysis. Anaesthesia 2019, 74, 651–662. [Google Scholar] [CrossRef]
- Neuman, M.D.; Bateman, B.T.; Wunsch, H. Inappropriate opioid prescription after surgery. Lancet 2019, 393, 1547–1557. [Google Scholar] [CrossRef]
- Manchikanti, L.; Helm, S., 2nd; Fellows, B.; Janata, J.W.; Pampati, V.; Grider, J.S.; Boswell, M.V. Opioid epidemic in the United States. Pain Physician 2012, 15, Es9–Es38. [Google Scholar] [CrossRef]
- Young, J.C.; Dasgupta, N.; Chidgey, B.A.; Stürmer, T.; Pate, V.; Hudgens, M.; Jonsson Funk, M. Impacts of Initial Prescription Length and Prescribing Limits on Risk of Prolonged Postsurgical Opioid Use. Med. Care 2022, 60, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, T.A.; Robinson, R. Multimodal Analgesia in the Era of the Opioid Epidemic. Surg. Clin. N. Am. 2022, 102, 105–115. [Google Scholar] [CrossRef]
- Shanthanna, H.; Ladha, K.S.; Kehlet, H.; Joshi, G.P. Perioperative Opioid Administration. Anesthesiology 2021, 134, 645–659. [Google Scholar] [CrossRef]
- McCurdy, M.A.; Burt, C.I.; Schneider, M.B.; Zhang, T.; Foster, M.J.; Aneizi, A.; Gilotra, M.N.; Hasan, S.A.; Henn Iii, R.F. Preoperative opioid use correlates with worse patient-reported outcomes two years after elective shoulder surgery. J. Orthop. 2021, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Yahiaoui-Doktor, M.; Meissner, W.; Zahn, P.K.; Pogatzki-Zahn, E.M. Predicting poor postoperative acute pain outcome in adults: An international, multicentre database analysis of risk factors in 50,005 patients. Pain Rep. 2020, 5, e831. [Google Scholar] [CrossRef] [PubMed]
- Hina, N.; Fletcher, D.; Poindessous-Jazat, F.; Martinez, V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: An observational case-control study. Eur. J. Anaesthesiol. 2015, 32, 255–261. [Google Scholar] [CrossRef]
- Angst, M.S.; Clark, J.D. Ketamine for managing perioperative pain in opioid-dependent patients with chronic pain: A unique indication? Anesthesiology 2010, 113, 514–515. [Google Scholar] [CrossRef]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef]
- Meyer-Frießem, C.H.; Lipke, E.; Weibel, S.; Kranke, P.; Reichl, S.; Pogatzki-Zahn, E.M.; Zahn, P.K.; Schnabel, A. Perioperative ketamine for postoperative pain management in patients with preoperative opioid intake: A systematic review and meta-analysis. J. Clin. Anesth. 2022, 78, 110652. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, H.W.; Kim, J.N. Effect of oral pregabalin on opioid-induced hyperalgesia in patients undergoing laparo-endoscopic single-site urologic surgery. Korean J. Anesth. 2013, 64, 19–24. [Google Scholar] [CrossRef][Green Version]
- Mercieri, M.; Palmisani, S.; De Blasi, R.A.; D’Andrilli, A.; Naccarato, A.; Silvestri, B.; Tigano, S.; Massullo, D.; Rocco, M.; Arcioni, R. Low-dose buprenorphine infusion to prevent postoperative hyperalgesia in patients undergoing major lung surgery and remifentanil infusion: A double-blind, randomized, active-controlled trial. Br. J. Anaesth. 2017, 119, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Richebé, P.; Pouquet, O.; Jelacic, S.; Mehta, S.; Calderon, J.; Picard, W.; Rivat, C.; Cahana, A.; Janvier, G. Target-controlled dosing of remifentanil during cardiac surgery reduces postoperative hyperalgesia. J. Cardiothorac. Vasc. Anesth. 2011, 25, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Sun, Z.; Shadhiya, F.; Arulthas, R.; Priya, G.V.; Christopher, P.; Muhammad, Z.; Yu, Y. The influence of dexmedetomidine on remifentanil-induced hyperalgesia and the sex differences. Exp. Med. 2018, 16, 3596–3602. [Google Scholar] [CrossRef]
- Hong, B.H.; Lee, W.Y.; Kim, Y.H.; Yoon, S.H.; Lee, W.H. Effects of intraoperative low dose ketamine on remifentanil-induced hyperalgesia in gynecologic surgery with sevoflurane anesthesia. Korean J. Anesth. 2011, 61, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Contreras, F.; Blanch, A.; Bravo, D.; Egaña, J.I.; Rappoport, D.; Cabané, P.; Rodríguez, F.; Penna, A. Remifentanil-Induced Secondary Hyperalgesia Is Not Prevented By Preoperative Acetazolamide Administration In Patients Undergoing Total Thyroidectomy: A Randomized Controlled Trial. J. Pain Res. 2019, 12, 2991–2997. [Google Scholar] [CrossRef]
- Choi, E.; Lee, H.; Park, H.S.; Lee, G.Y.; Kim, Y.J.; Baik, H.J. Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia. Korean J. Anesth. 2015, 68, 476–480. [Google Scholar] [CrossRef]
- Hang, L.H.; Shao, D.H.; Gu, Y.P. The ED50 and ED95 of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy. Swiss Med. Wkly. 2011, 141, w13195. [Google Scholar] [CrossRef]
- Lee, M.H.; Chung, M.H.; Han, C.S.; Lee, J.H.; Choi, Y.R.; Choi, E.M.; Lim, H.K.; Cha, Y.D. Comparison of effects of intraoperative esmolol and ketamine infusion on acute postoperative pain after remifentanil-based anesthesia in patients undergoing laparoscopic cholecystectomy. Korean J. Anesth. 2014, 66, 222–229. [Google Scholar] [CrossRef][Green Version]
- Rupniewska-Ladyko, A.; Malec-Milewska, M. A High Dose of Fentanyl May Accelerate the Onset of Acute Postoperative Pain. Anesth. Pain Med. 2019, 9, e94498. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Sikandar, S.; Bauer, C.S.; Dolphin, A.C.; Porreca, F.; Dickenson, A.H. Pregabalin suppresses spinal neuronal hyperexcitability and visceral hypersensitivity in the absence of peripheral pathophysiology. Anesthesiology 2011, 115, 144–152. [Google Scholar] [CrossRef]
- Soffin, E.M.; Wetmore, D.S.; Beckman, J.D.; Sheha, E.D.; Vaishnav, A.S.; Albert, T.J.; Gang, C.H.; Qureshi, S.A. Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: A retrospective matched cohort study. Neurosurg. Focus 2019, 46, E8. [Google Scholar] [CrossRef] [PubMed]
- Kars, M.S.; Villacres Mori, B.; Ahn, S.; Merwin, S.; Wendolowski, S.; Gecelter, R.; Rothman, A.; Poon, S. Fentanyl versus remifentanil-based TIVA for pediatric scoliosis repair: Does it matter? Reg. Anesth. Pain Med. 2019, 44, 627–631. [Google Scholar] [CrossRef]
- Martins, I.; Tavares, I. Reticular Formation and Pain: The Past and the Future. Front. Neuroanat. 2017, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.; Almeida, A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Prog. Neurobiol. 2002, 66, 81–108. [Google Scholar] [CrossRef]
- Costa, A.R.; Carvalho, P.; Flik, G.; Wilson, S.P.; Reguenga, C.; Martins, I.; Tavares, I. Neuropathic Pain Induced Alterations in the Opioidergic Modulation of a Descending Pain Facilitatory Area of the Brain. Front. Cell. Neurosci. 2019, 13, 287. [Google Scholar] [CrossRef]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav. Immun. 2018, 72, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Berta, T.; Liu, T.; Tan, P.H.; Ji, R.R. Acute morphine induces matrix metalloproteinase-9 up-regulation in primary sensory neurons to mask opioid-induced analgesia in mice. Mol. Pain 2012, 8, 19. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Lewis, S.S.; Coats, B.D.; Rezvani, N.; Zhang, Y.; Wieseler, J.L.; Somogyi, A.A.; Yin, H.; Maier, S.F.; Rice, K.C.; et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 2010, 167, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, V.; Sidorova, Y.; Viisanen, H.; Suleymanova, I.; Tiilikainen, H.; Li, Z.; Lilius, T.O.; Mätlik, K.; Anttila, J.E.; Airavaara, M.; et al. Differential Spinal and Supraspinal Activation of Glia in a Rat Model of Morphine Tolerance. Neuroscience 2018, 375, 10–24. [Google Scholar] [CrossRef]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Urban, D.J.; Wang, X.; Baratta, M.V.; Fabisiak, T.J.; Anderson, N.D.; Cheng, K.; Greene, L.I.; et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E3441–E3450. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Chen, L.; Gong, X.; Ma, B.; Gan, W.; Si, Y.; Xiao, H.; Li, C. Upregulation of P2X2 and P2X3 receptors in rats with hyperalgesia induced by heroin withdrawal. Neuroreport 2018, 29, 678–684. [Google Scholar] [CrossRef]
- Doyle, T.M.; Largent-Milnes, T.M.; Chen, Z.; Staikopoulos, V.; Esposito, E.; Dalgarno, R.; Fan, C.; Tosh, D.K.; Cuzzocrea, S.; Jacobson, K.A.; et al. Chronic Morphine-Induced Changes in Signaling at the A(3) Adenosine Receptor Contribute to Morphine-Induced Hyperalgesia, Tolerance, and Withdrawal. J. Pharm. Exp. 2020, 374, 331–341. [Google Scholar] [CrossRef]
- White, F.; Wilson, N. Opiate-induced hypernociception and chemokine receptors. Neuropharmacology 2010, 58, 35–37. [Google Scholar] [CrossRef]
- Wilson, N.M.; Jung, H.; Ripsch, M.S.; Miller, R.J.; White, F.A. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav. Immun. 2011, 25, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Janes, K.; Chen, Z.; Grace, P.M.; Esposito, E.; Cuzzocrea, S.; Largent-Milnes, T.M.; Neumann, W.L.; Watkins, L.R.; Spiegel, S.; et al. Activation of sphingosine-1-phosphate receptor subtype 1 in the central nervous system contributes to morphine-induced hyperalgesia and antinociceptive tolerance in rodents. Pain 2020, 161, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The blockade of CC chemokine receptor type 1 influences the level of nociceptive factors and enhances opioid analgesic potency in a rat model of neuropathic pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; He, Q.; Matsuda, M.; Han, Q.; Wang, K.; Bang, S.; Ding, H.; Ko, M.C.; Ji, R.R. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci. Transl. Med. 2020, 12, eaaw6471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Z.; Donnelly, C.R.; Wang, K.; Andriessen, A.S.; Tao, X.; Matsuda, M.; Zhao, J.; Ji, R.R. PD-1 Regulates GABAergic Neurotransmission and GABA-Mediated Analgesia and Anesthesia. Iscience 2020, 23, 101570. [Google Scholar] [CrossRef] [PubMed]
- Drdla-Schutting, R.; Benrath, J.; Wunderbaldinger, G.; Sandkühler, J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science 2012, 335, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Favorov, O.V.; Tommerdahl, M.; Lee, C.J.; Whitsel, B.L. Attenuated Glial K(+) Clearance Contributes to Long-Term Synaptic Potentiation Via Depolarizing GABA in Dorsal Horn Neurons of Rat Spinal Cord. Exp. Neurobiol. 2014, 23, 53–64. [Google Scholar] [CrossRef][Green Version]
- Liu, J.P.; He, Y.T.; Duan, X.L.; Suo, Z.W.; Yang, X.; Hu, X.D. Enhanced Activities of δ Subunit-containing GABA(A) Receptors Blocked Spinal Long-term Potentiation and Attenuated Formalin-induced Spontaneous Pain. Neuroscience 2018, 371, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Chartoff, E.H.; Connery, H.S. It’s MORe exciting than mu: Crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front. Pharmacol. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, W.; Lei, Y.; Cui, Y.; Chu, S.; Gu, X.; Ma, Z. Activation of spinal alpha-7 nicotinic acetylcholine receptor shortens the duration of remifentanil-induced postoperative hyperalgesia by upregulating KCC2 in the spinal dorsal horn in rats. Mol. Pain 2017, 13, 1744806917704769. [Google Scholar] [CrossRef]
- Van Berkel, M.A.; Elefritz, J.L. Evaluating off-label uses of acetazolamide. Bull. Am. Soc. Hosp. Pharm. 2018, 75, 524–531. [Google Scholar] [CrossRef]
- Costa, A.R.; Sousa, M.; Wilson, S.P.; Reguenga, C.; Teixeira-Pinto, A.; Tavares, I.; Martins, I. Shift of µ-opioid Receptor Signaling in the Dorsal Reticular Nucleus Is Implicated in Morphine-induced Hyperalgesia in Male Rats. Anesthesiology 2020, 133, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharm. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef]
- Gorosito, S.V.; Cambiasso, M.J. Axogenic effect of estrogen in male rat hypothalamic neurons involves Ca(2+), protein kinase C, and extracellular signal-regulated kinase signaling. J. Neurosci. Res. 2008, 86, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Carvalho, P.; de Vries, M.G.; Teixeira-Pinto, A.; Wilson, S.P.; Westerink, B.H.; Tavares, I. Increased noradrenergic neurotransmission to a pain facilitatory area of the brain is implicated in facilitation of chronic pain. Anesthesiology 2015, 123, 642–653. [Google Scholar] [CrossRef]
- Busserolles, J.; Lolignier, S.; Kerckhove, N.; Bertin, C.; Authier, N.; Eschalier, A. Replacement of current opioid drugs focusing on MOR-related strategies. Pharmacol. Ther. 2020, 210, 107519. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates mu Opioid Receptor Functional Properties in vivo. Front. Pharm. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, M.; Massotte, D. Therapeutic potential of opioid receptor heteromers in chronic pain and associated comorbidities. Br. J. Pharm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; He, Q.H.; Han, J.S.; Su, L.; Wan, Y. Heteromerization of μ-opioid receptor and cholecystokinin B receptor through the third transmembrane domain of the μ-opioid receptor contributes to the anti-opioid effects of cholecystokinin octapeptide. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Smith, H.S. Opioids and neuropathic pain. Pain Physician 2012, 15, Es93–Es110. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Bierbower, S.M.; Eskander, M.A.; Szteyn, K.; Por, E.D.; Gomez, R.; Veldhuis, N.; Bunnett, N.W.; Jeske, N.A. Activation of mu opioid receptors sensitizes transient receptor potential vanilloid type 1 (TRPV1) via β-arrestin-2-mediated cross-talk. PLoS ONE 2014, 9, e93688. [Google Scholar] [CrossRef]
- Narayan, A.; Hunkele, A.; Xu, J.; Bassoni, D.L.; Pasternak, G.W.; Pan, Y.X. Mu Opioids Induce Biased Signaling at the Full-Length Seven Transmembrane C-Terminal Splice Variants of the mu Opioid Receptor Gene, Oprm1. Cell. Mol. Neurobiol. 2021, 41, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, M.; Brown, T.; Rossi, G.C.; Hurd, Y.L.; Inturrisi, C.E.; Pasternak, G.W.; Pan, Y.-X. Stabilization of the μ-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J. Biol. Chem. 2013, 288, 21211–21227. [Google Scholar] [CrossRef] [PubMed]
- Bolan, E.A.; Pan, Y.X.; Pasternak, G.W. Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse 2004, 51, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Zhang, W.; Jin, X.; Liu, Y.; Xu, S.; Lei, L.; Shen, X.; Guo, X.; Xia, X.; et al. Opioid-induced redistribution of 6TM and 7TM μ opioid receptors: A hypothesized mechanistic facilitator model of opioid-induced hyperalgesia. Pharm. Rep. 2016, 68, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, F.A.; Conrad, M.S.; O’Buckley, S.C.; Rashid, N.U.; Slade, G.D.; Nackley, A.G. Mu Opioid Splice Variant MOR-1K Contributes to the Development of Opioid-Induced Hyperalgesia. PLoS ONE 2015, 10, e0135711. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, M.; Sánchez-Blázquez, P.; Vicente-Sánchez, A.; Berrocoso, E.; Garzón, J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: Implications in pain control. Neuropsychopharmacology 2012, 37, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Zhao, X.; Shen, S.; Luo, X.; Zhang, Y. Association between MDR(1)/CYP3A4/OPRM(1) gene polymorphisms and the post-caesarean fentanyl analgesic effect on Chinese women. Gene 2018, 661, 78–84. [Google Scholar] [CrossRef]
- Guastella, A.; Latchman, J.; Tofthagen, C.S. Opioid-Induced Hyperalgesia: Clinical Implications for Advanced Practice Nurses in Oncology. Clin. J. Oncol. Nurs. 2017, 21, 294–296. [Google Scholar] [CrossRef]
- Candido, K.D.; Kusper, T.M.; Knezevic, N.N. New Cancer Pain Treatment Options. Curr. Pain Headache Rep. 2017, 21, 12. [Google Scholar] [CrossRef]
- Chazan, S.; Ekstein, M.P.; Marouani, N.; Weinbroum, A.A. Ketamine for acute and subacute pain in opioid-tolerant patients. J. Opioid Manag. 2008, 4, 173–180. [Google Scholar] [CrossRef]
- Omar, E.; Wallon, G.; Bauer, C.; Axiotis, G.; Bouix, C.; Soubirou, J.L.; Aubrun, F. Evaluation of intravenous lidocaine in head and neck cancer surgery: Study protocol for a randomized controlled trial. Trials 2019, 20, 220. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Ribeiro, J.; Martins, I.; Tavares, I. Role of Spinal Cord α(2)-Adrenoreceptors in Noradrenergic Inhibition of Nociceptive Transmission During Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2019, 13, 1413. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Serrão, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2020, 51, 1756–1769. [Google Scholar] [CrossRef]
- Liang, D.Y.; Li, X.; Clark, J.D. 5-hydroxytryptamine type 3 receptor modulates opioid-induced hyperalgesia and tolerance in mice. Anesthesiology 2011, 114, 1180–1189. [Google Scholar] [CrossRef]
- Inacio, M.C.S.; Hansen, C.; Pratt, N.L.; Graves, S.E.; Roughead, E.E. Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: A retrospective cohort study. BMJ Open 2016, 6, e010664. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio-Cunha, T.J.; Martins, I. Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia. J. Clin. Med. 2022, 11, 6161. https://doi.org/10.3390/jcm11206161
Sampaio-Cunha TJ, Martins I. Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia. Journal of Clinical Medicine. 2022; 11(20):6161. https://doi.org/10.3390/jcm11206161
Chicago/Turabian StyleSampaio-Cunha, Tiago J., and Isabel Martins. 2022. "Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia" Journal of Clinical Medicine 11, no. 20: 6161. https://doi.org/10.3390/jcm11206161
APA StyleSampaio-Cunha, T. J., & Martins, I. (2022). Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia. Journal of Clinical Medicine, 11(20), 6161. https://doi.org/10.3390/jcm11206161






