Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience
Abstract
1. Introduction
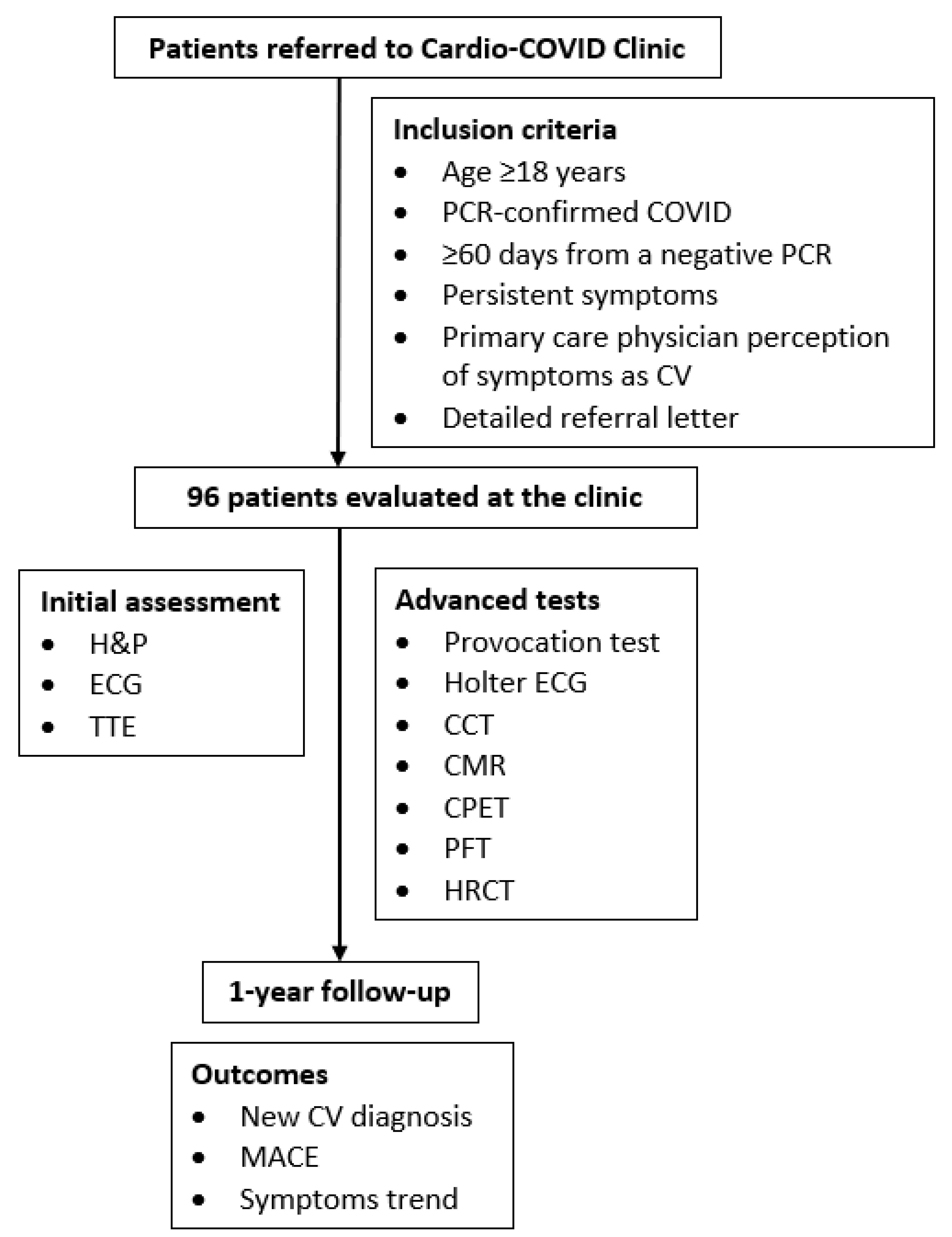
2. Methods
2.1. Study Design and Participants
2.2. Assessment and Follow-Up
2.3. Outcomes and Exposures
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
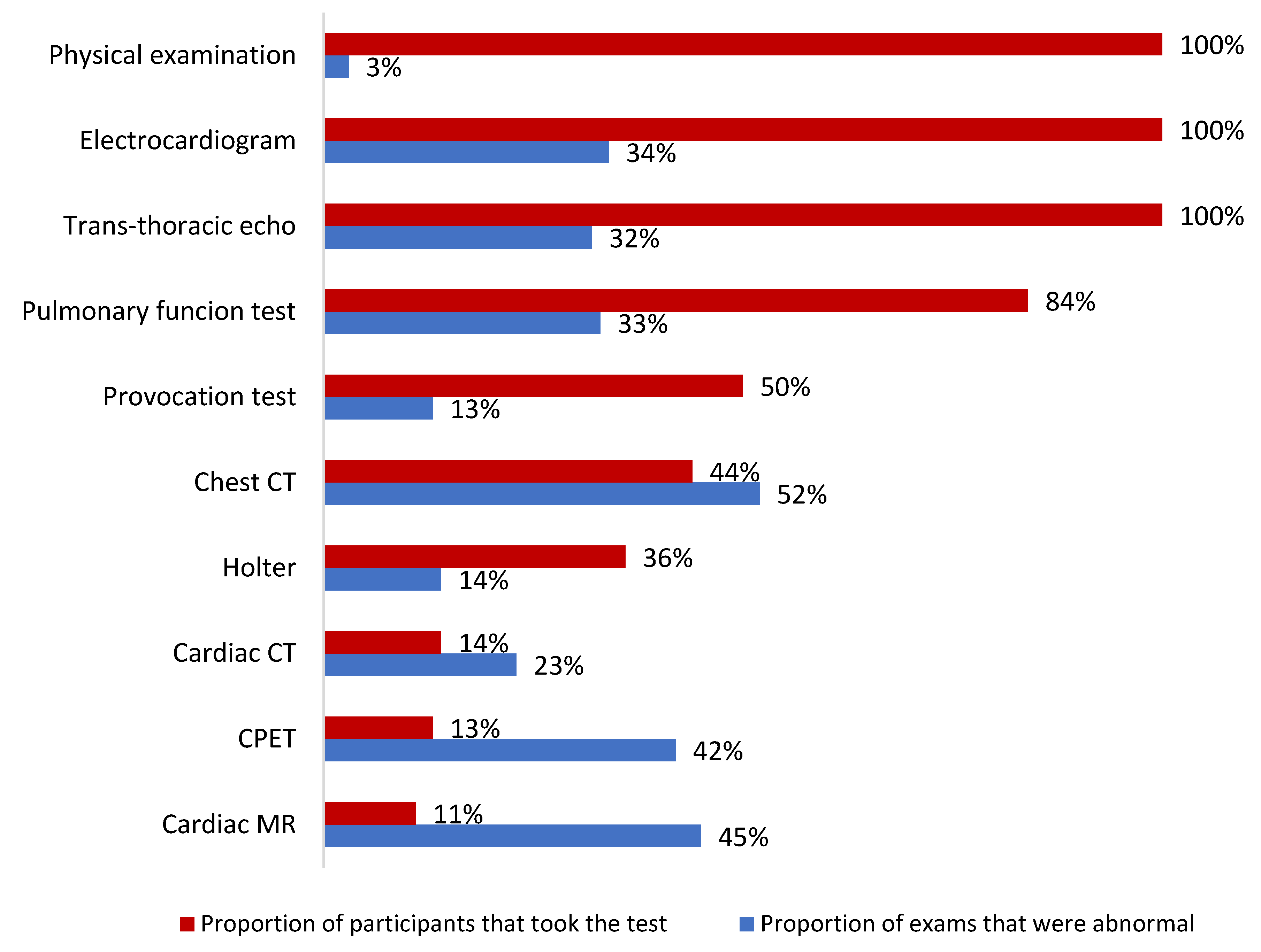
3.2. Long-COVID Manifestations and Findings
3.3. Cardiovascular Morbidity
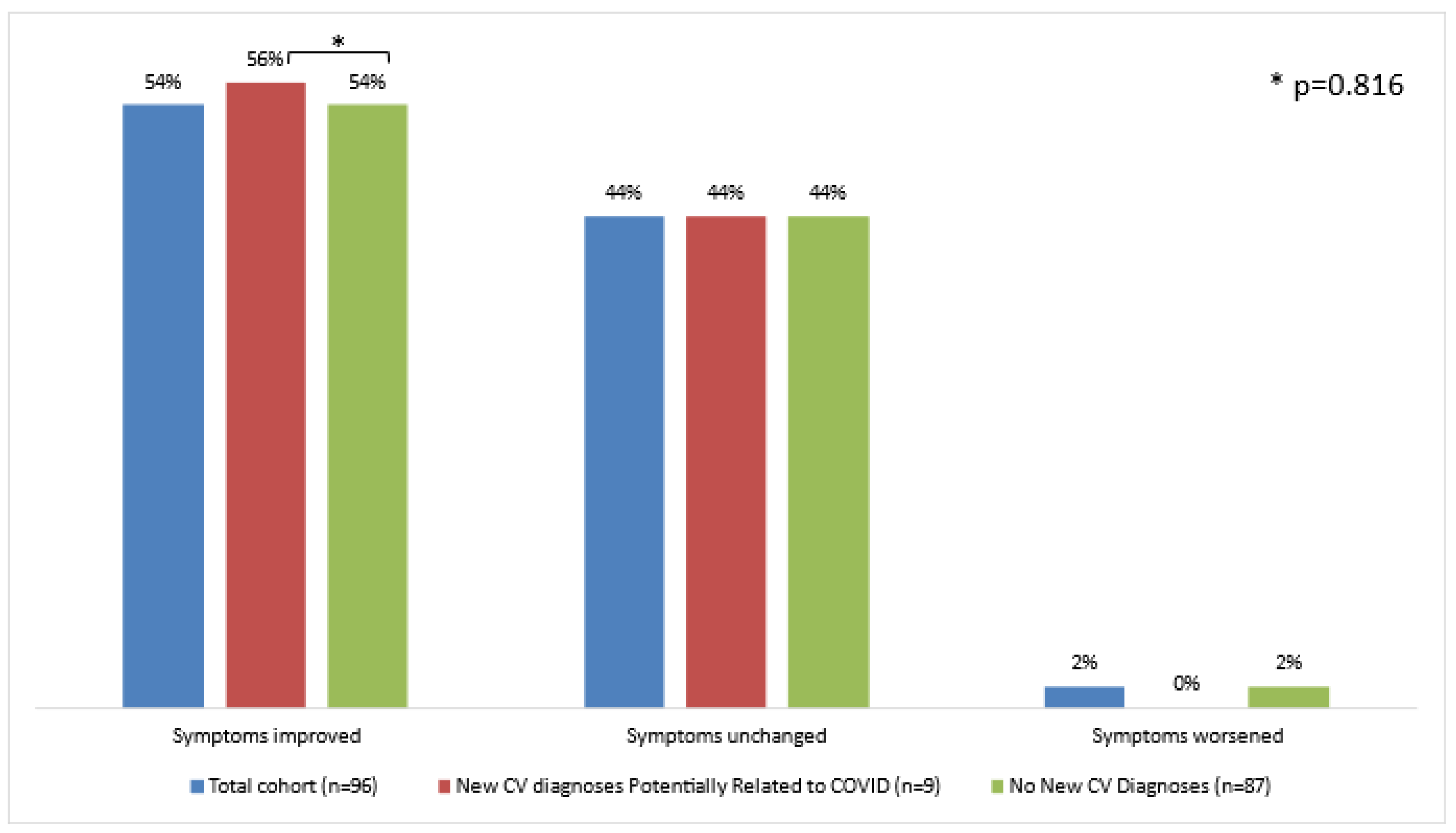
3.4. Symptomatic Course
3.5. Predictors of Presumably COVID-Related New CV Diagnoses and Symptom Non-Improvement
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, J.H.; Liu, Y.X.; Yuan, J.; Wang, F.X.; Wu, W.B.; Li, J.X.; Wang, L.F.; Gao, H.; Wang, Y.; Dong, C.F.; et al. First Case of COVID-19 Infection with Fulminant Myocarditis Complication: Case Report and Insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Kim, I.-C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 2020, 324, 799–801. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- O’Brien, C.; Ning, N.; McAvoy, J.; Mitchell, J.E.; Kalwani, N.; Wang, P.; Nguyen, D.; Reejhsinghani, R.; Rogers, A.; Lorenzo, J. Electrical Storm in COVID-19. JACC Case Rep. 2020, 2, 1256–1260. [Google Scholar] [CrossRef]
- Cimino, G.; Pascariello, G.; Bernardi, N.; Calvi, E.; Arabia, G.; Salghetti, F.; Bontempi, L.; Vizzardi, E.; Metra, M.; Curnis, A. Sinus Node Dysfunction in a Young Patient With COVID-19. JACC Case Rep. 2020, 2, 1240–1244. [Google Scholar] [CrossRef]
- Minhas, A.S.; Scheel, P.; Garibaldi, B.; Liu, G.; Horto, M.; Jennings, M.; Jones, S.R.; Michos, E.D.; Hays, A.G. Takotsubo Syndrome in the Setting of COVID-19 Infection. J. Am. Coll. Cardiol. Case Rep. 2020, 2, 1321–1325. [Google Scholar]
- Giustino, G.; Croft, L.B.; Oates, C.P.; Rahman, K.; Lerakis, S.; Reddy, V.Y.; Goldman, M. Takotsubo Cardiomyopathy in COVID-19. J. Am. Coll. Cardiol. 2020, 76, 628–629. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of Viral SARS-CoV-2 Genomes and Histopathological Changes in Endomyocardial Biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Fox, S.E.; Li, G.; Akmatbekov, A.; Harbert, J.L.; Lameira, F.S.; Brown, J.Q.; Heide, R.S.V. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation 2020, 142, 1123–1125. [Google Scholar] [CrossRef]
- Halpin, S.J.; Mclvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and Rehabilitation Needs in Survivors of COVID-19 Infection. A Cross-Sectional Evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, Signs and Symptoms, and Criteria Adopted for Long COVID-19: A Systematic Review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2020, 27, 89–95. [Google Scholar] [CrossRef]
- Nehme, M.; Braillard, O.; Alcoba, G.; Perone, S.A.; Courvoisier, D.; Chappuis, F.; Guessous, I. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Ann. Intern. Med. 2021, 174, 723–772. [Google Scholar] [CrossRef]
- The Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Eiros, R.; Barreiro-Perez, M.; Martin-Garcia, A.; Almeida, J.; Villacorta, E.; Perez-Pons, A.; Merchan, S.; Torres-Valle, A.; Pablo, C.S.; González-Calle, D.; et al. Pericarditis and Myocarditis Long After SARS-CoV-2 infection: A Cross-Sectional Descriptive Study in Health-care Workers. medRxiv 2020. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 1 April 2022).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, H.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.; Gilboe, H.; Einvik, G. Prevalence and Determinants of Fatigue after COVID-19 in Non-Hospitalized Subjects: A Population-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 2030. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. eClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Estabragh, Z.R.; Mamas, M.A. The cardiovascular manifestations of influenza: A systematic review. Int. J. Cardiol. 2013, 167, 2397–2403. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Habtemicael, Y.G.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, O.M.; Nadruz, W., Jr.; Querejeta Roca, G.; Claggett, B.; Solomon, S.D.; Mirabelli, M.C.; London, S.J.; Loehr, L.R.; Shah, A.M. Declining Lung Function and Cardiovascular Risk: The ARIC Study. J. Am. Coll. Cardiol. 2018, 72, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort (n = 96) | |
|---|---|
| Demographic Details | |
| Age, years | 54 (44–64) |
| Male | 44 (46) |
| Cardiovascular Background | |
| Prior CV Disease | 15 (16) |
| CV Risk Factors | |
| Any | 69 (72) |
| Pre-diabetes/Diabetes Mellitus | 34 (35) |
| Hypertension | 22 (23) |
| Dyslipidemia | 56 (58) |
| Obesity | 30 (31) |
| Present Smoking | 10 (10) |
| Acute Covid Data | |
| Symptoms During Acute COVID | 95 (99) |
| Acute COVID Severity, per NIH Criteria | |
| Mild | 40 (42) |
| Moderate | 30 (31) |
| Severe | 26 (27) |
| Hospitalization | |
| Frequency | 28 (29) |
| Length, days | 8 (5–18) |
| Invasive Ventilation | 1 (3.4) |
| Abnormal Tests Results | |
| ECG | 10 (45) |
| TTE | 2 (25) |
| Serum Biomarkers (hs-cTn ± NT-proBNP) | 3 (13) |
| Time from COVID Diagnosis to Examination, days | 142 (111–197) |
| COVID Vaccination Doses | |
| 0 | 8 (10) |
| 1 | 27 (35) |
| 2 | 42 (55) |
| Total Cohort (n = 96) | |
|---|---|
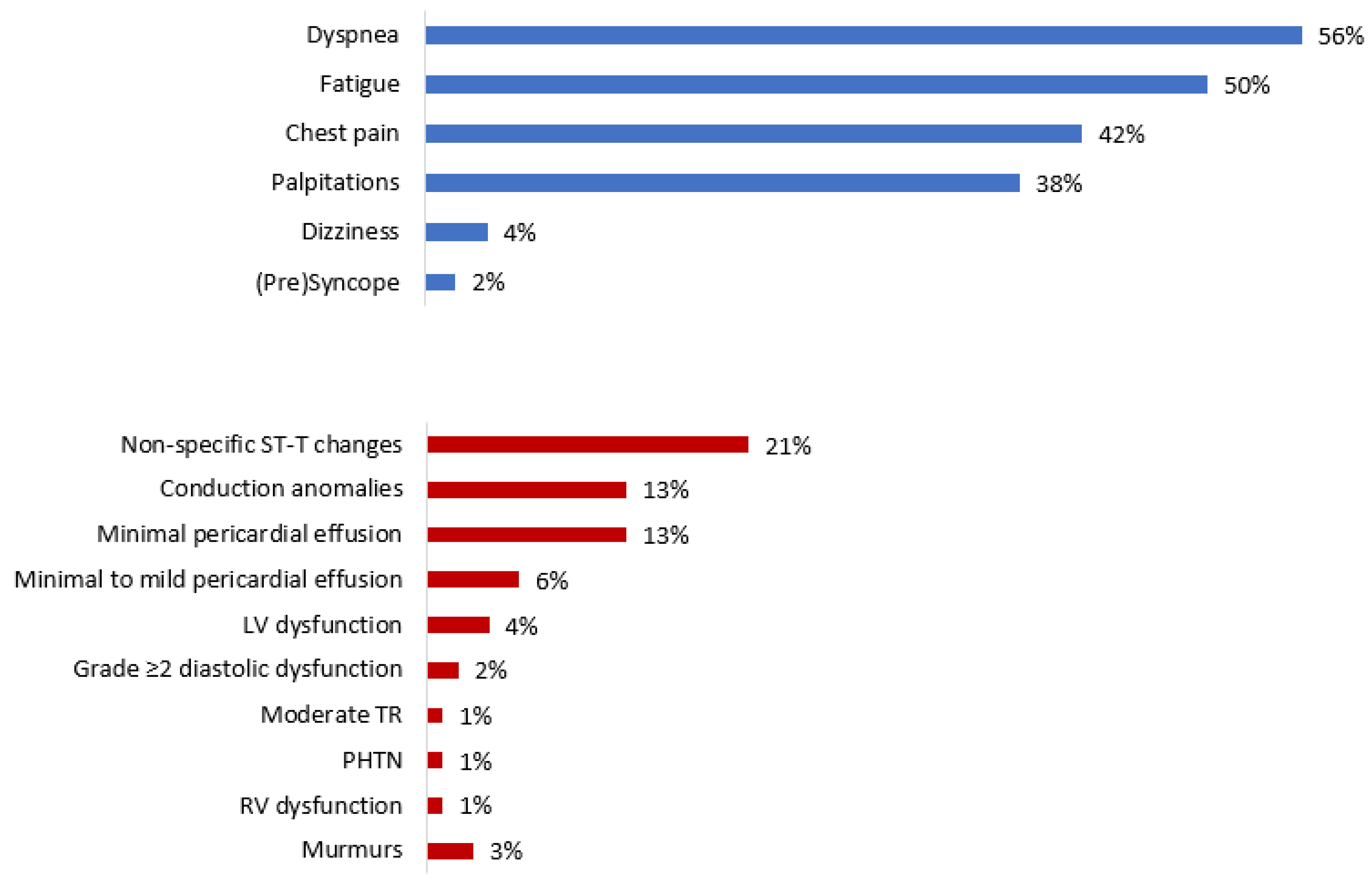
| Symptoms | |
| Any | 96 (100) |
| Fatigue | 48 (50) |
| Dyspnea | 54 (56) |
| Chest Pain | 40 (42) |
| Palpitations | 36 (38) |
| Dizziness/Vertigo | 4 (4) |
| Pre-syncope/Syncope | 2 (2) |
| NYHA Class Functional Status | |
| I | 43 (45) |
| II | 41 (43) |
| III | 12 (12) |
| IV | 0 (0) |
| ECG Findings | |
| Any | 32 (34) |
| Rhythm Disorders | 3 (3) |
| Rate Disorders | 0 (0) |
| Conduction Disorders | 12 (13) |
| ST-T changes | 20 (21) |
| TTE Findings | |
| Any | 31 (32) |
| LV Systolic Dysfunction | 4 (4) |
| RV Systolic Dysfunction | 1 (1) |
| Grade2 and up Diastolic Dysfunction | 2 (2) |
| LA Dilatation | 6 (6) |
| Moderate and up Valvular Dysfunction | 1 (1) |
| Systolic Pulmonary Hypertension | 1 (1) |
| Pericardial Effusion | 18 (19) |
| Cardiac Provocation Test Findings | |
| Any | 6 (13) |
| Ischemic Findings | 2 (4) |
| Atrial Fibrillation | 1 (2) |
| Chronotropic Incompetence | 3 (6) |
| Age-Adjusted CV Fitness | |
| Good | 15 (39) |
| Average | 14 (36) |
| Low | 10 (26) |
| Holter ECG findings | |
| Any | 5 (14) |
| Inappropriate Sinus Tachycardia | 1 (3) |
| Atrial Tachycardia | 1 (3) |
| Non-Sustained Ventricular Tachycardia | 1 (3) |
| Couplet Ventricular Premature Beats | 1 (3) |
| 1st degree AV block | 1 (3) |
| CCT findings | |
| Any | 3 (23) |
| Non-Obstructive Coronary Artery Disease | 2 (15) |
| Aberrant Coronary Artery | 1 (8) |
| CMR findings | |
| Any | 5 (45) |
| Myocarditis | 3 (27) |
| Myopericarditis | 1 (9) |
| Hypertrophic obstructive cardiomyopathy | 1 (9) |
| CPET findings | |
| CV Restraint | 5 (42) |
| PFT findings | |
| Any | 27 (33) |
| Obstruction | 13 (16) |
| Restriction | 5 (6) |
| Reduced Diffusing Capacity | 9 (11) |
| HRCT findings | |
| Any | 22 (51) |
| Interstitial Changes | 9 (21) |
| Fibrotic Changes | 4 (10) |
| Ground Glass Opacities | 7 (17) |
| Lung Nodules | 2 (5) |
| New CV Diagnoses (n = 9) | No New CV Diagnoses (n = 87) | p-Value | |
|---|---|---|---|
| Demographic Details | |||
| Age, years | 52 (41–54) | 56 (45–66) | 0.139 |
| Male | 2 (22) | 42 (48) | 0.173 |
| Cardiovascular Background | |||
| Prior CV Disease | 0 (0) | 15 (17) | 0.346 |
| CV Risk Factors | |||
| Any | 5 (56) | 64 (74) | 0.263 |
| Pre-diabetes/Diabetes Mellitus | 1 (11) | 33 (38) | 0.152 |
| Hypertension | 2 (22) | 20 (23) | 0.949 |
| Dyslipidemia | 2 (22) | 54 (62) | 0.069 |
| Obesity | 4 (44) | 26 (30) | 0.454 |
| Present Smoking | 1 (11) | 9 (10) | 1.000 |
| Acute COVID Data | |||
| Symptoms During Acute COVID | 9 (100) | 86 (99) | 1.000 |
| Acute COVID Severity, per NIH Criteria | 0.450 | ||
| Mild | 2 (22) | 38 (44) | |
| Moderate | 4 (44) | 26 (30) | |
| Severe | 3 (33) | 23 (26) | |
| Hospitalization | |||
| Frequency | 2 (22) | 26 (30) | 1.000 |
| Length, days | 18 (17–20) | 8 (5–18) | 0.784 |
| Invasive Ventilation | 0 (0.0) | 1 (4) | NA |
| Abnormal Tests Results | |||
| ECG | 1 (33) | 9 (47) | 1.000 |
| TTE | 1 (50) | 1 (17) | 0.464 |
| Serum Biomarkers (hs-cTn and/or NT-proBNP) | 1 (25) | 2 (11) | 0.453 |
| Time from COVID Diagnosis to Examination, days | 152 (75–214) | 141 (112–195) | 0.912 |
| COVID Vaccination Doses | 0.399 | ||
| 0 | 2 (25) | 6 (9) | |
| 1 | 2 (25) | 25 (36) | |
| 2 | 4 (50) | 38 (55) | |
| New CV Diagnoses (n = 9) | No New CV Diagnoses (n = 87) | p-Value | |
|---|---|---|---|
| Symptoms | |||
| Any | 8 (89) | 86 (99) | 1.000 |
| Fatigue | 7 (78) | 41 (47) | 0.159 |
| Dyspnea | 7 (78) | 47 (54) | 0.291 |
| Chest Pain | 4 (44) | 36 (41) | 1.000 |
| Palpitations | 6 (67) | 30 (35) | 0.076 |
| Dizziness/Vertigo | 1 (11) | 3 (3) | 0.330 |
| Pre-syncope/Syncope | 0 (0) | 2 (2) | 1.000 |
| NYHA Class Functional Status | 0.444 | ||
| I | 4 (44) | 39 (45) | |
| II | 5 (56) | 36 (41) | |
| III | 0 (0) | 12 (14) | |
| IV | 0 (0) | 0 (0) | |
| Symptom Non-Improvement | 4 (44) | 40 (46) | 0.816 |
| ECG Findings | |||
| Any | 4 (44) | 28 (32) | 0.475 |
| Rhythm Disorders | 0 (0) | 3 (3) | 1.000 |
| Rate Disorders | 0 (0) | 0 (0) | 1.000 |
| Conduction Disorders | 2 (22) | 10 (11) | 0.319 |
| ST-T changes | 3 (33) | 17 (20) | 0.387 |
| TTE Findings | |||
| Any | 4 (44) | 27 (31) | 0.471 |
| LV Systolic Dysfunction | 1 (11) | 3 (3) | 0.246 |
| RV Systolic Dysfunction | 0 (0) | 1 (1) | 1.000 |
| Grade2 and up Diastolic Dysfunction | 0 (0) | 2 (2) | 0.863 |
| LA Dilatation | 2 (22) | 4 (5) | 0.017 |
| ≥Moderate Valvular Dysfunction | 1 (11) | 0 (0) | 1.000 |
| Systolic Pulmonary Hypertension | 1 (11) | 0 (0) | 0.700 |
| Pericardial Effusion | 2 (22) | 16 (18) | 0.124 |
| Cardiac Provocation Test Findings | |||
| Any | 3 (50) | 3 (7) | 0.200 |
| Ischemic Findings | 0 (0) | 2 (5) | 1.000 |
| Atrial Fibrillation | 0 (0) | 1 (2) | 1.000 |
| Chronotropic Incompetence | 3 (50) | 0 (0) | 1.000 |
| Age-Adjusted CV Fitness | 0.453 | ||
| Good | 1 (17) | 14 (42) | |
| Average | 2 (33) | 12 (36) | |
| Low | 3 (50) | 7 (21) | |
| Holter ECG findings | 0.139 | ||
| Any | 2 (40) | 3 (10) | |
| Inappropriate Sinus Tachycardia | 1 (20) | 0 (0) | |
| Atrial Tachycardia | 1 (20) | 0 (0) | |
| Non-Sustained Ventricular Tachycardia | 0 (0) | 1 (3) | |
| Couplet Ventricular Premature Beats | 0 (0) | 1 (3) | |
| 1st degree AV block | 0 (0) | 1 (3) | |
| CCT findings | 1.000 | ||
| Any | 0 (0) | 3 (27) | |
| Non-Obstructive Coronary Artery Disease | 0 (0) | 2 (18) | |
| Aberrant Coronary Artery | 0 (0) | 1 (9) | |
| CMR findings | 0.242 | ||
| Any | 4 (67) | 1 (20) | |
| Myocarditis | 3 (50) | 0 (0) | |
| Myopericarditis | 1 (17) | 0 (0) | |
| Hypertrophic obstructive cardiomyopathy | 0 (0) | 1 (20) | |
| CPET findings | |||
| CV Restraint | 2 (100) | 3 (30) | 0.152 |
| PFT findings | |||
| Any | 6 (67) | 21 (29) | 0.054 |
| Obstruction | 5 (56) | 8 (11) | 0.084 |
| Restriction | 0 (0) | 5 (69) | 0.076 |
| Reduced Diffusing Capacity | 1 (11) | 8 (11) | 0.243 |
| HRCT findings | |||
| Any | 3 (43) | 19 (54) | 0.691 |
| Interstitial Changes | 1 (14) | 8 (23) | 0.362 |
| Fibrotic Changes | 1 (14) | 3 (9) | 0.846 |
| Ground Glass Opacities | 0 (0) | 7 (20) | 0.124 |
| Lung Nodules | 1 (14) | 1 (3) | 0.632 |
| New, Potentially COVID-Related CV Diagnoses | Symptom Non-Improvement | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Baseline Clinical Variables | ||||
| Age (continuous) | 0.96 (0.92–1.01) | 0.144 | 1.01 (0.97–1.04) | 0.755 |
| Sex Male | 0.31 (0.06–1.56) | 0.154 | 1.04 (0.39–2.76) | 0.939 |
| Prior CV Disease | 0.90 (0.86–1.23) | 0.655 | 0.38 (0.09–1.57) | 0.179 |
| CV Risk Factors | 0.45 (0.11–1.82) | 0.262 | 1.31 (0.43–4.03) | 0.632 |
| Acute COVID Parameters | ||||
| Severity | ||||
| Moderate vs. Mild | 2.92 (0.50–17.15) | 0.235 | 1.40 (0.54–3.61) | 0.491 |
| Severe vs. Mild | 2.48 (0.39–15.96) | 0.340 | 0.76 (0.28–2.09) | 0.600 |
| ≥Moderate vs. Mild | 2.71 (0.53–13.82) | 0.229 | 0.68 (0.25–1.86) | 0.456 |
| Severe vs. Non-Severe | 1.39 (0.32–6.02) | 0.659 | 0.82 (0.29–2.34) | 0.713 |
| Hospitalization | 0.67 (0.13–3.45) | 0.632 | 0.49 (0.17–1.39) | 0.177 |
| Hospitalization Length | 1.11 (0.93–1.34) | 0.251 | 0.93 (0.82–1.05) | 0.218 |
| Abnormal In-Hospital ECG | 0.56 (0.04–7.21) | 0.653 | 5.33 (0.62–45.99) | 0.128 |
| Abnormal In-Hospital TTE | 5.00 (0.15–166.59) | 0.368 | 2.00 (0.05–78.25) | 0.711 |
| Abnormal In-Hospital Cardiac Biomarkers | 2.83 (0.19–41.99) | 0.449 | 0.57 (0.04–7.74) | 0.674 |
| COVID Vaccination Status | ||||
| COVID Vaccination | 0.29 (0.05–1.74) | 0.174 | 1.57 (0.24–10.24) | 0.640 |
| Long COVID Presentation | ||||
| Fatigue | 3.93 (0.77–19.98) | 0.099 | 2.00 (0.74–5.39) | 0.170 |
| Dyspnea | 2.98 (0.59–15.16) | 0.189 | 1.75 (0.63–4.90) | 0.286 |
| Chest Pain | 1.13 (0.28–4.52) | 0.859 | 1.96 (0.73–5.28) | 0.182 |
| Palpitations | 3.8 (0.89–16.23) | 0.072 | 1.68 (0.62–4.56) | 0.311 |
| Dizziness/Vertigo | 3.50 (0.33–37.69) | 0.302 | 0.79 (0.33–4.28) | 0.673 |
| Pre-syncope/Syncope | 2.21 (0.89–10.23) | 0.872 | 3.21 (0.05–5.48) | 0.915 |
| NYHA Class Functional Status | ||||
| Continuous | 0.53 (0.16–1.74) | 0.294 | 2.38 (1.26–4.49) | 0.008 |
| Class II-III vs. I | 0.59 (0.14–2.50) | 0.472 | 2.70 (1.17–6.25) | 0.020 |
| Class III vs. I-II | 1.00 (0.99–1.01) | 0.999 | 4.20 (1.06–16.64) | 0.041 |
| Work-Up Findings | ||||
| Heart Murmur | 0.67 (0.40–2.89) | 0.203 | 1.17 (0.07–19.59) | 0.912 |
| Abnormal ECG | 1.69 (0.41–6.94) | 0.473 | 0.50 (0.14–1.76) | 0.280 |
| Abnormal TTE | 1.72 (0.43–6.91) | 0.446 | 2.84 (0.94–8.56) | 0.064 |
| LVEF (continuous) | 0.96 (0.84–1.11) | 0.582 | 0.98 (0.89–1.09) | 0.737 |
| LV Systolic Dysfunction | 1.02 (0.24–19.53) | 0.534 | 0.76 (0.32–4.54) | 0.863 |
| LA Dilatation | 2.71 (0.48–15.34) | 0.259 | 1.67 (0.26–10.74) | 0.591 |
| LAVi (continuous) | 0.97 (0.89–1.12) | 0.940 | 1.00 (0.93–1.08) | 0.976 |
| More than Mild Valvular Dysfunction | 2.00 (0.21–19.23) | 0.549 | 4.92 (0.52–46.78) | 0.165 |
| PASP (continuous) | 0.91 (0.75–1.11) | 0.368 | 1.18 (0.97–1.45) | 0.104 |
| Pericardial Effusion | 2.33 (0.52–10.39) | 0.266 | 6.64 (1.3–33.88) | 0.023 |
| Abnormal Provocation Test * | 0.17 (0.02–1.71) | 0.133 | ||
| Average/Low (vs Good) Age-Adjusted CV Fitness | 3.68 (0.39–35.14) | 0.257 | 0.90 (0.21–3.82) | 0.886 |
| CV Restraint per CPET | 2.89 (0.86–2.56) | 0.811 | 0.75 (0.06–8.83) | 0.819 |
| Abnormal Holter ECG | NA | NA | 0.97 (0.64–3.42) | 0.723 |
| Abnormal CCT | NA | NA | 1 (0.06–15.99) | 1.000 |
| Abnormal CMR | NA | NA | 0.44 (0.04–5.58) | 0.530 |
| Abnormal PFT | 4.86 (1.11–21.26) | 0.036 | 2.26 (0.75–6.80) | 0.148 |
| Abnormal HRCT | 0.63 (0.12–3.25) | 0.582 | 0.29 (0.07–1.22) | 0.091 |
| Outcomes | ||||
| New CV Diagnosis | NA | NA | 1.19 (0.27–5.24) | 0.816 |
| Symptom Non-Improvement | 1.19 (0.27–5.24) | 0.816 | NA | NA |
| New, Potentially COVID-Related CV Diagnoses | Symptom Non-Improvement | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Fatigue | 2.89 (0.50–16.72) | 0.235 | ||
| Palpitations | 2.60 (0.53–12.73) | 0.240 | ||
| NYHA Class Functional Status | ||||
| Continuous | 1.76 (0.60–5.14) | 0.303 | ||
| Class II-III vs. I | 1.55 (0.40–6.06) | 0.530 | ||
| Class III vs. I-II | 3.97 (0.34–46.24) | 0.271 | ||
| Pericardial Effusion | ||||
| NYHA Class Continuous | 2.26 (0.40–12.70) | 0.353 | ||
| NYHA Class II-III vs. I | 2.40 (0.43–13.31) | 0.316 | ||
| NYHA Class III vs. I-II | 1.92 (0.33–11.16) | 0.470 | ||
| Abnormal PFT | 5.16 (1.12–23.68) | 0.035 | ||
| Abnormal HRCT | ||||
| NYHA Class Continuous | 1.17 (0.26–4.79) | 0.882 | ||
| NYHA Class II-III vs. I | 0.98 (0.24–4.02) | 0.974 | ||
| NYHA Class III vs. I-II | 1.17 (0.27–4.78) | 0.872 | ||
| Symptom Improvement (n = 52) | Symptom Non-Improvement (n = 44) | p-Value | |
|---|---|---|---|
| Demographic Details | |||
| Age, years | 53 (42–66) | 57 (45–62) | 0.517 |
| Male | 22 (42) | 22 (50) | 0.451 |
| Cardiovascular Background | |||
| Prior CV Disease | 10 (19) | 5 (11) | 0.290 |
| CV Risk Factors | |||
| Any | 34 (65) | 35 (80) | 0.124 |
| Pre-diabetes/Diabetes Mellitus | 19 (37) | 15 (34) | 0.803 |
| Hypertension | 9 (17) | 13 (30) | 0.155 |
| Dyslipidemia | 30 (58) | 26 (59) | 0.890 |
| Obesity | 17 (33) | 13 (30) | 0.740 |
| Present Smoking | 3 (9) | 7 (16) | 0.178 |
| Acute Covid Data | |||
| Symptoms During Acute COVID | 52 (100) | 43 (98) | 0.458 |
| Acute COVID Severity, per NIH Criteria | 0.533 | ||
| Mild | 22 (23) | 18 (41) | |
| Moderate | 14 (27) | 16 (36) | |
| Severe | 16 (31) | 10 (23) | |
| Hospitalization | |||
| Frequency | 18 (35) | 10 (23) | 0.202 |
| Length, days | 11 (5–19) | 7 (5–16) | 0.586 |
| Invasive Ventilation | 1 (6) | 0 (0) | NA |
| Abnormal Tests Results | |||
| ECG | 4 (33) | 6 (60) | 0.391 |
| TTE | 1 (25) | 1 (25) | 1.000 |
| Serum Biomarkers (hs-cTn and/or NT-proBNP) | 2 (15) | 1 (10) | 0.704 |
| Time from COVID Diagnosis to Examination, days | 140 (105–179) | 143 (113–214) | 0.405 |
| COVID Vaccination Doses | 0.314 | ||
| 0 | 6 (15) | 2 (5) | |
| 1 | 12 (30) | 15 (41) | |
| 2 | 22 (55) | 20 (54) | |
| Symptom Improvement (n = 52) | Symptom Non-Improvement (n = 44) | p-Value | |
|---|---|---|---|
| Symptoms | |||
| Any | 52 (100) | 44 (100) | 1.000 |
| Fatigue | 22 (42) | 26 (59) | 0.101 |
| Dyspnea | 27 (52) | 27 (61) | 0.353 |
| Chest Pain | 20 (39) | 20 (46) | 0.489 |
| Palpitations | 18 (35) | 18 (41) | 0.526 |
| Dizziness/Vertigo | 0 (0) | 4 (9) | 0.041 |
| Pre-syncope/Syncope | 1 (1) | 1 (2) | 0.207 |
| NYHA Class Functional Status | 0.022 | ||
| I | 29 (56) | 14 (32) | |
| II | 20 (39) | 21 (48) | |
| III | 3 (6) | 9 (21) | |
| IV | 0 (0) | 0 (0) | |
| ECG Findings | |||
| Any | 17 (33) | 15 (34) | 0.658 |
| Rhythm Disorders | 2 (4) | 1 (2) | 0.498 |
| Conduction Disorders | 6 (12) | 6 (14) | 0.871 |
| ST-T changes | 13 (25) | 8 (18) | 0.387 |
| TTE Findings | |||
| Any | 14 (27) | 17 (39) | 0.274 |
| LV Systolic Dysfunction | 3 (6) | 1 (2) | 0.245 |
| RV Systolic Dysfunction | 0 (0) | 1 (2) | 0.468 |
| Grade2 and up Diastolic Dysfunction | 1 (2) | 1 (2) | 1.000 |
| LA Dilatation | 3 (6) | 3 (6) | 0.509 |
| ≥Moderate Valvular Dysfunction | 1 (2) | 0 (0) | 1.000 |
| Systolic Pulmonary Hypertension | 0 (0) | 1 (2) | 0.471 |
| Pericardial Effusion | 6 (12) | 12 (27) | 0.060 |
| Cardiac Provocation Test Findings | |||
| Any | 4 (17) | 2 (8) | 0.666 |
| Ischemic Findings | 1 (4) | 1 (4) | 1.000 |
| Atrial Fibrillation | 0 (0) | 1 (4) | 0.700 |
| Chronotropic Incompetence | 3 (12) | 0 (0) | 0.121 |
| Age-Adjusted CV Fitness | 0.831 | ||
| Good | 9 (43) | 6 (33) | |
| Average | 7 (33) | 7 (39) | |
| Low | 5 (24) | 5 (28) | |
| Holter ECG findings | 0.141 | ||
| Any | 1 (5) | 4 (27) | |
| Inappropriate Sinus Tachycardia | 1 (5) | 0 (0) | |
| Atrial Tachycardia | 0 (0) | 1 (7) | |
| Non-Sustained Ventricular Tachycardia | 0 (0) | 1 (7) | |
| Couplet Ventricular Premature Beats | 0 (0) | 1 (7) | |
| 1st degree AV block | 0 (0) | 1 (7) | |
| CCT findings | 1.000 | ||
| Any | 1 (25) | 2 (22) | |
| Non-Obstructive Coronary Artery Disease | 0 (0) | 2 (22) | |
| Aberrant Coronary Artery | 1 (25) | 0 (0) | |
| CMR findings | 1.000 | ||
| Any | 3 (50) | 2 (40) | |
| Myocarditis | 2 (33) | 1 (20) | |
| Myopericarditis | 0 (0) | 1 (20) | |
| Hypertrophic obstructive cardiomyopathy | 1 (17) | 0 (0) | |
| CPET findings | |||
| CV Restraint | 2 (40) | 3 (43) | 1.000 |
| PFT findings | |||
| Any | 12 (27) | 15 (42) | 0.155 |
| Obstruction | 4 (9) | 9 (25) | 0.168 |
| Restriction | 3 (7) | 2 (6) | 0.628 |
| Reduced Diffusing Capacity | 5 (11) | 4 (12) | 0.448 |
| HRCT findings | |||
| Any | 13 (59) | 9 (45) | 0.361 |
| Interstitial Changes | 6 (27) | 3 (15) | 0.346 |
| Fibrotic Changes | 2 (9) | 2 (10) | 1.000 |
| Ground Glass Opacities | 4 (18) | 3 (15) | 0.876 |
| Lung Nodules | 1 (5) | 1 (5) | 1.000 |
| New, Potentially COVID-Related CV Diagnosis | 5 (10) | 4 (9) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shechter, A.; Yelin, D.; Margalit, I.; Abitbol, M.; Morelli, O.; Hamdan, A.; Vaturi, M.; Eisen, A.; Sagie, A.; Kornowski, R.; et al. Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience. J. Clin. Med. 2022, 11, 6123. https://doi.org/10.3390/jcm11206123
Shechter A, Yelin D, Margalit I, Abitbol M, Morelli O, Hamdan A, Vaturi M, Eisen A, Sagie A, Kornowski R, et al. Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience. Journal of Clinical Medicine. 2022; 11(20):6123. https://doi.org/10.3390/jcm11206123
Chicago/Turabian StyleShechter, Alon, Dana Yelin, Ili Margalit, Merry Abitbol, Olga Morelli, Ashraf Hamdan, Mordehay Vaturi, Alon Eisen, Alex Sagie, Ran Kornowski, and et al. 2022. "Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience" Journal of Clinical Medicine 11, no. 20: 6123. https://doi.org/10.3390/jcm11206123
APA StyleShechter, A., Yelin, D., Margalit, I., Abitbol, M., Morelli, O., Hamdan, A., Vaturi, M., Eisen, A., Sagie, A., Kornowski, R., & Shapira, Y. (2022). Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience. Journal of Clinical Medicine, 11(20), 6123. https://doi.org/10.3390/jcm11206123








