Effect of Choline Alphoscerate on the Survival of Glioblastoma Patients: A Retrospective, Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Surgery and Adjuvant Treatment
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Patients and Tumors
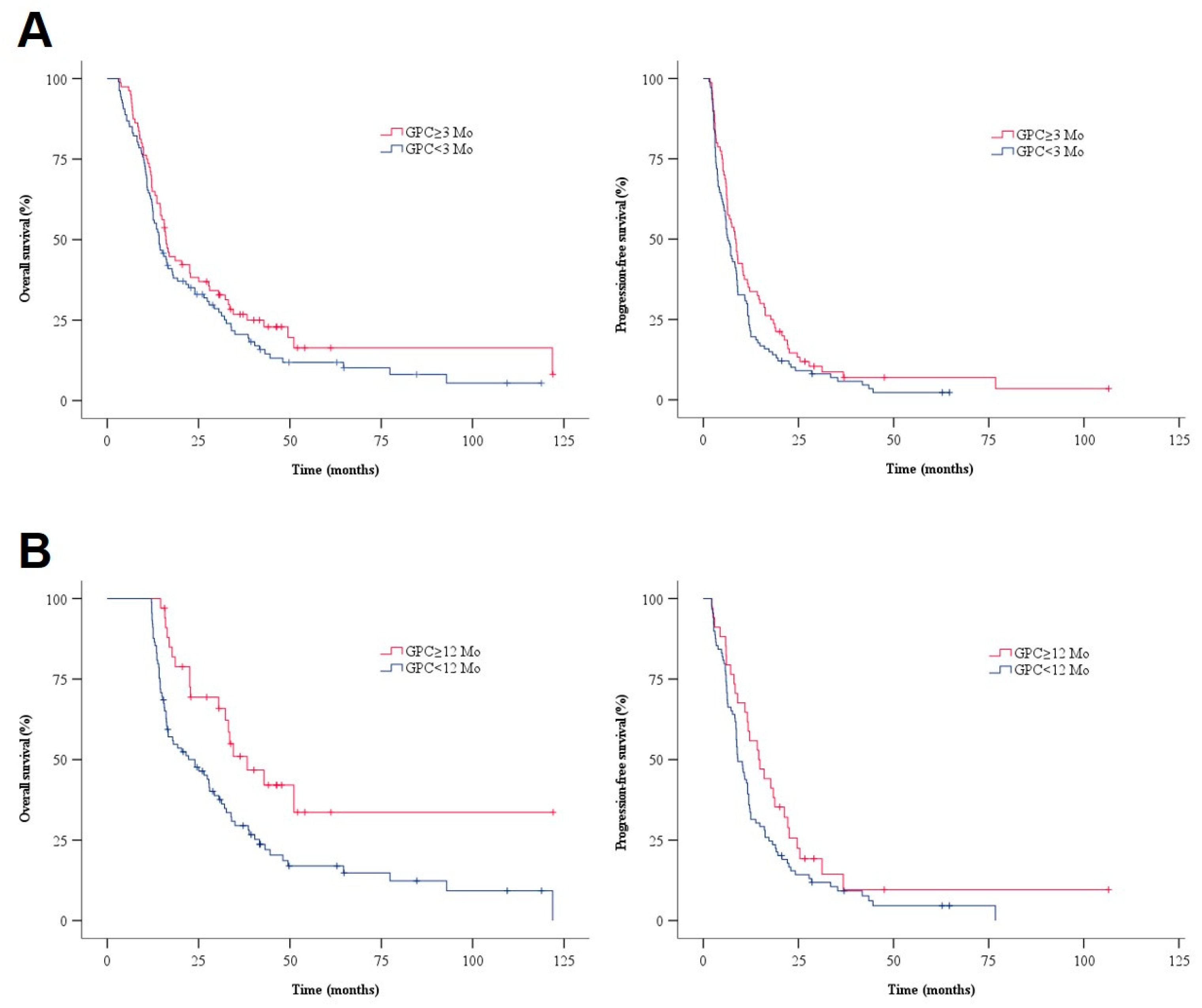
3.2. Survival Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartman, H.E.; Sun, Y.; Devasia, T.P.; Chase, E.C.; Jairath, N.K.; Dess, R.T.; Jackson, W.C.; Morris, E.; Li, P.; Hochstedler, K.A.; et al. Integrated Survival Estimates for Cancer Treatment Delay Among Adults with Cancer during the COVID-19 Pandemic. JAMA Oncol. 2020, 6, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shibui, S.; Narita, Y.; Mizusawa, J.; Beppu, T.; Ogasawara, K.; Sawamura, Y.; Kobayashi, H.; Nishikawa, R.; Mishima, K.; Muragaki, Y.; et al. Randomized trial of chemoradiotherapy and adjuvant chemotherapy with nimustine (ACNU) versus nimustine plus procarbazine for newly diagnosed anaplastic astrocytoma and glioblastoma (JCOG0305). Cancer Chemother. Pharmacol. 2013, 71, 511–521. [Google Scholar] [CrossRef]
- Kim, B.S.; Seol, H.J.; Nam, D.H.; Park, C.K.; Kim, I.H.; Kim, T.M.; Kim, J.H.; Cho, Y.H.; Yoon, S.M.; Chang, J.H.; et al. Concurrent Chemoradiotherapy with Temozolomide Followed by Adjuvant Temozolomide for Newly Diagnosed Glioblastoma Patients: A Retrospective Multicenter Observation Study in Korea. Cancer Res. Treat. 2017, 49, 193–203. [Google Scholar] [CrossRef]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Choucair, A.K.; Levin, V.A.; Gutin, P.H.; Davis, R.L.; Silver, P.; Edwards, M.S.; Wilson, C.B. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J. Neurosurg. 1986, 65, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Bronzetti, E.; Mancini, M.; Vega, J.A.; Zaccheo, D. Choline acetyltransferase and acetylcholinesterase in the hippocampus of aged rats: Sensitivity to choline alphoscerate treatment. Mech. Ageing Dev. 1994, 74, 47–58. [Google Scholar] [CrossRef]
- Sigala, S.; Imperato, A.; Rizzonelli, P.; Casolini, P.; Missale, C.; Spano, P. L-alpha-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur. J. Pharmacol. 1992, 211, 351–358. [Google Scholar] [CrossRef]
- Amenta, F.; Tayebati, S.K.; Vitali, D.; Di Tullio, M.A. Association with the cholinergic precursor choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech. Ageing Dev. 2006, 127, 173–179. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Di Tullio, M.A.; Tomassoni, D.; Amenta, F. Neuroprotective effect of treatment with galantamine and choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J. Neurol. Sci. 2009, 283, 187–194. [Google Scholar] [CrossRef]
- Amenta, F.; Tayebati, S.K. Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr. Med. Chem. 2008, 15, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Poggesi, A.; Donnini, I.; Rinnoci, V.; Chiti, G.; Squitieri, M.; Tudisco, L.; Fierini, F.; Melone, A.; Pescini, F.; et al. Efficacy and Safety of the Association of Nimodipine and Choline Alphoscerate in the Treatment of Cognitive Impairment in Patients with Cerebral Small Vessel Disease. The CONIVaD Trial. Drugs Aging 2021, 38, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Youn, Y.C. Quantitative electroencephalography changes in patients with mild cognitive impairment after choline alphoscerate administration. J. Clin. Neurosci. 2022, 102, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Iacopino, D.G.; Azzarello, G.; Gaggiotti, C.; Graziano, F.; Gulì, C.; Pino, M.A.; Maugeri, R. End-of-Life Care in High-Grade Glioma Patients. The Palliative and Supportive Perspective. Brain Sci. 2018, 30, 125. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.; Chang, J.; Choi, D.; Son, J.S.; Kim, K.; Kim, S.M.; Jeong, S.; Park, S.M. Association of L-α Glycerylphosphorylcholine with Subsequent Stroke Risk After 10 Years. JAMA Netw. Open 2021, 4, e2136008. [Google Scholar] [CrossRef]
- Thompson, E.G.; Sontheimer, H. Acetylcholine Receptor Activation as a Modulator of Glioblastoma Invasion. Cells 2019, 8, 1203. [Google Scholar] [CrossRef]
- Bordey, A.; Sontheimer, H.; Trouslard, J. Muscarinic activation of BK channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration. J. Membr. Biol. 2000, 176, 31–40. [Google Scholar] [CrossRef]
- Cristofaro, I.; Alessandrini, F. Cross Interaction between M2 Muscarinic Receptor and Notch1/EGFR Pathway in Human Glioblastoma Cancer Stem Cells: Effects on Cell Cycle Progression and Survival. Cells 2020, 9, 657. [Google Scholar] [CrossRef]
- Nelson, S.J. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn. Reson. Med. 2001, 46, 228–239. [Google Scholar] [CrossRef]
- Glunde, K.; Jacobs, M.A.; Bhujwalla, Z.M. Choline metabolism in cancer: Implications for diagnosis and therapy. Expert Rev. Mol. Diagn. 2006, 6, 821–829. [Google Scholar] [CrossRef]
- Amenta, F.; Carotenuto, A.; Fasanaro, A.M.; Rea, R.; Traini, E. The ASCOMALVA (Association between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in Alzheimer’s Disease) Trial: Interim results after two years of treatment. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S281–S288. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.A.; Smith, J.A.; Bezjak, A.; Mehta, M.P.; Liebmann, J.; Illidge, T.; Kunkler, I.; Caudrelier, J.M.; Eisenberg, P.D.; Meerwaldt, J.; et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. J. Clin. Oncol. 2004, 22, 157–165. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Ligibel, J.A.; Are, M.; Baker, K.S.; Demark-Wahnefried, W.; Dizon, D.; Friedman, D.L.; Goldman, M.; Jones, L.; King, A.; et al. Survivorship: Screening for cancer and treatment effects, version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 1526–1531. [Google Scholar] [CrossRef]
- Traini, E.; Bramanti, V.; Amenta, F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent. Curr. Alzheimer Res. 2013, 10, 1070–1079. [Google Scholar] [CrossRef]
- Parnetti, L.; Mignini, F.; Tomassoni, D.; Traini, E.; Amenta, F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J. Neurol. Sci. 2007, 257, 264–269. [Google Scholar] [CrossRef]
- Parnetti, L.; Amenta, F.; Gallai, V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech. Ageing Dev. 2001, 122, 2041–2055. [Google Scholar] [CrossRef]
- De Jesus Moreno Moreno, M. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin. Ther. 2003, 25, 178–193. [Google Scholar] [CrossRef]
- Glunde, K.; Jiang, L.; Moestue, S.A.; Gribbestad, I.S. MRS and MRSI guidance in molecular medicine: Targeting and monitoring of choline and glucose metabolism in cancer. NMR Biomed. 2011, 24, 673–690. [Google Scholar] [CrossRef]
- Shinoura, N.; Nishijima, M.; Hara, T.; Haisa, T.; Yamamoto, H.; Fujii, K.; Mitsui, I.; Kosaka, N.; Kondo, T.; Hara, T. Brain tumors: Detection with C-11 choline PET. Radiology 1997, 202, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Pucci, S.; Fasoli, F.; Moretti, M.; Benfante, R.; Di Lascio, S.; Viani, P.; Daga, A.; Gordon, T.J.; McIntosh, M.; Zoli, M.; et al. Choline and nicotine increase glioblastoma cell proliferation by binding and activating α7- and α9-containing nicotinic receptors. Pharmacol. Res. 2021, 163, 105336. [Google Scholar] [CrossRef]
- Ishiuchi, S.; Tsuzuki, K.; Yoshida, Y.; Yamada, N.; Hagimura, N.; Okado, H.; Miwa, A.; Kurihara, H.; Nakazato, Y.; Tamura, M.; et al. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 2002, 8, 971–978. [Google Scholar] [CrossRef]
- Takano, T.; Lin, J.H.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- Akaike, A. Preclinical evidence of neuroprotection by cholinesterase inhibitors. Alzheimer Dis. Assoc. Disord. 2006, 20, S8–S11. [Google Scholar] [CrossRef]
- Wehrwein, E.; Thompson, S.A.; Coulibaly, S.F.; Linn, D.M.; Linn, C.L. Acetylcholine protection of adult pig retinal ganglion cells from glutamate-induced excitotoxicity. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1531–1543. [Google Scholar] [CrossRef]
| Parameter | No. of Patients (%) | p-Value | ||
|---|---|---|---|---|
| Total (n = 187) | GPC Group (n = 80) | Non-GPC Group (n = 107) | ||
| Median age at diagnosis, yrs (IQR) | 64.0 (54–72) | 63.0 (54–70) | 66.0 (53–74) | 0.686 |
| Gender (male) | 101 (54) | 42 (53) | 59 (55) | 0.768 |
| Preoperative KPS score (mean ± SD) | 80 ± 13 | 82 ± 11 | 79 ± 14 | 0.119 |
| Postoperative KPS score (mean ± SD) | 81 ± 10 | 83 ± 7 | 79 ± 12 | 0.033 |
| Median tumor volume (cm3, IQR) | 31.6 (16–57) | 29.6 (15–49) | 33.4 (16–62) | 0.372 |
| Location of tumor | 0.014 | |||
| Supratentorial | 168 (90) | 77 (96) | 91 (85) | |
| Infratentorial | 19 (10) | 3 (4) | 16 (15) | |
| Extent of resection | 0.080 | |||
| Gross total resection | 79 (42) | 39 (49) | 40 (37) | |
| Subtotal resection | 63 (34) | 28 (35) | 35 (33) | |
| Biopsy | 45 (24) | 13 (16) | 32 (30) | |
| MGMT promoter methylation | 0.878 | |||
| Methylated | 97 (56) | 44 (57) | 53 (56) | |
| Unmethylated | 75 (44) | 33 (43) | 42 (44) | |
| Not available | 15 | 3 | 12 | |
| CCRT | 130 (70) | 68 (85) | 62 (58) | <0.001 |
| Median PFS, mos (95% CI) | 7.2 (5.8–8.6) | 8.4 (6.6–10.1) | 6.5 (5.4–8.0) | 0.092 |
| Tumor relapse | 177 (94) | 74 (93) | 103 (96) | |
| Median OS, mos (95% CI) | 15.5 (14.1–17.0) | 16.1 (14.6–17.6) | 14.2 (11.8–16.6) | 0.158 |
| Death | 154 (82) | 62 (78) | 92 (86) | |
| Factor | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| Age | <0.001 | 1.046 (1.031–1.062) | <0.001 | 1.036 (1.018–1.054) | 0.001 | 1.025 (1.010–1.039) | 0.046 | 1.016 (1.000–1.033) |
| Preop. KPS | 0.031 | 0.988 (0.978–0.999) | 0.457 | - | 0.033 | 0.988 (0.978–0.999) | 0.632 | - |
| Postop. KPS | 0.001 | 0.977 (0.964–0.990) | 0.052 | 0.983 (0.967–1.000) | 0.092 | 0.988 (0.975–1.002) | 0.838 | - |
| Supratentorial location | 0.001 | 0.430 (0.260–0.711) | 0.702 | 0.002 | 0.457 (0.281–0.744) | 0.53 | - | |
| Complete resection | <0.001 | 0.466 (0.333–0.650) | <0.001 | 0.482 (0.337–0.725) | 0.002 | 0.613 (0.452–0.830) | 0.008 | 0.647 (0.469–0.892) |
| Methylated MGMT promoter | 0.083 | 0.745 (0.535–1.039) | <0.001 | 0.507 (0.354–0.725) | 0.012 | 0.669 (0.489–0.915 | 0.004 | 0.616 (0.443–0.855) |
| CCRT | <0.001 | 0.257 (0.180–0.366) | 0.001 | 0.453 (0.286–0.719) | <0.001 | 0.365 (0.260–0.512) | 0.002 | 0.526 (0.348–0.797) |
| GPC usage (≥3 mos) | 0.159 | 0.792 (0.573–1.096) | 0.799 | - | 0.094 | 0.774 (0.573–1.045) | 0.886 | - |
| Parameter | No. of Patients (%) | p-Value | ||
|---|---|---|---|---|
| Total (n = 123) | Long-Term GPC Group (n = 34) | Non-Long-Term GPC Group (n = 89) | ||
| Median age at diagnosis, yrs (IQR) | 59.0 (52–68) | 59.0 (52–65) | 59.0 (52–70) | 0.561 |
| Gender (male) | 64 (52) | 18 (53) | 46 (52) | 1.000 |
| Preoperative KPS score (mean ± SD) | 82 ± 12 | 83 ± 13 | 81 ± 12 | 0.192 |
| Postoperative KPS score (mean ± SD) | 83 ± 8 | 85 ± 6 | 82 ± 8 | 0.097 |
| Median tumor volume (cm3, IQR) | 30.0(15–57) | 31.5 (16–52) | 28.0 (15–58) | 0.653 |
| Location of tumor | 0.105 | |||
| Supratentorial | 115 (93) | 34 (100) | 81 (91) | |
| Infratentorial | 8 (7) | 0 | 8 (9) | |
| Extent of resection | 0.138 | |||
| Gross total resection | 63 (51) | 20 (59) | 43 (49) | |
| Subtotal resection | 39 (32) | 12 (35) | 27 (30) | |
| Biopsy | 21 (17) | 2 (6) | 19 (21) | |
| MGMT promoter methylation | 1.000 | |||
| Methylated | 70 (60) | 20 (61) | 50 (60) | |
| Unmethylated | 46 (40) | 13 (39) | 33 (40) | |
| Not available | 7 | 1 | 1 | |
| CCRT | 105 (85) | 33 (97) | 72 (81) | 0.042 |
| Median OS, mos (95% CI) | 27.8 (20.9–34.7) | 38.3 (27.5–49.1) | 24.0 (15.7–32.3) | 0.004 |
| Tumor relapse | 90 (73) | 18 (53) | 72 (81) | |
| Median PFS, mos (95% CI) | 11.0 (8.7–13.3) | 14.6 (9.2–20.0) | 9.1 (7.2–11.0) | 0.082 |
| Death | 113 (92) | 29 (85) | 84 (94) | |
| Factor | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| Age | 0.002 | 1.031 (1.011–1.051) | <0.001 | 1.041 (1.020–1.064) | 0.575 | 1.005 (0.988–1.022) | 0.48 | - |
| Preop. KPS | 0.391 | 0.993 (0.978–1.009) | 0.667 | - | 0.413 | 0.994 (0.979–1.009) | 0.626 | - |
| Postop. KPS | 0.814 | 0.997 (0.975–1.020) | 0.556 | - | 0.505 | 1.008 (0.985–1.031) | 0.181 | - |
| Supratentoral location | 0.024 | 0.403 (0.183–0.885) | 0.014 | 0.304 (0.118–0.787) | 0.033 | 0.451 (0.216–0.939) | 0.639 | - |
| Complete resection | 0.002 | 0.520 (0.342–0.792) | 0.001 | 0.454 (0.289–0.715) | 0.094 | 0.728 (0.502–1.056) | 0.064 | 0.696 (0.475–1.021) |
| Methylated MGMT promoter | 0.311 | 0.800 (0.520–1.231) | 0.01 | 0.525 (0.321–0.859) | 0.05 | 0.677 (0.458–1.000 | 0.065 | 0.692 (0.468–1.023) |
| CCRT | 0.001 | 0.360 (0.207–0.627) | 0.169 | - | 0.001 | 0.406 (0.241–0.684) | 0.003 | 0.445 (0.263–0.752) |
| Long-term GPC usage UUU (≥ 12 mos) | 0.005 | 0.477 (0.284–0.801) | 0.019 | 0.532 (0.314–0.900) | 0.085 | 0.689 (0.451–1.053) | 0.358 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Lee, T.-K.; Noh, M.-G.; Jung, T.-Y.; Kim, I.-Y.; Jung, S.; Lee, K.-H.; Moon, K.-S. Effect of Choline Alphoscerate on the Survival of Glioblastoma Patients: A Retrospective, Single-Center Study. J. Clin. Med. 2022, 11, 6052. https://doi.org/10.3390/jcm11206052
Kim YJ, Lee T-K, Noh M-G, Jung T-Y, Kim I-Y, Jung S, Lee K-H, Moon K-S. Effect of Choline Alphoscerate on the Survival of Glioblastoma Patients: A Retrospective, Single-Center Study. Journal of Clinical Medicine. 2022; 11(20):6052. https://doi.org/10.3390/jcm11206052
Chicago/Turabian StyleKim, Yeong Jin, Tae-Kyu Lee, Myung-Giun Noh, Tae-Young Jung, In-Young Kim, Shin Jung, Kyung-Hwa Lee, and Kyung-Sub Moon. 2022. "Effect of Choline Alphoscerate on the Survival of Glioblastoma Patients: A Retrospective, Single-Center Study" Journal of Clinical Medicine 11, no. 20: 6052. https://doi.org/10.3390/jcm11206052
APA StyleKim, Y. J., Lee, T.-K., Noh, M.-G., Jung, T.-Y., Kim, I.-Y., Jung, S., Lee, K.-H., & Moon, K.-S. (2022). Effect of Choline Alphoscerate on the Survival of Glioblastoma Patients: A Retrospective, Single-Center Study. Journal of Clinical Medicine, 11(20), 6052. https://doi.org/10.3390/jcm11206052







