Abstract
The association of well-differentiated gastro-entero-pancreatic neuroendocrine neoplasia (WD GEP-NEN) with metabolic syndrome (MetS), abdominal obesity, and fasting glucose abnormalities was recently described. However, whether obesity and metabolic syndrome risk factors are associated with GEP-NEN adverse outcomes and the poorer prognosis was unknown. The present study aimed to evaluate whether the presence of MetS or any of its individual components at WD GEP-NEN diagnosis influenced disease outcomes. A cohort of patients with non-localized WD GEP-NETs (n = 81), was classified according to the primary tumor site (gastrointestinal or pancreatic), pathological grading (G1 (Ki67 ≤ 2%) and G2 (3% ≤ Ki67 ≤ 20%) (WHO 2010)), disease extension (loco-regional or metastatic disease), presence of hormonal secretion syndrome (functioning or non-functioning), and evaluated for the presence of MetS criteria at diagnosis. MetS was present in 48 (59.3%) patients. During a median follow-up of 95.0 months (16.8–262.5), 18 patients died of the disease (10 with MetS vs. 8 without MetS). Overall survival (OS) at 5 years was 87.1% (95% CI: 73.6–94.0) for MetS and 90.9% (95% CI: 74.4–97.0) for non-Mets group, while OS at 10 years was 72.5% (95% CI: 55.3–84.0) for MetS, and 76.4% (95% CI: 53.6–89.0) for non-MetS group. Progression-Free Survival (PFS) at 5 years was 45.9% (95% CI: 30.8–59.8) for MetS and 40.0% (95% CI: 21.3–58.1) for non-MetS group, and PFS at 10 years was 18.1% (95% CI: 7.0–33.5) for MetS and 24.4% (95% CI: 9.0–43.7) for non-MetS group. Waist circumference (WC), a surrogate measure for visceral obesity, was associated with significantly shorter PFS (HR = 1.03; 95% CI: 1.01–1.06), although did not influence OS (HR = 1.01; 95% CI: 0.97–1.06). The findings of this study reinforce a potential link between visceral obesity and GEP-NEN and further suggest that obesity could influence disease prognosis.
1. Introduction
Gastro-entero-pancreatic neuroendocrine neoplasia (GEP-NEN) was considered a rare entity before the 460% increase in incidence observed over the past four decades [1]. The association between environmental factors and cancer is well established for several neoplasias, although with different levels of evidence according to the type of tumor considered [2,3,4,5]. Unhealthy dietary patterns, sedentary life, obesity, and diabetes are the modifiable risk factors (RFs) that were recognized to contribute to nearly 30% of new cancer diagnoses [6,7,8,9,10]. Less well-characterized is the influence of the above-mentioned RFs on cancer outcomes, although the association with a higher recurrence and mortality rate has been reported for colorectal, breast, uterus, stomach, prostate, and pancreas, among other neoplasias [11,12,13,14,15,16]. Although MetS and MetS individual components, namely abdominal obesity and abnormal fasting plasma glucose (FPG), have been recently described to be associated with well-differentiated GEP-NEN by our group [17], data on how obesity, diabetes, and other metabolic syndrome RF’s influence GEP-NEN’s prognosis was lacking.
The aim of the current study was to evaluate whether the presence of obesity, MetS and MetS individual components, at the time of WD GEP-NEN diagnosis influenced overall survival (OS) and progression-free survival (PFS) from the first treatment intervention in individuals with non-localized disease.
2. Materials and Methods
Patients with confirmed WD GEP-NETs were recruited from the Endocrine Tumors Clinic at a single national tertiary referral center for oncologic diseases (Portuguese Oncology Institute of Porto; IPOFG, Porto, Portugal). The criteria for inclusion were WD GEP-NEN diagnosis confirmed by histopathology and/or PET-68Ga-DOTA-NOC. Patients under 18 years old at diagnosis, harboring familial GEP-NEN, neuroendocrine carcinoma (NEC), and type 1 gastric endocrine neoplasia (T1-GEN) were excluded from the study, as these tumors have a distinct biological behavior and/or established etiologies [18].
Among patients with WD GEP-NEN, who consented to participate in the study (n = 136), those who did not fulfill the inclusion criteria or had insufficient data for analysis were excluded (n = 55). All the remaining eligible patients were included for statistical analysis (n = 81). Tumors were classified according to primary tumor site: gastrointestinal (GI-NEN) or pancreatic (pNEN); hormonal secretion: functioning or non-functioning (F or non-F); pathological WHO 2010 grade: G1 (<2 mitotic count; Ki-67 ≤ 2) and G2 (2–20 mitotic count; 3% ≤ Ki67 ≤ 20%) and disease extension (loco-regional or metastatic disease) [19]. Non-localized disease extension was categorized as locoregional or metastatic, to enable the grouping of WD GEP-NENs, since ENETS staging categories diverge according to the primary tumor site. Patients with insufficient data to allow grading were classified as WD GEP-NEN only if found to express somatostatin receptors on 68Ga-DOTA-NOC-PET/CT (n = 2). Patients with WD GEP-NEN-presenting metastatic disease and carcinoid syndrome without any visible pancreatic or thoracic lesions on imaging studies were assumed as having midgut primary tumors (n = 2). No insulinoma or rare functional pancreatic NEN presenting with hyperglycemia, such as glucagonoma, VIPoma, or somatostatinoma, were included in this patient series [20].
Patients with WD GEP-NENs were assessed for body mass index (BMI) class [21], fasting plasma glucose (FPG) category [22], and the presence of MetS diagnostic criteria or any individual MetS component [23].
Data for analysis were collected during face-to-face patient interviews, to assess past medical history of type 2 diabetes (T2D), hypertension, dyslipidemia, ongoing medications, and family history of T2D. Anthropometric parameters, such as height, weight, waist circumference (WC), and blood pressure (BP), and biochemical data, including FPG and lipid profile, were evaluated after blood sampling at our institution for treatment-naïve patients, or retrospectively through data-files recollection of parameters before initiation of any treatment intervention at other healthcare institutions, whenever the patient was already under treatment by referral to our center.
Patients were classified into three categories according to BMI: normal weight (BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), or obese (BMI ≥ 30 kg/m2) [21]. Patients were also classified according to FPG levels: euglycemic (NG; FPG < 100 mg/dL), impaired fasting glucose (IFG; 100 mg/dL ≤ FPG < 126 mg/dL), or T2D (FPG ≥ 126 mg/dL) [22]. MetS was classified according to the Joint Interim Statement (JIS) of IDFTFEP (International Diabetes Federation Task Force on Epidemiology and Prevention)/NHLBI (National Heart, Lung, and Blood Institute)/AHA (American Heart Association)/WHF (World Heart Federation)/IAS (International Atherosclerosis Society)/IASO (International Association for the Study of Obesity) criteria [23]: WC ≥ 88 cm (female) or 102 cm (male); systolic BP ≥ 130 or diastolic BP ≥ 85 mmHg or previous history of high BP or under BP-lowering medication; HDL-cholesterol (HDL-c) < 40 mg/dL (male) or ≤ 50 mg/dL (female) or drug treatment to reduce HDL-c; triglycerides (TG) ≥ 150 mg/dL or on triglyceride-lowering drugs; FPG ≥ 100 mg/dL or ongoing treatment with glucose-lowering drugs.
OS was defined as the time in months between the date of first treatment intervention and the date of last contact or death. PFS was defined as the time in months between the date of the first treatment and the date of first progression detection, date of the last contact, or death.
Statistical analysis was performed using R software v4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were summarized as frequencies and percentages. Continuous variables were presented as median, minimum, and maximum. Chi-square or Fisher’s exact tests were used to evaluate the association between two categorical variables. Medians between groups were compared using the Mann–Whitney or Kruskal–Wallis test. OS, PFS, and the corresponding 95% confidence intervals (CIs) were estimated using the Kaplan–Meier method. Survival between groups was compared by the log-rank test. Cox proportional-hazards regression analysis was used to compute hazard ratios (HR) with the corresponding 95% CIs. For the Cox proportional-hazards regression analysis, univariate models were performed, and each variable was considered separately. Then, the variables that were considered statistically significant in the univariate models were retained for the multivariable model. The final multivariable model contains the variables that were statistically significant. All tests of statistical significance were two-sided; a p value < 0.05 was considered significant.
3. Results
3.1. Baseline Characteristics of Patient Population with WD GEP-NEN
Patients with WD GEP-NEN (n = 81) were divided into two groups, according to baseline characteristics considering the absence (n = 33; 40.7%) or presence of MetS (n = 48; 59.3%) at diagnosis (Table 1). Patients in the MetS group were older (63 years for MetS vs. 53 years for non-MetS; p = 0.002), but there was no significant difference in gender distribution (p = 0.262). As expected, patients with MetS group had higher median BMI, WC, SBP, DBP, triglycerides and FPG levels than non-MetS group.

Table 1.
Patients and well-differentiated gastro-entero-pancreatic neuroendocrine neoplasia (WD GEP-NEN) tumor characteristics (n = 81), according to the presence of metabolic syndrome (MetS) criteria.
When comparing patients with or without MetS, there were no differences in the primary tumor site, presence of hormonal secretion syndrome, or metastatic disease. G1 tumors were more frequent in patients with MetS (64.6%) than non-MetS (51.5%), although this difference was not statistically significant (p = 0.241). There were also no statistically significant differences in follow-up time for OS (101.7 months for non-MetS vs. 84.7 months for. MetS; p = 0.204), nor in follow-up time for PFS (50.6 months for non-MetS vs. 50.7 months for MetS; p = 0.966), between the two groups.
3.2. Overall Survival and Progression-Free Survival at 5 and 10 Years according to the Presence of Metabolic Syndrome
No statistically significant differences were found in OS and PFS rates at 5 and 10 years between patients with MetS and without MetS (Table 2). The OS rate for patients with MetS was 87.1% (95% CI: 73.6–94.0) at 5 years, whereas the rate was 90.9% (95% CI: 74.4–97.0) for patients without MetS. The OS rate at 10 years was 72.5% (95% CI: 55.3–84.0) for patients with MetS and 76.4% (95% CI: 53.6–89.0) for patients without MetS. ThPFS rate for patients with MetS was 45.9% (95% CI: 30.8–59.8) at 5 years, whereas the rate was 40.0% (95% CI: 21.3–58.1) for patients without MetS. The PFS rate at 10 years was 18.1% (95% CI: 7.0–33.5) for patients with MetS and 24.4% (95% CI: 9.0–43.7) for patients without MetS.

Table 2.
WD GEP-NEN Mortality Overall Survival and Progression-free Survival at 5 and 10 years.
There were no significant differences in the presence of MetS individual components namely, waist circumference, hypertension, elevated triglycerides, low HDL-c, and elevated FPG (Table 2) in OS and PFS at 5 and 10 years.
3.3. Median Overall Survival and Progression-Free Survival of Patients with WD GEP-NEN according to the Presence of MetS
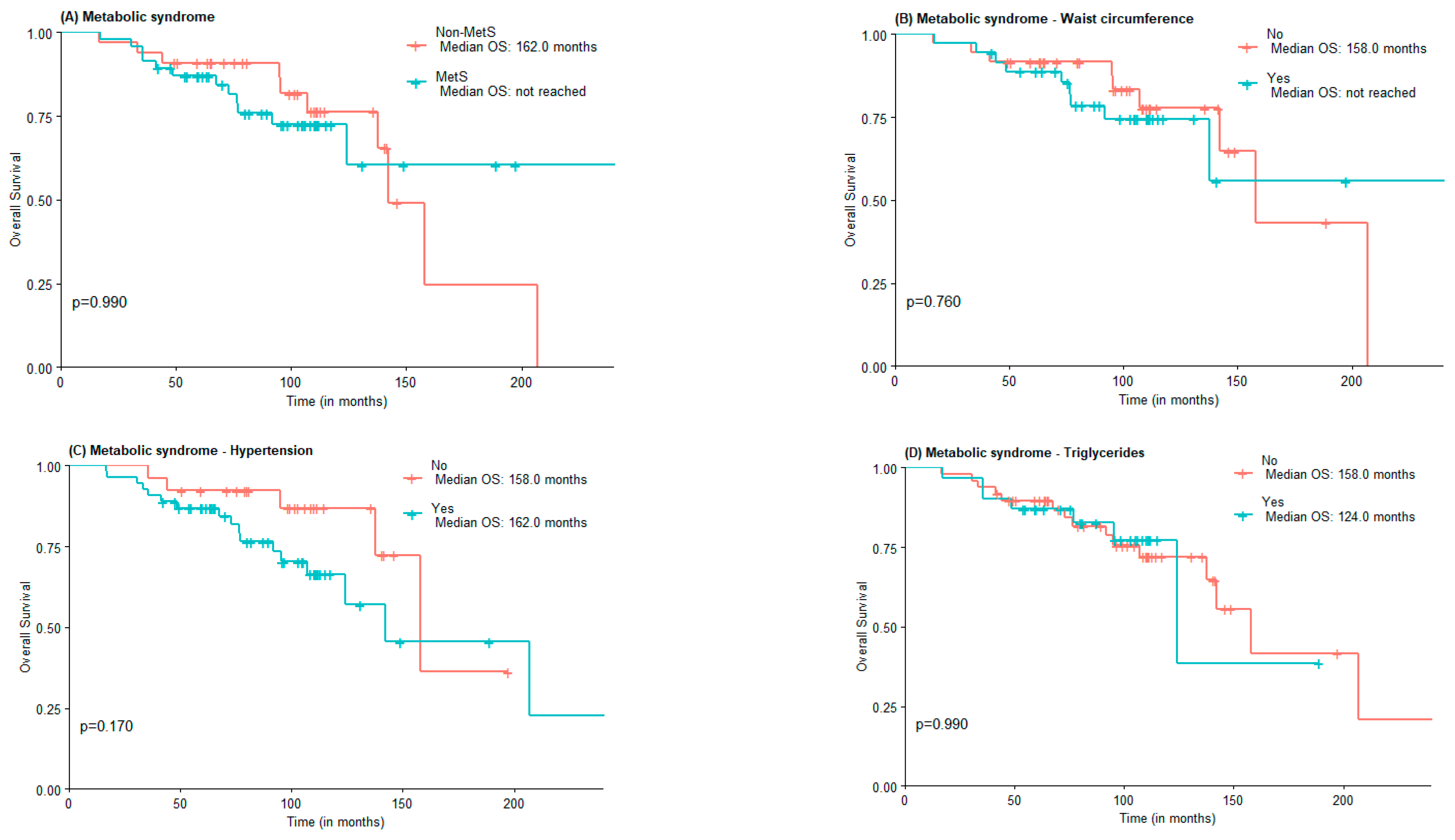
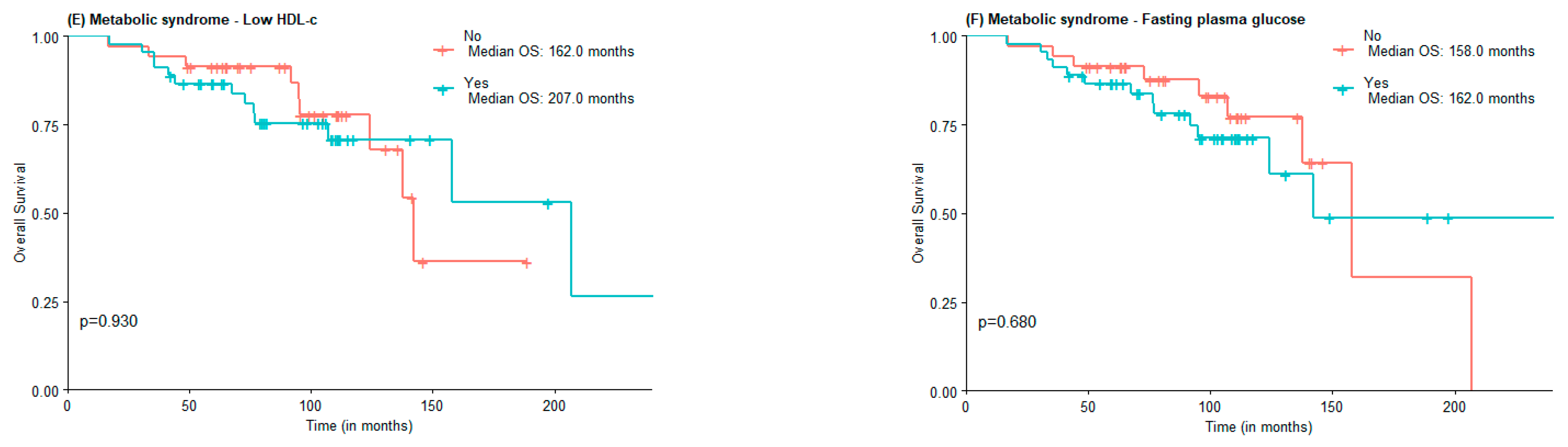
The median OS was 142.0 months for the non-MetS group and was not reached for the MetS one (Figure 1A). For abdominal obesity measured by WC, the median OS was 158.0 months for patients without abdominal obesity and not reached for patients with abdominal obesity (Figure 1B). The median OS was lower in the group with MetS criteria for hypertension vs. non-MetS group (142.0 vs. 158 months) (Figure 1C). The median OS for elevated triglycerides and FPG was lower in the MetS group, although not statistically significant (124 vs. 158 months and 142 vs. 158 months, respectively). Interestingly, the median OS of patients with low HDL-c was higher when compared to those of patients with normal or elevated HDL-c (207.0 months vs. 142.0 months), in the low HDL-c group there was also a greater proportion of patients under statin treatment (Figure 1E).
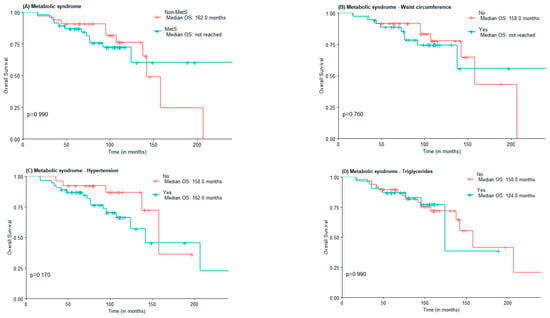
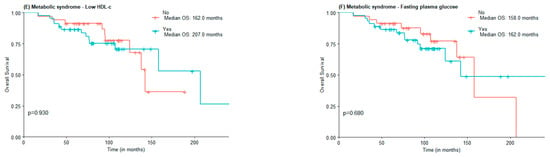
Figure 1.
OS according to the presence of Metabolic Syndrome (A) and Metabolic Syndrome components waist circumference (B), hypertension (C), high triglycerides (D), low HDL-c (E), and Fasting plasma glucose (F).
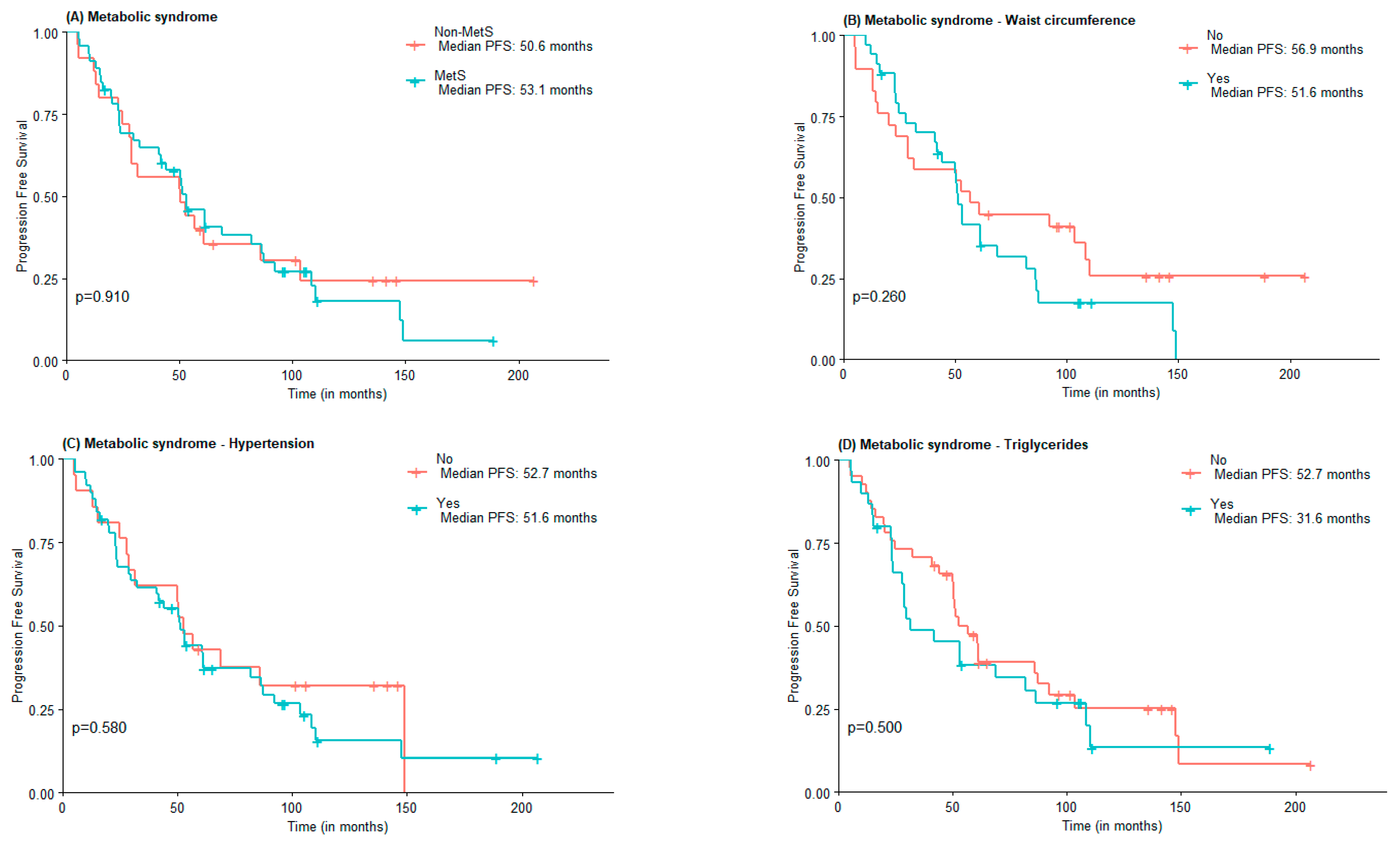
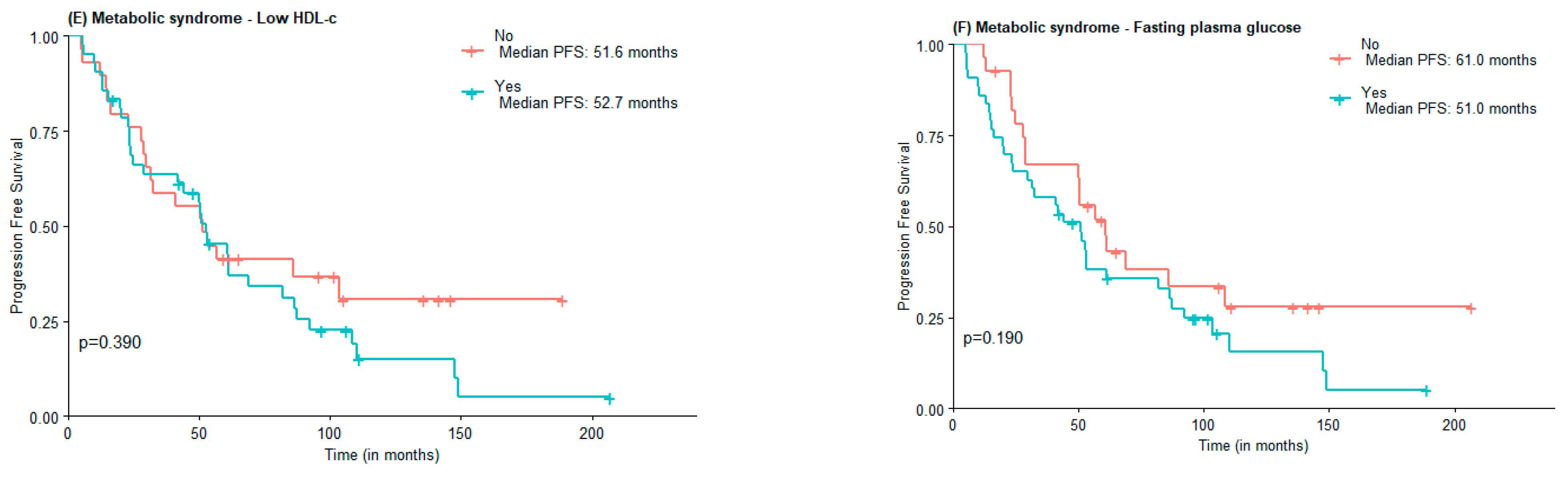
Regarding PFS, no statistically significant differences were found between MetS and non-MetS groups (53.1 vs. 50.6 months), nor concerning MetS components elevated WC (51.6 vs. 56.9 months), hypertension (51.6 vs. 52.7 months), and HDL-c (52.76 vs. 51.6 months). Subjects with elevated triglycerides and FPG had lower PFS compared to those with normal triglycerides and FPG, although not statistically significant (31.6 vs. 52.7 months and 51.0 vs. 61.0 months) (Figure 2).
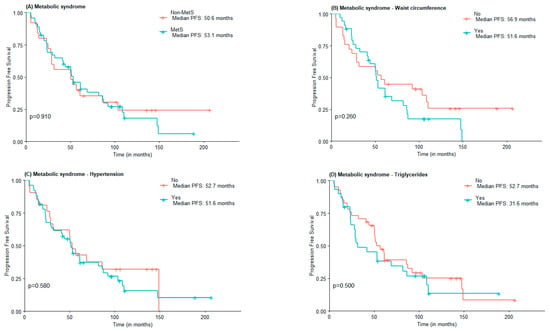
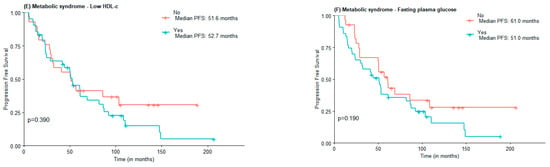
Figure 2.
PFS according to the presence of Metabolic Syndrome (A) and Metabolic Syndrome components waist circumference (B), hypertension (C), high triglycerides (D), low HDL-c (E), and Fasting plasma glucose (F).
3.4. Influence of Tumor Pathological Features on Patient Outcomes
When considering all the characteristics individually, WHO tumor grade was the only characteristic that influenced OS, as patients diagnosed with grade 2 tumors had shorter OS than patients diagnosed with grade 1 tumors (HR = 3.84; 95% CI: 1.41–10.50). PFS was influenced by WHO grading (patients who presented grade 2 tumors had a shorter PFS than patients diagnosed with grade 1 (HR = 2.34; 95% CI: 1.30–4.22)), stage (patients diagnosed with metastatic disease had a shorter PFS when compared to patients diagnosed with locoregional disease; HR = 3.11; 95% CI: 1.33–7.30), and waist circumference (patients with elevated waist circumference had a shorter PFS than patients with normal waist circumference; HR = 1.03; 95% CI: 1.01–1.06).
When considering a multivariable model only WHO grading (HR = 2.17; 95% CI: 1.15–4.11) and waist circumference (HR = 1.03; 95% CI: 1.01–1.06) influenced survival outcomes. Table 3 presents the hazard ratios for OS and PFS according to patient and tumor characteristics.

Table 3.
Hazard Ratios and 95% confidence intervals for Overall Survival and Progression-free Survival, according to patient and tumor characteristics, using univariate and multivariate logistic regression.
4. Discussion
To the best of our knowledge, this is one of the first studies addressing the influence of obesity and MetS on GEP-NEN outcomes and the first that includes abdominal obesity. In our patient cohort with WD GEP-NEN, using waist circumference as a surrogate marker for visceral obesity at diagnosis, was shown to have a prognostic value for PFS in patients with non-localized WD GEP-NEN (HR = 1.01; 95% CI: 1.01–1.06), although not affecting OS. Additionally, this study also confirmed previous findings that intrinsic tumor factors, namely WHO grade G2 influences OS (HR = 3.84; 95% CI: 1.41–10.50) and PFS, both at univariate (HR = 2.34; 95% CI: 1.30–4.22) and multivariate (HR = 2.17; 95% CI: 1.11–4.11) analysis. Metastatic disease at diagnosis was also shown to influence PFS (HR = 3.11; 95% CI: 1.33–7.30), but not OS (HR = 3.60; 95% CI: 0.84–15.51).
The association of obesity, MetS, and T2D with cancer risk is well established. The International Agency for Research on Cancer (IARC) group and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recognized the influence of overweight and obesity as RF for 13 different types of cancers, including breast, colorectal, endometrial, esophageal adenocarcinoma, gallbladder, gastric, renal cell, liver, multiple myeloma, ovarian, pancreas, and thyroid, despite different levels of evidence applying for each type of tumor [7]. Indeed, 5.6% of all incident cancers can be attributed to the combined effects of diabetes and BMI as independent risk factors, among which high BMI was responsible for twice as many cancer cases as diabetes [24]. Additionally, there is now enough evidence to state that not only is MetS a RF for cancer, but also MetS individual components, such as visceral obesity, hyperglycemia, dyslipidemia, and hypertension, independently of BMI [25]. Moreover, a recent meta-analysis concluded that central adiposity is a stronger predictor of non-neuroendocrine tumor risk than overall body size [26].
In parallel with obesity and MetS, an exponential rise in NEN incidence has been noticed over the last forty years, which has traditionally been attributed to improved diagnostic skills and imaging techniques. However, little is known about how extrinsic RFs have influenced NEN’s burden. Our group has recently described an association between MetS and some MetS individual components, such as visceral obesity and abnormal FPG with WD GEP-NEN [17]. We have also found that patients with WD GEP-NEN and MetS had a higher risk of presenting a lower tumor grade yet disseminated disease at diagnosis, independently of primary tumor site and hormonal status [27]. In support of our previous study’s findings, a metanalysis published by Leoncini et. al. [28] concluded that obesity conferred an estimate OR = 1.37 (95% CI: 0.25–7.69; I2 = 98.5; p < 0.0001) for pancreatic NEN, although this conclusion was not supported by all studies included. The available data also suggested that diabetes conferred an increased risk for pancreatic NEN (OR = 2.76; 95% CI: 1.65–4.64; I2 = 55.3; p = 0.090), the risk being even higher for recent onset diabetes (OR = 12.80; 95% CI: 2.47–66.42; I2 = 55.3; p = 0.135). A recent Italian case-control study, that evaluated the influence of chronotype on WD GEP-NEN, concluded that a higher proportion of evening chronotype and unhealthy metabolic profile, along with smoking and sedentary habits, was observed in patients with GEP-NEN when compared to healthy controls [29].
Another matter of debate is the influence of obesity and metabolic diseases on cancer outcomes. In contrast to epidemiological studies seeking to identify RFs for cancer incidence, studies aimed to identify RFs for patient outcomes have been less conclusive. Additionally, these studies are often hampered by the multitude of variables to be considered, such as patient overall condition, anti-cancer treatments, and treatment side effects, as well as other ongoing treatments for chronic conditions. Notwithstanding, a recent meta-analysis was able to conclude that the survival of patients with digestive tract cancer was negatively affected by the presence of MetS [13]. MetS was also found to be associated with a higher risk of death from colorectal (HR = 3.48; 95% CI: 1.68–7.22) and breast cancer in women (HR = 11.90; 95% CI: 2.25–62.84) [14]. Moreover, overall cancer mortality was found to be higher in patients with abdominal adiposity, independently of BMI, over a mean follow-up of 9.7 years [30]. Concerning NEN, despite several pathological and molecular tumor features that have been identified to be relevant for prognosis [31,32,33,34,35], data on how extrinsic factors, such as obesity and metabolic diseases influence disease outcomes is very scarce.
A recent post-hoc analysis of the CLARINET study on the impact of diabetes and metformin at GEP-NEN PFS concluded that diabetes was not a negative prognostic factor. However, patients in the placebo group who were under metformin had longer PFS (85.7 weeks for metformin vs. 38.7 weeks for no-metformin, p = 0.017), an effect that was not observed in the lanreotide treatment arm [36]; a finding which favors the hypothesis that the anti-tumor effect of metformin is abrogated in the presence of mTORC1 axis inhibition by somatostatin analogues. Metformin is an anti-diabetic drug with direct action on phosphoinositide 3-kinase/serine/threonine kinase/mTor (Pi3k/Akt/mTor pathway) via Adenosine Monophosphate-Activated Protein Kinase (AMPK) suppressive activity on Mammalian Target of Rapamycin Complex 1 (mTORC1) and indirect effects on insulin secretion and insulin-like growth factor-1 (IGF-1) activity, which was also demonstrated to depict anti-cancer effects [37]. Indeed, the anti-proliferative effects of metformin in NEN, as an adjuvant treatment to conventional therapy, have been described in vitro [38] and suggested by retrospective studies [39,40,41].
The PRIME-NET Study conclusions contrast with those of CLARINET post-hoc. In the former study, patients with diabetes treated with everolimus and somatostatin analogues under metformin, had longer PFS than patients under other anti-diabetic drugs [42]. It is noteworthy that our study cohort differs from the ones of the abovementioned studies. When compared to CLARINET post-hoc, the follow-up time is longer (95.0 months vs. 24 months) and included mainly carcinoid syndrome-associated GI-tract primary tumors (55.6% midgut tumors) vs. non-functioning pNEN [36], a feature that also contrasts with the PRIME-NET study that included only patients with pNEN, some of which in the context of MEN1, while the hormonal status was not described [42]. Our study is one of the first to describe the association of MetS with functioning GI-NEN, namely midgut NEN, while most of our previous data documented the association of metabolic abnormalities with non-functioning pNEN [28]. In fact, our cohort included predominantly functioning tumors (60.5%). Moreover, our study population consisted of well-differentiated tumors (WHO 2010 NET G1 and NET G2), while CLARINET post-hoc only included tumors with Ki67 < 10% and PRIME-NET included all WHO categories, which could have biased the results. Additionally, although the percentage of patients who were overweight (40.7%) in our study was similar to the PRIME-NET and CLARINET studies, we found that almost half of our patients had abdominal obesity (45.7%), a parameter which was not assessed in the two previous studies. Diabetes was present in 23.7% of our patient cohort, vs. 38.7% in CLARINET post-hoc and 100% in PRIME-NET; while FGA was noticed in 19.8% of our cohort, and neither study evaluated intermediate hyperglycemia. Notwithstanding, our results show that elevated waist circumference was associated with shorter PFS, regardless of the fact that BMI, MetS, hypertension, dyslipidemia, and hyperglycemia were not found to be associated with poorer WD-GEP-NEN prognosis [43]. A 2021 analysis on the Surveillance, Epidemiology, and End Results (SEER) databases and linked Medicare claims (1991–2016), compared the risk for T2D in somatostatin analogues (SSA) treated vs. no-SSA treated patients, did not find significant differences, which suggests that GEP-NEN patients have a higher risk for T2D, independently of the hyperglycemic effects of treatment [44]. Similar findings were published by our group [17]. Evening chronotype also seems to influence GEP-NEN prognosis, as it showed to be associated with distant metastasis, a higher grade, and progressive disease [29].
Data on the influence of dyslipidemia on GEP-NEN prognosis is limited to few studies. Disease progression was associated with elevated triglycerides in advanced pNEN treated with everolimus, and reversed with dietary and pharmacological intervention with statins and metformin [45]. IL-6 expression was found to be influenced by low HDL-c, and positively associated with disease progression in a cohort of WD GEP-NEN [46]. In vitro experiments with neuroendocrine pancreatic and midgut cells also showed the anti-proliferative effects of statins, pointing to a direct anti-tumor effect besides the lipid-lowering action [40,47].
Our study has some strengths and limitations. One of the strengths is having collected all data at diagnosis, overcoming any biases related to the effect of treatment on glycemic profile and body weight. The effect of abdominal obesity independently of BMI on disease prognosis was also evaluated. The limitations include the fact that this study was conducted in a relatively small patient cohort (n = 81). The majority of patients with MetS were under anti-hypertensive and statins drugs, which biased the evaluation of blood pressure, as well as lipid levels as RFs for WD GEP-NEN, especially for total cholesterol, LDL-c and HDL-c. The small number of patients under metformin treatment did not allow analyzing the influence of that antidiabetic drug on WD GEP-NEN outcomes. Another limitation is that the influence of the different tumor and metabolic targeted treatments on patient outcomes were not evaluated, nor were BMI trajectory and metabolic parameters within the follow-up.
5. Conclusions
In summary, a new era for oncological diseases is emerging, where lifestyle-related modifiable risk factors for cancer play an important role not only in the pathogenesis and incidence, but also in prognosis. Neuroendocrine neoplasia burden in the last four decades is likely to be part of this problem. In this study, we demonstrate that waist circumference, a surrogate measure for visceral obesity, is associated with significantly shorter PFS in WD GEP-NEN. This finding reinforces the potential link between visceral obesity and GEP-NEN, and further suggests that obesity could influence disease prognosis.
Author Contributions
Conceptualization, A.P.S. and M.P.M.; methodology, A.P.S. and J.R.; software, J.R.; validation, M.P.M., R.H. and M.H.C.; investigation, A.P.S.; writing—original draft preparation A.P.S. and J.R.; writing—review and editing, A.P.S., J.R., R.H., M.H.C. and M.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
Jéssica Rocha Rodrigues is funded by the Foundation for Science and Technology (FCT) through the following PhD grant: UI/BD/152282/2021.This work is funded by the Foundation for Science and Technology (FCT) through the following funds: UIDB/00215/2020, UIDP/00215/2020 and LA/P/0064/2020.
Institutional Review Board Statement
The study was approved by the Institutional Ethics Committee of the Portuguese Oncology Institute of Porto (IPO Porto)-(IPOP/366/2013). All participants provided informed consent prior to study enrolment.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors will make available the anonymized data used in this work upon request.
Acknowledgments
The authors are grateful to Sofia Pereira for revising and support the formatting of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- NCD Countdown Collaborators. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xu, C.; Yang, H.; Li, S.; Wang, Y. The role of healthy lifestyle in cancer incidence and temporal transitions to cardiometabolic disease. Cardio Oncol. 2021, 3, 663–674. [Google Scholar] [CrossRef]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Garg, S.K.; Maurer, H.; Reed, K.; Selagamsetty, R. Diabetes and cancer: Two diseases with obesity as a common risk factor. Diabetes Obes. Metab. 2014, 16, 97–110. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Agalliu, I.; Lin, D.W.; Stanford, J.L.; Kristal, A.R. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer 2007, 109, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhang, M.; Zhang, H.; Xia, Y.; Lin, J.; Zheng, X.; Peng, F.; Niu, W. Prediction of Metabolic Syndrome for the Survival of Patients With Digestive Tract Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Kakehi, E.; Kotani, K.; Kayaba, K.; Nakamura, Y.; Ishikawa, S. Metabolic syndrome is a risk factor for cancer mortality in the general Japanese population: The Jichi Medical School Cohort Study. Diabetol. Metab. Syndr. 2019, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, W.Y.; Zhu, G.Q.; Wang, O.C.; Ma, R.M.; Huang, G.Q.; Shi, K.Q.; Guo, G.L.; Braddock, M.; Zheng, M.H. Metabolic syndrome contributes to an increased recurrence risk of non-metastatic colorectal cancer. Oncotarget 2015, 6, 19880–19890. [Google Scholar] [CrossRef]
- Balentine, C.J.; Enriquez, J.; Fisher, W.; Hodges, S.; Bansal, V.; Sansgiry, S.; Petersen, N.J.; Berger, D.H. Intra-abdominal fat predicts survival in pancreatic cancer. J. Gastrointest. Surg. 2010, 14, 1832–1837. [Google Scholar] [CrossRef]
- Santos, A.P.; Santos, A.C.; Castro, C.; Raposo, L.; Pereira, S.S.; Torres, I.; Henrique, R.; Cardoso, H.; Monteiro, M.P. Visceral Obesity and Metabolic Syndrome Are Associated with Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2018, 10, 293. [Google Scholar] [CrossRef]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- O’Toole, D.; Kianmanesh, R.; Caplin, M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology 2016, 103, 117–118. [Google Scholar] [CrossRef]
- O’Toole, D.; Salazar, R.; Falconi, M.; Kaltsas, G.; Couvelard, A.; de Herder, W.W.; Hyrdel, R.; Nikou, G.; Krenning, E.; Vullierme, M.P.; et al. Rare functioning pancreatic endocrine tumors. Neuroendocrinology 2006, 84, 189–195. [Google Scholar] [CrossRef]
- Borrell, L.N.; Samuel, L. Body mass index categories and mortality risk in US adults: The effect of overweight and obesity on advancing death. Am. J. Public Health 2014, 104, 512–519. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 2018, 6, e6–e15. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Barberio, A.M.; Alareeki, A.; Viner, B.; Pader, J.; Vena, J.E.; Arora, P.; Friedenreich, C.M.; Brenner, D.R. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat. Commun. 2019, 10, 383. [Google Scholar] [CrossRef]
- Santos, A.P.; Castro, C.; Antunes, L.; Henrique, R.; Cardoso, M.H.; Monteiro, M.P. Disseminated Well-Differentiated Gastro-Entero-Pancreatic Tumors Are Associated with Metabolic Syndrome. J. Clin. Med. 2019, 8, 1479. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G.; La Vecchia, C.; Boccia, S.; Rindi, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 68–81. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Modica, R.; Laudisio, D.; Aprano, S.; Faggiano, A.; Colao, A.; Savastano, S. Chronotype: What role in the context of gastroenteropancreatic neuroendocrine tumors? J. Transl. Med. 2021, 19, 324. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef]
- Pape, U.F.; Berndt, U.; Muller-Nordhorn, J.; Bohmig, M.; Roll, S.; Koch, M.; Willich, S.N.; Wiedenmann, B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2008, 15, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tan, Y.; Ge, W.; Ding, K.; Hu, H. Pattern and risk factors for distant metastases in gastrointestinal neuroendocrine neoplasms: A population-based study. Cancer Med. 2018, 7, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Tsai, K.L.; Liang, C.M.; Tai, W.C.; Rau, K.M.; Wu, K.L.; Huang, C.C.; Chuah, S.K. Prognostic factors of patients with gastroenteropancreatic neuroendocrine neoplasms. Kaohsiung J. Med. Sci. 2018, 34, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, A.; Pea, A.; Chang, D.K.; Jamieson, N.B. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front. Med. 2020, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, L.; Dai, S.; Chen, M.; Li, F.; Sun, J.; Luo, F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw. Open 2021, 4, e2124750. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Prinzi, N.; Torchio, M.; Corti, F.; Coppa, J.; Buzzoni, R.; Di Bartolomeo, M.; Milione, M. Impact of Diabetes and Metformin Use on Enteropancreatic Neuroendocrine Tumors: Post Hoc Analysis of the CLARINET Study. Cancers 2022, 14, 69. [Google Scholar] [CrossRef]
- Lei, Y.; Yi, Y.; Liu, Y.; Liu, X.; Keller, E.T.; Qian, C.-N.; Zhang, J.; Lu, Y. Metformin targets multiple signaling pathways in cancer. Chin. J. Cancer 2017, 36, 17. [Google Scholar] [CrossRef]
- Vlotides, G.; Tanyeri, A.; Spampatti, M.; Zitzmann, K.; Chourdakis, M.; Spöttl, G.; Maurer, J.; Nölting, S.; Göke, B.; Auernhammer, C.J. Anticancer effects of metformin on neuroendocrine tumor cells in vitro. Hormones 2014, 13, 498–508. [Google Scholar] [CrossRef]
- Pusceddu, S.; Buzzoni, R.; Vernieri, C.; Concas, L.; Marceglia, S.; Giacomelli, L.; Milione, M.; Leuzzi, L.; Femia, D.; Formisano, B. Metformin with everolimus and octreotide in pancreatic neuroendocrine tumor patients with diabetes. Future Oncol. 2016, 12, 1251–1260. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Pedraza-Arevalo, S.; L-López, F.; Gahete, M.D.; Gálvez-Moreno, M.A.; Castaño, J.P.; Luque, R.M. Type 2 diabetes in neuroendocrine tumors: Are biguanides and statins part of the solution? J. Clin. Endocrinol. Metab. 2019, 104, 57–73. [Google Scholar] [CrossRef]
- Vernieri, C.; Pusceddu, S.; de Braud, F. Impact of metformin on systemic metabolism and survival of patients with advanced pancreatic neuroendocrine tumors. Front. Oncol. 2019, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Marconcini, R.; Spada, F.; Massironi, S.; Ibrahim, T.; Brizzi, M.P.; Campana, D.; Faggiano, A.; et al. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 2018, 155, 479–489.e477. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Marais, R. Metformin: A diabetes drug for cancer, or a cancer drug for diabetics? J. Clin. Oncol. 2012, 30, 2698–2700. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Yang, J.Y.; Baeg, K.; Leiter, A.C.; Mhango, G.; Gallagher, E.J.; Wisnivesky, J.P.; Kim, M.K. Association between somatostatin analogues and diabetes mellitus in gastroenteropancreatic neuroendocrine tumor patients: A Surveillance, Epidemiology, and End Results-Medicare analysis of 5235 patients. Cancer Rep. 2021, 4, e1387. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Pusceddu, S.; Fucà, G.; Indelicato, P.; Centonze, G.; Castagnoli, L.; Ferrari, E.; Ajazi, A.; Pupa, S.; Casola, S.; et al. Impact of systemic and tumor lipid metabolism on everolimus efficacy in advanced pancreatic neuroendocrine tumors (pNETs). Int. J. Cancer 2019, 144, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Pereira, R.; Santos, A.P.; Costa, M.M.; Morais, T.; Sampaio, P.; Machado, B.; Afonso, L.P.; Henrique, R.; Monteiro, M.P. Higher IL-6 peri-tumoural expression is associated with gastro-intestinal neuroendocrine tumour progression. Pathology 2019, 51, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Nölting, S.; Maurer, J.; Spöttl, G.; Aristizabal Prada, E.T.; Reuther, C.; Young, K.; Korbonits, M.; Göke, B.; Grossman, A.; Auernhammer, C.J. Additive Anti-Tumor Effects of Lovastatin and Everolimus In Vitro through Simultaneous Inhibition of Signaling Pathways. PLoS ONE 2015, 10, e0143830. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).