Are Cirrhotic Patients Receiving Invasive Mechanical Ventilation at Risk of Abundant Microaspiration
Abstract
:1. Introduction
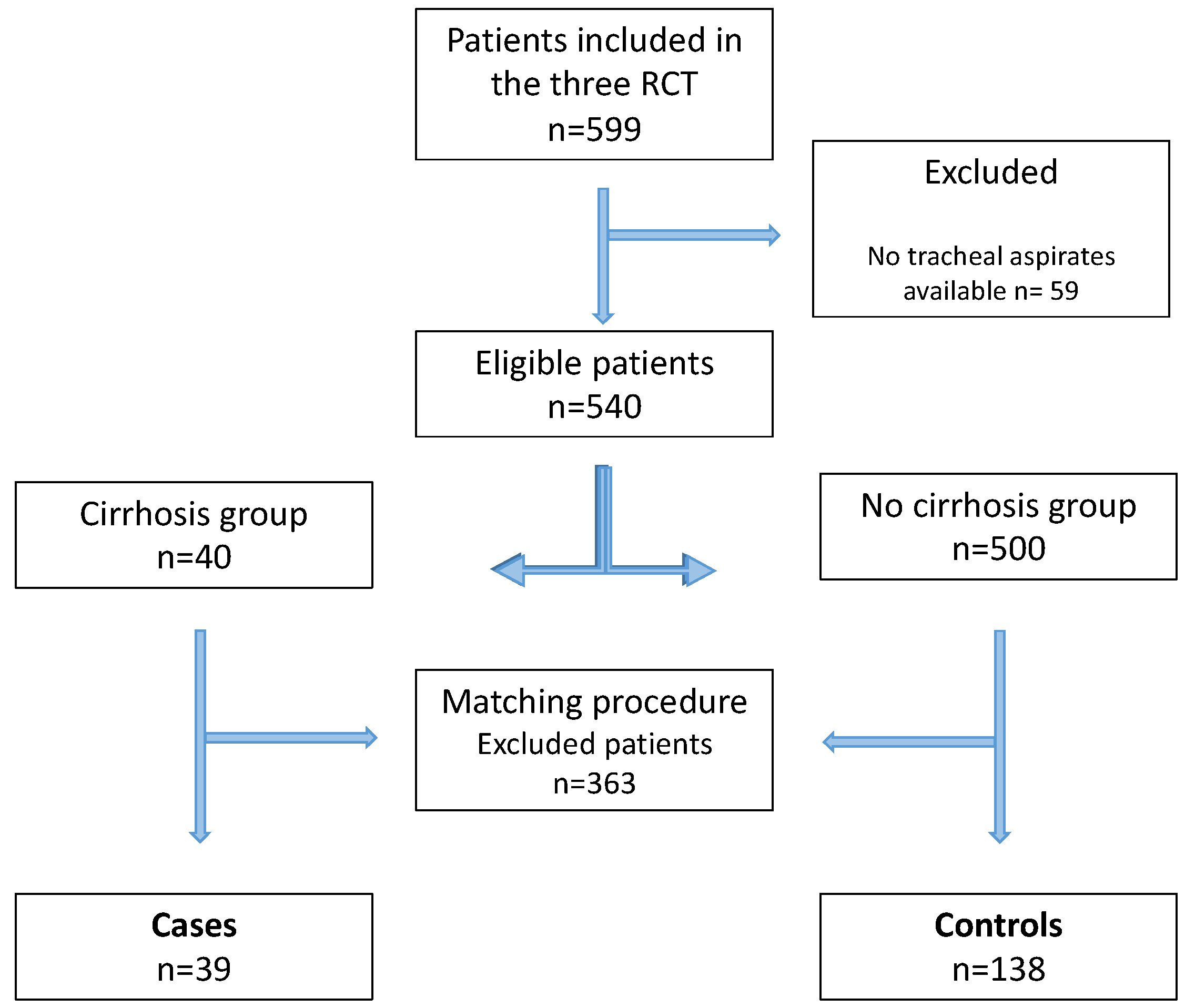
2. Materials and Methods
3. Results
3.1. At Inclusion
3.2. Primary Outcome
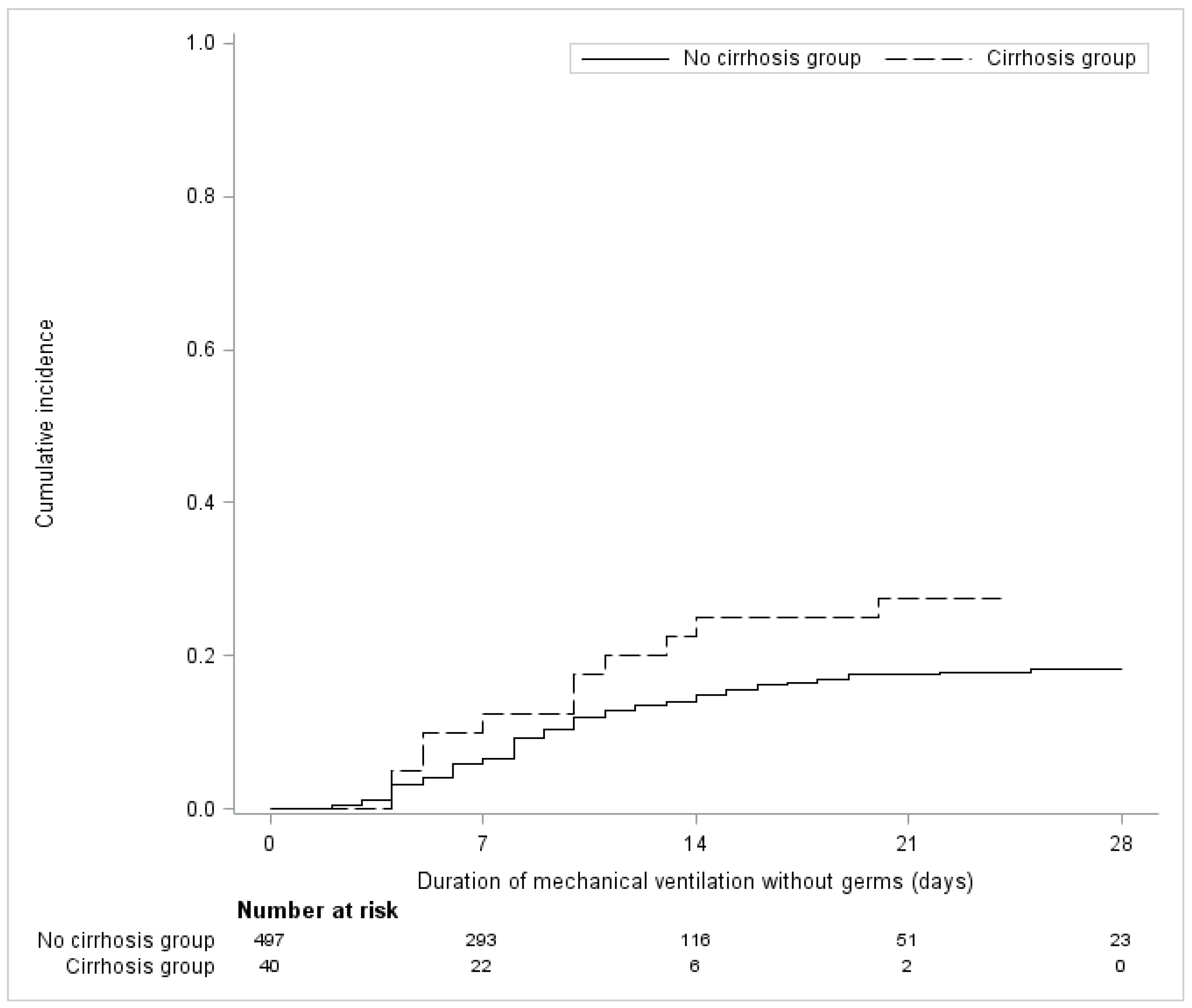
3.3. Secondary Outcomes
3.4. Characteristics of VAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar]
- Rello, J.; Ramírez-Estrada, S.; Romero, A.; Arvaniti, K.; Koulenti, D.; Nseir, S.; Oztoprak, N.; Bouadma, L.; Vidaur, L.; Lagunes, L.; et al. Factors associated with ventilator-associated events: An international multicenter prospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1693–1699. [Google Scholar]
- Rouzé, A.; Jaillette, E.; Nseir, S. Relationship between microaspiration of gastric contents and ventilator-associated pneumonia. Ann. Transl. Med. 2018, 6, 428. [Google Scholar]
- Majumdar, A.; Bailey, M.; Kemp, W.M.; Bellomo, R.; Roberts, S.K.; Pilcher, D. Declining mortality in critically ill patients with cirrhosis in Australia and New Zealand between 2000 and 2015. J. Hepatol. 2017, 67, 1185–1193. [Google Scholar]
- Otero Sanchez, L.; Karakike, E.; Njimi, H.; Putignano, A.; Degré, D.; Hites, M.; Jacobs, F.; Moreno, C.; Trepo, E.; Gustot, T.; et al. Clinical Course and Risk Factors for Infection in Severe Forms of Alcohol-Associated Liver Disease. Hepatology 2021, 74, 2714–2724. [Google Scholar]
- Levesque, E.; Saliba, F.; Ichaï, P.; Samuel, D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014, 60, 570–578. [Google Scholar]
- Papakrivou, E.; Makris, D.; Manoulakas, E.; Karvouniaris, M.; Zakynthinos, E. Intra-Abdominal Hypertension is a Risk Factor for Increased VAP Incidence: A Prospective Cohort Study in the ICU of a Tertiary Hospital. J. Intensive Care Med. 2020, 35, 700–707. [Google Scholar]
- Lima, R.; Silva, P.L.; Capelozzi, V.L.; Oliveira, M.G.; Santana, M.C.E.; Cruz, F.F.; Pelosi, P.; Schanaider, A.; Malbrain, M.L.; Rocco, P.R.; et al. Early impact of abdominal compartment syndrome on liver, kidney and lung damage in a rodent model. Anaesthesiol. Intensive Ther. 2017, 49, 130–138. [Google Scholar]
- Rouzé, A.; De Jonckheere, J.; Zerimech, F.; Labreuche, J.; Parmentier-Decrucq, E.; Voisin, B.; Jaillette, E.; Maboudou, P.; Balduyck, M.; Nseir, S. Efficiency of an electronic device in controlling tracheal cuff pressure in critically ill patients: A randomized controlled crossover study. Ann. Intensive Care 2016, 6, 93. [Google Scholar]
- Jaillette, E.; BestCuff Study Group and the BoRéal Network; Girault, C.; Brunin, G.; Zerimech, F.; Behal, H.; Chiche, A.; Broucqsault-Dedrie, C.; Fayolle, C.; Minacori, F.; et al. Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: A multicenter cluster-randomized cross-over controlled trial. Intensive Care Med. 2017, 43, 1562–1571. [Google Scholar]
- Nseir, S.; Le Gouge, A.; Lascarrou, J.B.; Lacherade, J.C.; Jaillette, E.; Mira, J.P.; Mercier, E.; Declercq, P.-L.; Sirodot, M.; Piton, G.; et al. Impact of nutrition route on microaspiration in critically ill patients with shock: A planned ancillary study of the NUTRIREA-2 trial. Crit. Care 2019, 23, 111. [Google Scholar]
- De Pietri, L.; Bianchini, M.; Montalti, R.; De Maria, N.; Di Maira, T.; Begliomini, B.; Gerunda, G.; Di Benedetto, F.; Garcia-Tsao, G.; Villa, E. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology 2016, 63, 566–573. [Google Scholar]
- Dewavrin, F.; Zerimech, F.; Boyer, A.; Maboudou, P.; Balduyck, M.; Duhamel, A.; Nseir, S. Accuracy of alpha amylase in diagnosing microaspiration in intubated critically-ill patients. PLoS ONE 2014, 9, e90851. [Google Scholar]
- Zhou, B.; Fine, J.; Latouche, A.; Labopin, M. Competing risks regression for clustered data. Biostatistics 2012, 13, 371–383. [Google Scholar]
- Boivin, Z.; Perez, M.F.; Atuegwu, N.C.; Anzueto, A.; Mortensen, E.M. Impact of Cirrhosis on Pneumonia-Related Outcomes in Hospitalized Older Veterans. Am. J. Med. Sci. 2019, 357, 296–301. [Google Scholar]
- Bonnel, A.R.; Bunchorntavakul, C.; Reddy, K.R. Immune dysfunction and infections in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 727–738. [Google Scholar]
- Herrero, R.; Sánchez, G.; Asensio, I.; López, E.; Ferruelo, A.; Vaquero, J.; Moreno, L.; de Lorenzo, A.; Bañares, R.; Lorente, J.A. Liver-lung interactions in acute respiratory distress syndrome. Intensive Care Med. Exp. 2020, 8 (Suppl. 1), 48. [Google Scholar]
- Bauer, M. The liver-gut-axis: Initiator and responder to sepsis. Curr. Opin. Crit. Care 2022, 28, 216–220. [Google Scholar]
- Di Pasquale, M.; Esperatti, M.; Crisafulli, E.; Ferrer, M.; Bassi, G.L.; Rinaudo, M.; Escorsell, A.; Fernandez, J.; Mas, A.; Blasi, F.; et al. Impact of chronic liver disease in intensive care unit acquired pneumonia: A prospective study. Intensive Care Med. 2013, 39, 1776–1784. [Google Scholar]
- Dong, V.; Sun, K.; Gottfried, M.; Cardoso, F.S.; McPhail, M.J.; Stravitz, R.T.; Lee, W.M.; Karvellas, C.J. Significant lung injury and its prognostic significance in acute liver failure: A cohort analysis. Liver Int. 2020, 40, 654–663. [Google Scholar]
- Qadir, N.; Wang, T.; Barjaktarevic, I.; Chang, S.Y. Acute Respiratory Failure and Pulmonary Complications in End-Stage Liver Disease. Semin. Respir. Crit. Care Med. 2018, 39, 546–555. [Google Scholar]
- Metheny, N.A.; Clouse, R.E.; Chang, Y.H.; Stewart, B.J.; Oliver, D.A.; Kollef, M.H. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: Frequency, outcomes, and risk factors. Crit. Care Med. 2006, 34, 1007–1015. [Google Scholar]
- Peñuelas, O.; Frutos-Vivar, F.; Fernández, C.; Anzueto, A.; Epstein, S.K.; Apezteguía, C.; González, M.; Nin, N.; Raymondos, K.; Tomicic, V.; et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am. J. Respir. Crit. Care Med. 2011, 184, 430–437. [Google Scholar]
| No Cirrhosis Group N = 500 | Cirrhosis Group N = 40 | ASD (%) | |
|---|---|---|---|
| Age (years), mean ± SD | 63.0 ± 14.7 | 57.0 ± 12.4 | 44.0 |
| Men | 335 (67.0) | 28 (70.0) | 6.5 |
| SAPS II, mean ± SD | 51.6 ± 17.8 | 50.6 ± 17.7 | 5.7 |
| Medical admission α | 352 (70.4) | 30 (75.0) | 15.5 |
| Shock | 331 (66.2) | 34 (85.0) | 44.9 |
| Chronic Renal Failure | 45 (9.0) | 3 (7.5) | 5.5 |
| Gastric Reflux | 26 (5.2) | 4 (10.0) | 18.2 |
| Immunodepression | 83 (16.6) | 5 (12.5) | 11.6 |
| Diabetes | 130 (26.0) | 8 (20.0) | 14.3 |
| COPD | 115 (23.0) | 6 (15.0) | 20.5 |
| Heart failure | 105 (21.0) | 3 (7.5) | 39.4 |
| Enteral feeding | 413 (82.6) | 34 (85.0) | 6.5 |
| Stress ulcer prophylaxis | 409 (81.8) | 33 (82.5) | 1.8 |
| Pump proton inhibitor 1 | 281 (56.5) | 27 (67.5) | 22.7 |
| Sedation | 400 (80.0) | 31 (77.5) | 6.1 |
| Prone positioning | 29 (5.8) | 8 (20.0) | 43.3 |
| Mean PEEP, median (IQR) | 6 (5 to 8) | 6 (5 to 10) | 17.2 |
| Mean GCS 2, median (IQR) | 14 (7 to 15) | 13 (6 to 15) | 19.8 |
| Number of tracheal aspirates 3 median (IQR) | 14 (8 to 23) | 16 (10 to 20) | 18.4 |
| No Cirrhosis Group N = 138 | Cirrhosis Group N = 39 | ASD (%) | |
|---|---|---|---|
| Age (years), mean ± SD | 58.8 ± 10.9 | 57.8 ± 11.6 | 9.0 |
| Men | 104 (75.4) | 28 (71.8) | 8.1 |
| SAPS II, mean ± SD | 50.1 ± 16.4 | 50.3 ± 17.9 | 1.3 |
| Medical admission α | 107 (77.5) | 30 (76.9) | 20.3 |
| Shock | 94 (68.1) | 33 (84.6) | 39.6 |
| Chronic Renal Failure | 8 (5.8) | 3 (7.7) | 7.6 |
| Gastric Reflux | 5 (3.6) | 4 (10.3) | 26.3 |
| Immunodepression | 27 (19.6) | 5 (12.8) | 18.4 |
| Diabetes | 29 (21.0) | 8 (20.5) | 1.2 |
| COPD | 27 (19.6) | 6 (15.4) | 11.0 |
| Heart failure | 22 (15.9) | 3 (7.7) | 25.8 |
| Enteral feeding | 114 (82.6) | 33 (84.6) | 5.4 |
| Stress Ulcer Prophylaxis | 116 (84.1) | 32 (82.1) | 5.4 |
| Pump Proton Inhibitor | 86 (62.3) | 26 (66.7) | 9.1 |
| Sedation | 113 (81.9) | 30 (76.9) | 12.3 |
| Prone positioning | 8 (5.8) | 7 (17.9) | 38.2 |
| Mean PEEP, median (IQR) | 6 (5 to 8) | 6 (5 to 10) | 13.0 |
| Mean GCS 1, median (IQR) | 13 (6 to 15) | 13 (6 to 15) | 13.3 |
| Number of tracheal aspirates 2 median (IQR) | 16 (8 to 25) | 16 (10 to 20) | 4.9 |
| No Cirrhosis Group N = 138 | Cirrhosis Group N = 39 | Effect Size | Value (95%CI) | p-Value | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Abundant gastric micro aspiration | 117/138 (84.8) | 30/39 (76.9) | Relative risk | 0.91 (0.75 to 1.10) | 0.31 |
| Secondary outcomes | |||||
| Abundant alpha-amylase measurements | 95/137 (69.3) | 23/38 (60.5) | Relative risk | 0.87 (0.65 to 1.16) | 0.34 |
| VAP, number of events (cumulative incidence) at 28 days | 22/137 (16.1) | 11/39 (28.2) | Hazard ratio | 1.91 (0.89 to 4.07) | 0.094 |
| Mechanical ventilation duration (days), median (IQR) | 9 (5 to 25) | 11 (5 to not reach) | Hazard ratio | 0.75 (0.48 to 1.17) | 0.20 |
| length of ICU stay (days), median (IQR) | 16 (9 to not reach) | 17 (9 to not reach) | Hazard ratio | 0.83 (0.50 to 1.37) | 0.45 |
| ICU mortality, number of events (cumulative incidence) at 28 days | 34/138 (24.6) | 13/39 (33.3) | Hazard ratio | 1.41 (0.79 to 2.51) | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, C.; Gaudet, A.; Jaillette, E.; Reignier, J.; Lassailly, G.; Balduyck, M.; Cailliau, E.; Rouze, A.; Nseir, S.; on behalf of the Micro Cirrhosis Study Group. Are Cirrhotic Patients Receiving Invasive Mechanical Ventilation at Risk of Abundant Microaspiration. J. Clin. Med. 2022, 11, 5994. https://doi.org/10.3390/jcm11205994
Levy C, Gaudet A, Jaillette E, Reignier J, Lassailly G, Balduyck M, Cailliau E, Rouze A, Nseir S, on behalf of the Micro Cirrhosis Study Group. Are Cirrhotic Patients Receiving Invasive Mechanical Ventilation at Risk of Abundant Microaspiration. Journal of Clinical Medicine. 2022; 11(20):5994. https://doi.org/10.3390/jcm11205994
Chicago/Turabian StyleLevy, Clementine, Alexandre Gaudet, Emmanuelle Jaillette, Jean Reignier, Guillaume Lassailly, Malika Balduyck, Emeline Cailliau, Anahita Rouze, Saad Nseir, and on behalf of the Micro Cirrhosis Study Group. 2022. "Are Cirrhotic Patients Receiving Invasive Mechanical Ventilation at Risk of Abundant Microaspiration" Journal of Clinical Medicine 11, no. 20: 5994. https://doi.org/10.3390/jcm11205994
APA StyleLevy, C., Gaudet, A., Jaillette, E., Reignier, J., Lassailly, G., Balduyck, M., Cailliau, E., Rouze, A., Nseir, S., & on behalf of the Micro Cirrhosis Study Group. (2022). Are Cirrhotic Patients Receiving Invasive Mechanical Ventilation at Risk of Abundant Microaspiration. Journal of Clinical Medicine, 11(20), 5994. https://doi.org/10.3390/jcm11205994





