Decision Making during the Learning Curve of Minimally Invasive Mitral Valve Surgery: A Focused Review for the Starting Minimally Invasive Surgeon
Abstract
1. Introduction
2. Learning Curve of Minimally Invasive Mitral Valve Surgery
3. Benefits and Limitations of Minimally Invasive Access
3.1. Benefits of Minimally Invasive Access
3.2. Limitations of Minimally Invasive Access
4. Considerations in Pre-Operative Planning and Patient Selection during the Learning Curve
4.1. Pre-Operative Planning and Imaging
4.2. Patient Selection
4.3. Considerations of Mitral Valve Pathology during Learning Curve of MIMVS
5. Available Techniques and Safety Considerations
5.1. Safety of the Minimally Invasive Techniques
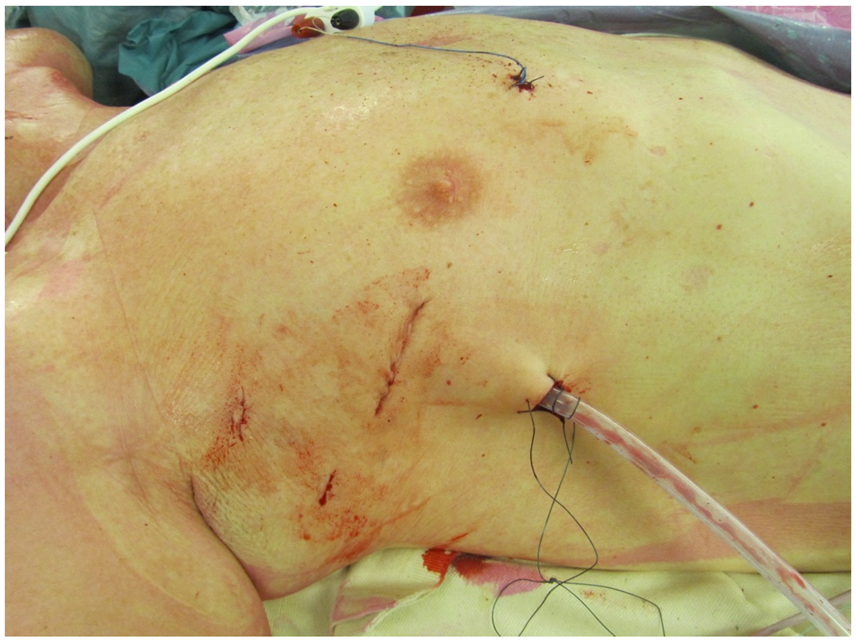
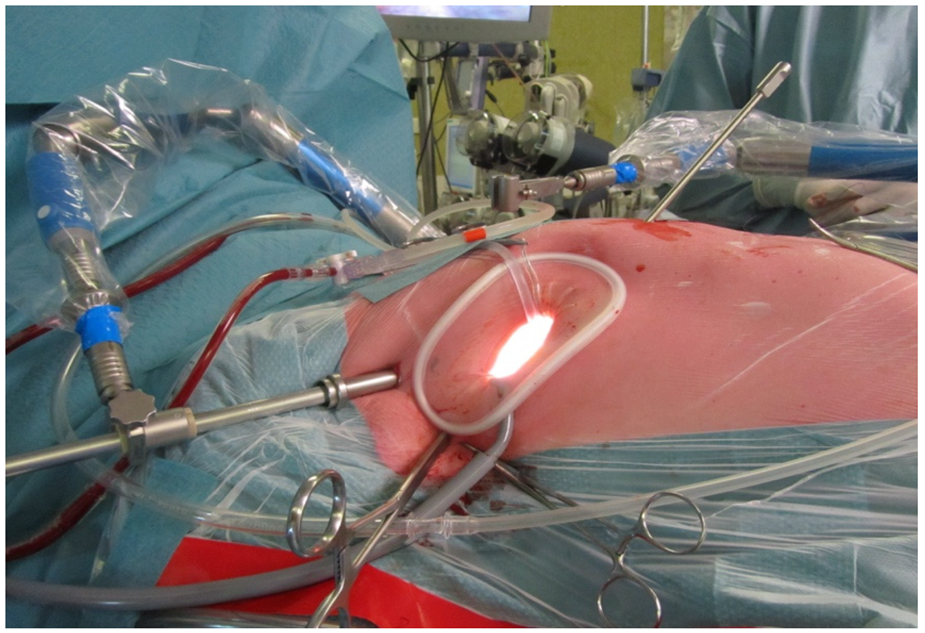
5.2. Less Invasive Surgical Access and Visualization of the Mitral Valve
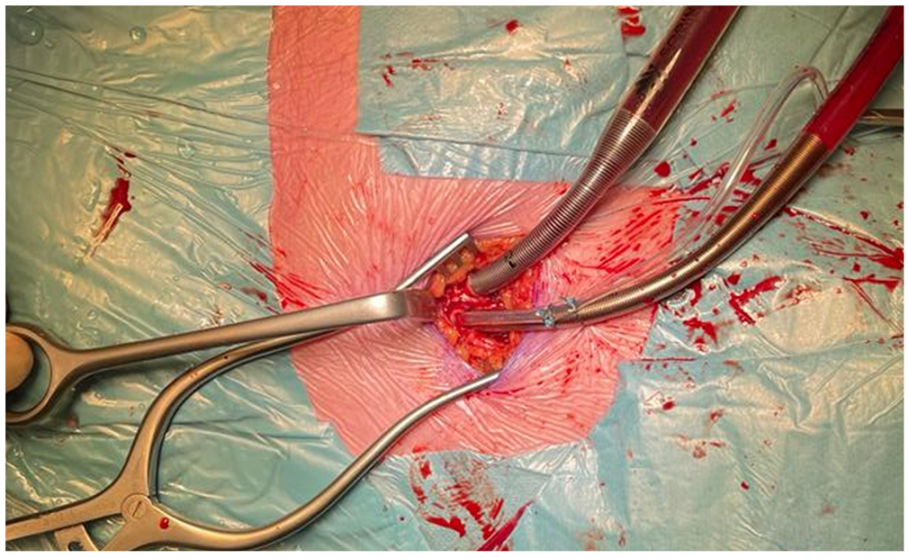
5.3. Cardiopulmonary Bypass: Arterial Cannulation
5.4. Cardiopulmonary Bypass: Venous Cannulation
5.5. Techniques for Aortic Occlusion and De-Airing
5.6. Hypothermic Fibrillary Arrest
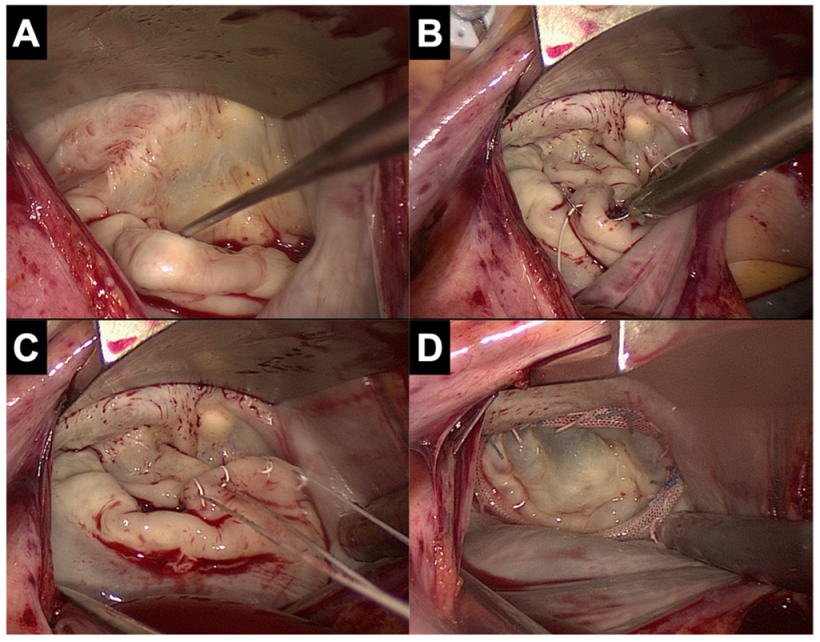
5.7. Valve Repair and Replacement Techniques
6. Conversion to Sternotomy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carpentier, A.; Loulmet, D.; Le Bret, E.; Haugades, B.; Dassier, P.; Guibourt, P. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C. R. Acad. Sci. 1996, 319, 219–223. [Google Scholar]
- Chitwood, W.R.; Elbeery, J.R.; Chapman, W.H.; Moran, J.M.; Lust, R.L.; Wooden, W.A.; Deaton, D.H. Video-assisted minimally invasive mitral valve surgery: The “micro-mitral” operation. J. Thorac. Cardiovasc. Surg. 1997, 113, 413–414. [Google Scholar] [CrossRef]
- Mohr, F.W.; Falk, V.; Diegeler, A.; Walther, T.; van Son, J.A.; Autschbach, R. Minimally invasive port-access mitral valve surgery. J. Thorac. Cardiovasc. Surg. 1998, 115, 567–574, discussion 574–566. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Seeburger, J.; Pfannmueller, B.; Garbade, J.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Minimally invasive mitral valve surgery: “The Leipzig experience”. Ann. Cardiothorac. Surg. 2013, 2, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Miceli, A.; Canarutto, D.; Lio, A.; Murzi, M.; Gilmanov, D.; Ferrarini, M.; Farneti, P.A.; Quaini, E.L.; Solinas, M. Early and long-term outcomes of minimally invasive mitral valve surgery through right minithoracotomy: A 10-year experience in 1604 patients. J. Cardiothorac. Surg. 2015, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; de Kroon, T.L.; Post, M.C.; Kelder, J.C.; Schut, K.F.; Saouti, N.; van Putte, B.P. Minimally invasive mitral valve surgery: A systematic safety analysis. Open Heart 2020, 7, e001393. [Google Scholar] [CrossRef]
- Ko, K.; de Kroon, T.L.; Kelder, J.C.; Saouti, N.; van Putte, B.P. Reoperative Mitral Valve Surgery Through Port Access. Semin. Thorac. Cardiovasc. Surg. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, J.; Borger, M.A.; Falk, V.; Passage, J.; Walther, T.; Doll, N.; Mohr, F.W. Minimally invasive mitral valve surgery after previous sternotomy: Experience in 181 patients. Ann. Thorac. Surg. 2009, 87, 709–714. [Google Scholar] [CrossRef]
- Murzi, M.; Miceli, A.; Di Stefano, G.; Cerillo, A.G.; Farneti, P.; Solinas, M.; Glauber, M. Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: A single institution experience with 173 patients. J. Thorac. Cardiovasc. Surg. 2014, 148, 2763–2768. [Google Scholar] [CrossRef]
- Romano, M.A.; Haft, J.W.; Pagani, F.D.; Bolling, S.F. Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: A safe and effective operative alternative. J. Thorac. Cardiovasc. Surg. 2012, 144, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Nissen, A.P.; Miller, C.C., 3rd; Thourani, V.H.; Woo, Y.J.; Gammie, J.S.; Ailawadi, G.; Nguyen, T.C. Less Invasive Mitral Surgery Versus Conventional Sternotomy Stratified by Mitral Pathology. Ann. Thorac. Surg. 2021, 111, 819–827. [Google Scholar] [CrossRef]
- Holzhey, D.M.; Seeburger, J.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Learning minimally invasive mitral valve surgery: A cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013, 128, 483–491. [Google Scholar] [CrossRef]
- Kastengren, M.; Svenarud, P.; Källner, G.; Franco-Cereceda, A.; Liska, J.; Gran, I.; Dalén, M. Minimally invasive versus sternotomy mitral valve surgery when initiating a minimally invasive programme. Eur. J. Cardiothorac. Surg. 2020, 58, 1168–1174. [Google Scholar] [CrossRef]
- Gammie, J.S.; Zhao, Y.; Peterson, E.D.; O’Brien, S.M.; Rankin, J.S.; Griffith, B.P.J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: Trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2010, 90, 1401–1408.e1, discussion 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Shih, E.; Squiers, J.J.; DiMaio, J.M. Systematic Review of Minimally Invasive Surgery for Mitral Valve Infective Endocarditis. Innovations 2021, 16, 244–248. [Google Scholar] [CrossRef]
- Kofler, M.; Van Praet, K.M.; Schambach, J.; Akansel, S.; Sundermann, S.; Schonrath, F.; Jacobs, S.; Falk, V.; Kempfert, J. Minimally invasive surgery versus sternotomy in native mitral valve endocarditis: A matched comparison. Eur. J. Cardiothorac. Surg. 2021, 61, 189–194. [Google Scholar] [CrossRef]
- Folkmann, S.; Seeburger, J.; Garbade, J.; Schon, U.; Misfeld, M.; Mohr, F.W.; Pfannmueller, B. Minimally Invasive Mitral Valve Surgery for Mitral Valve Infective Endocarditis. Thorac. Cardiovasc. Surg. 2018, 66, 525–529. [Google Scholar] [CrossRef]
- Luca, F.; van Garsse, L.; Rao, C.M.; Parise, O.; La Meir, M.; Puntrello, C.; Rubino, G.; Carella, R.; Lorusso, R.; Gensini, G.F.; et al. Minimally invasive mitral valve surgery: A systematic review. Minim. Invasive Surg. 2013, 2013, 179569. [Google Scholar] [CrossRef]
- Jebran, A.F.; Saha, S.; Waezi, N.; Al-Ahmad, A.; Niehaus, H.; Danner, B.C.; Baraki, H.; Kutschka, I. Design and training effects of a physical reality simulator for minimally invasive mitral valve surgery. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 409–415. [Google Scholar] [CrossRef]
- Sardari Nia, P.; Daemen, J.H.T.; Maessen, J.G. Development of a high-fidelity minimally invasive mitral valve surgery simulator. J. Thorac. Cardiovasc. Surg. 2019, 157, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.T.; Nguyen, D.H.; Van Hoang, S.; Le, K.M.; Nguyen, T.T.; Nguyen, V.L.; Nguyen, B.H.; Truong, B.Q. Learning curve in minimally invasive mitral valve surgery: A single-center experience. J. Cardiothorac. Surg. 2019, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Martin, J.; Lal, A.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J.; et al. Minimally invasive versus conventional open mitral valve surgery: A meta-analysis and systematic review. Innovations 2011, 6, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Falk, V.; Cheng, D.C.; Martin, J.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J. Minimally invasive versus open mitral valve surgery: A consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations 2011, 6, 66–76. [Google Scholar] [CrossRef]
- Moscarelli, M.; Lorusso, R.; Abdullahi, Y.; Varone, E.; Marotta, M.; Solinas, M.; Casula, R.; Parlanti, A.; Speziale, G.; Fattouch, K.; et al. The Effect of Minimally Invasive Surgery and Sternotomy on Physical Activity and Quality of Life. Heart Lung Circ. 2021, 30, 882–887. [Google Scholar] [CrossRef]
- Modi, P.; Hassan, A.; Chitwood, W.R., Jr. Minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur J. Cardiothorac. Surg. 2008, 34, 943–952. [Google Scholar] [CrossRef]
- Maier, R.H.; Kasim, A.S.; Zacharias, J.; Vale, L.; Graham, R.; Walker, A.; Laskawski, G.; Deshpande, R.; Goodwin, A.; Kendall, S.; et al. Minimally invasive versus conventional sternotomy for Mitral valve repair: Protocol for a multicentre randomised controlled trial (UK Mini Mitral). BMJ Open 2021, 11, e047676. [Google Scholar] [CrossRef]
- Park, C.B.; Suri, R.M.; Burkhart, H.M.; Greason, K.L.; Dearani, J.A.; Schaff, H.V.; Sundt, T.M., 3rd. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J. Thorac. Cardiovasc. Surg. 2010, 140, 1028–1035. [Google Scholar] [CrossRef]
- Paparella, D.; Fattouch, K.; Moscarelli, M.; Santarpino, G.; Nasso, G.; Guida, P.; Margari, V.; Martinelli, L.; Coppola, R.; Albertini, A.; et al. Current trends in mitral valve surgery: A multicenter national comparison between full-sternotomy and minimally-invasive approach. Int. J. Cardiol. 2020, 306, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, J. Concomitant minithoracotomy aortic and mitral valve surgery: The minimally invasive “Miami Method”. Ann. Cardiothorac. Surg. 2015, 4, 85–87. [Google Scholar] [CrossRef]
- Nakayama, T.; Nakamura, Y.; Kanamori, K.; Hirano, T.; Kuroda, M.; Nishijima, S.; Ito, Y.; Tsuruta, R.; Hori, T. Early and midterm results of minimally invasive aortic and mitral valve surgery via right mini-thoracotomy. J. Card. Surg. 2020, 35, 35–39. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Margari, V.; Paparella, D. Bilateral mini-thoracotomy for combined minimally invasive direct coronary artery bypass and mitral valve repair. Eur. J. Cardiothorac. Surg. 2022, 62, ezac306. [Google Scholar] [CrossRef]
- Sardari Nia, P.; Olsthoorn, J.R.; Heuts, S.; van Kuijk, S.M.J.; Vainer, J.; Streukens, S.; Schalla, S.; Segers, P.; Barenbrug, P.; Crijns, H.J.G.M.; et al. Effect of a dedicated mitral heart team compared to a general heart team on survival: A retrospective, comparative, non-randomized interventional cohort study based on prospectively registered data. Eur. J. Cardiothorac. Surg. 2021, 60, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Agnihotri, A.K.; Mehall, J.R.; Wolfe, J.A.; Hummel, B.W.; Fayers, T.M.; Farivar, R.S.; Grossi, E.A.; Guy, T.S.; Hargrove, W.C.; et al. Minimally Invasive Mitral Valve Surgery I: Patient Selection, Evaluation, and Planning. Innovations 2016, 11, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Moscarelli, M.; Fattouch, K.; Casula, R.; Speziale, G.; Lancellotti, P.; Athanasiou, T. What Is the Role of Minimally Invasive Mitral Valve Surgery in High-Risk Patients? A Meta-Analysis of Observational Studies. Ann. Thorac. Surg. 2016, 101, 981–989. [Google Scholar] [CrossRef]
- Santana, O.; Reyna, J.; Grana, R.; Buendia, M.; Lamas, G.A.; Lamelas, J. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann. Thorac. Surg. 2011, 91, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Kaeding, A.F.; Seeburger, J.; Melnitchouk, S.; Hoebartner, M.; Winkfein, M.; Misfeld, M.; Mohr, F.W. Minimally invasive mitral valve repair in Barlow’s disease: Early and long-term results. J. Thorac. Cardiovasc. Surg. 2014, 148, 1379–1385. [Google Scholar] [CrossRef]
- Muneretto, C.; Bisleri, G.; Bagozzi, L.; Repossini, A.; Berlinghieri, N.; Chiari, E. Results of minimally invasive, video-assisted mitral valve repair in advanced Barlow’s disease with bileaflet prolapse. Eur. J. Cardiothorac. Surg. 2015, 47, 46–50, discussion 50–41. [Google Scholar] [CrossRef]
- Vo, A.T.; Le, K.M.; Nguyen, T.T.; Vu, T.T.; Pham, C.V.T.; Ngo, H.Q.T.; Le, T.Q.; Nguyen, D.H. Minimally Invasive Mitral Valve Surgery for Rheumatic Valve Disease. Heart Surg. Forum 2019, 22, E390–E395. [Google Scholar] [CrossRef]
- Moscarelli, M.; Fattouch, K.; Gaudino, M.; Nasso, G.; Paparella, D.; Punjabi, P.; Athanasiou, T.; Benedetto, U.; Angelini, G.D.; Santarpino, G.; et al. Minimal Access Versus Sternotomy for Complex Mitral Valve Repair: A Meta-Analysis. Ann. Thorac. Surg. 2020, 109, 737–744. [Google Scholar] [CrossRef]
- Barac, Y.D.; Glower, D.D. Port-Access Mitral Valve Surgery-An Evolution of Technique. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 829–837. [Google Scholar] [CrossRef]
- Wolfe, J.A.; Malaisrie, S.C.; Farivar, R.S.; Khan, J.H.; Hargrove, W.C.; Moront, M.G.; Ryan, W.H.; Ailawadi, G.; Agnihotri, A.K.; Hummel, B.W.; et al. Minimally Invasive Mitral Valve Surgery II: Surgical Technique and Postoperative Management. Innovations 2016, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, K.M.; Stamm, C.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Montagner, M.; Nazari Shafti, T.Z.; Jacobs, S.; Falk, V.; Kempfert, J. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interv. Cardiol. 2018, 13, 14–19. [Google Scholar] [CrossRef]
- Poffo, R.; Montanhesi, P.K.; Toschi, A.P.; Pope, R.B.; Mokross, C.A. Periareolar Access for Minimally Invasive Cardiac Surgery: The Brazilian Technique. Innovations 2018, 13, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Marin Cuartas, M.; Javadikasgari, H.; Pfannmueller, B.; Seeburger, J.; Gillinov, A.M.; Suri, R.M.; Borger, M.A. Mitral valve repair: Robotic and other minimally invasive approaches. Prog. Cardiovasc. Dis. 2017, 60, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Toolan, C.; Palmer, K.; Al-Rawi, O.; Ridgway, T.; Modi, P. Robotic mitral valve surgery: A review and tips for safely negotiating the learning curve. J. Thorac. Dis. 2021, 13, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, J.; Aberle, C.; Macias, A.E.; Alnajar, A. Cannulation Strategies for Minimally Invasive Cardiac Surgery. Innovations 2020, 15, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.J.; Birla, R.; Vohra, H.A. Clinical outcomes associated with retrograde arterial perfusion in minimally invasive mitral valve surgery: A systematic review. Perfusion 2021, 36, 11–20. [Google Scholar] [CrossRef]
- Barbero, C.; Marchetto, G.; Ricci, D.; El Qarra, S.; Attisani, M.; Filippini, C.; Boffini, M.; Rinaldi, M. Right Minithoracotomy for Mitral Valve Surgery: Impact of Tailored Strategies on Early Outcome. Ann. Thorac. Surg. 2016, 102, 1989–1994. [Google Scholar] [CrossRef]
- Chitwood, W.R., Jr.; Elbeery, J.R.; Moran, J.F. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann. Thorac. Surg. 1997, 63, 1477–1479. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Sundermann, S.H.; Kempfert, J. Endoaortic Balloon Occlusion During Minimally Invasive Mitral Valve Surgery. Innovations 2022, 17, 83–87. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Nersesian, G.; Montagner, M.; Akansel, S.; Eggert-Doktor, D.; Kofler, M.; Sundermann, S.; Falk, V.; Kempfert, J. Endoaortic balloon occlusion in minimally invasive mitral valve surgery. Multimed. Man. Cardiothorac. Surg. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; Pellerin, M.; Lebon, J.S.; Dionne, P.O.; Jeanmart, H.; Bouchard, D. Minimally invasive mitral valve surgery: Influence of aortic clamping technique on early outcomes. Ann. Thorac. Surg. 2013, 96, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Casselman, F.; Aramendi, J.; Bentala, M.; Candolfi, P.; Coppoolse, R.; Gersak, B.; Greco, E.; Herijgers, P.; Hunter, S.; Krakor, R.; et al. Endoaortic Clamping Does Not Increase the Risk of Stroke in Minimal Access Mitral Valve Surgery: A Multicenter Experience. Ann. Thorac. Surg. 2015, 100, 1334–1339. [Google Scholar] [CrossRef]
- Rival, P.M.; Moore, T.H.M.; McAleenan, A.; Hamilton, H.; Du Toit, Z.; Akowuah, E.; Angelini, G.D.; Vohra, H.A. Transthoracic clamp versus endoaortic balloon occlusion in minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2019, 56, 643–653. [Google Scholar] [CrossRef]
- Bentala, M.; Heuts, S.; Vos, R.; Maessen, J.; Scohy, T.V.; Gerritse, B.M.; Sardari Nia, P. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bonaros, N.; Hoefer, D.; Oezpeker, C.; Gollmann-Tepekoeylue, C.; Holfeld, J.; Dumfarth, J.; Kilo, J.; Ruttmann-Ulmer, E.; Hangler, H.; Grimm, M.; et al. Predictors of safety and success in minimally invasive surgery for degenerative mitral disease. Eur. J. Cardiothorac. Surg. 2021, 61, 637–644. [Google Scholar] [CrossRef]
- Pfannmueller, B.; Misfeld, M.; Verevkin, A.; Garbade, J.; Holzhey, D.M.; Davierwala, P.; Seeburger, J.; Noack, T.; Borger, M.A. Loop neochord versus leaflet resection techniques for minimally invasive mitral valve repair: Long-term results. Eur. J. Cardiothorac. Surg. 2021, 59, 180–186. [Google Scholar] [CrossRef]
- Vollroth, M.; Seeburger, J.; Garbade, J.; Borger, M.A.; Misfeld, M.; Mohr, F.W. Conversion rate and contraindications for minimally invasive mitral valve surgery. Ann. Cardiothorac. Surg. 2013, 2, 853–854. [Google Scholar] [CrossRef]
| Significant Aortic, Iliac, or Femoral Disease That Prevents Safe Retrograde Arterial Perfusion |
|---|
| Left ventricular ejection fraction < 25% |
| Severe right ventricular dysfunction |
| Pulmonary artery pressure > 70 mmHg |
| Aorta > 4 cm if endo-aortic balloon being used |
| Significant mitral annular calcification |
| Patients with more than mild aortic regurgitation |
| Kyphoscoliosis and pectus excavatum |
| Morbidly obese and extremely muscular patients |
| Previous right thoracotomy or expected adhesions in the right chest |
| Advanced renal- or liver disease, significant pulmonary disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.; Verhagen, A.F.T.M.; de Kroon, T.L.; Morshuis, W.J.; van Garsse, L.A.F.M. Decision Making during the Learning Curve of Minimally Invasive Mitral Valve Surgery: A Focused Review for the Starting Minimally Invasive Surgeon. J. Clin. Med. 2022, 11, 5993. https://doi.org/10.3390/jcm11205993
Ko K, Verhagen AFTM, de Kroon TL, Morshuis WJ, van Garsse LAFM. Decision Making during the Learning Curve of Minimally Invasive Mitral Valve Surgery: A Focused Review for the Starting Minimally Invasive Surgeon. Journal of Clinical Medicine. 2022; 11(20):5993. https://doi.org/10.3390/jcm11205993
Chicago/Turabian StyleKo, Kinsing, Ad F. T. M. Verhagen, Thom L. de Kroon, Wim J. Morshuis, and Leen A. F. M. van Garsse. 2022. "Decision Making during the Learning Curve of Minimally Invasive Mitral Valve Surgery: A Focused Review for the Starting Minimally Invasive Surgeon" Journal of Clinical Medicine 11, no. 20: 5993. https://doi.org/10.3390/jcm11205993
APA StyleKo, K., Verhagen, A. F. T. M., de Kroon, T. L., Morshuis, W. J., & van Garsse, L. A. F. M. (2022). Decision Making during the Learning Curve of Minimally Invasive Mitral Valve Surgery: A Focused Review for the Starting Minimally Invasive Surgeon. Journal of Clinical Medicine, 11(20), 5993. https://doi.org/10.3390/jcm11205993





