Post-Implant Phosphodiesterase-5 Inhibitors in Patients with Left Ventricular Assist Device: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Article Selection
2.2. Definitions
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Quality and Publication Bias Assessment
3. Results
3.1. Search Strategy and Patient Demographics
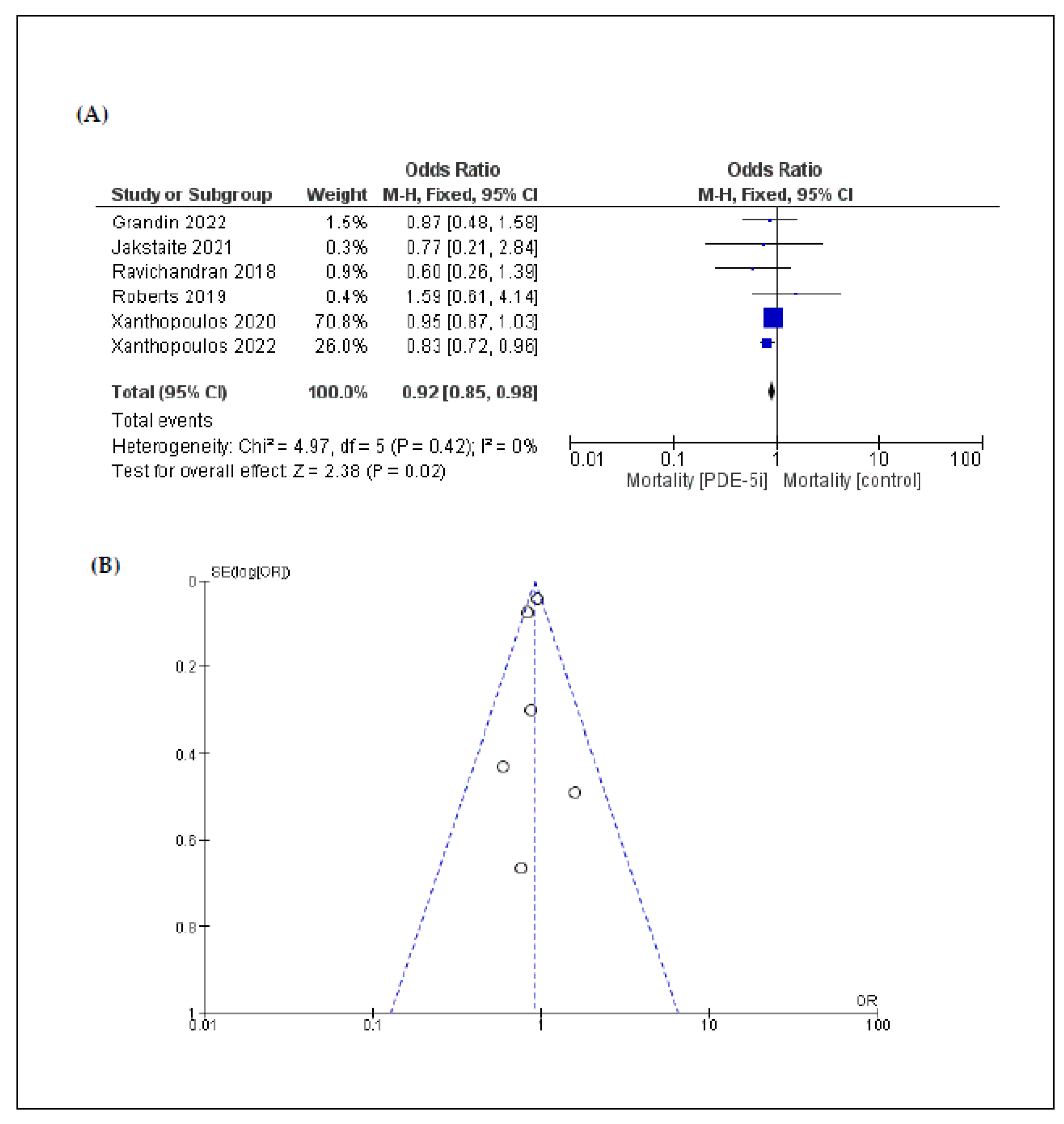
3.2. Primary Endpoint: Mortality
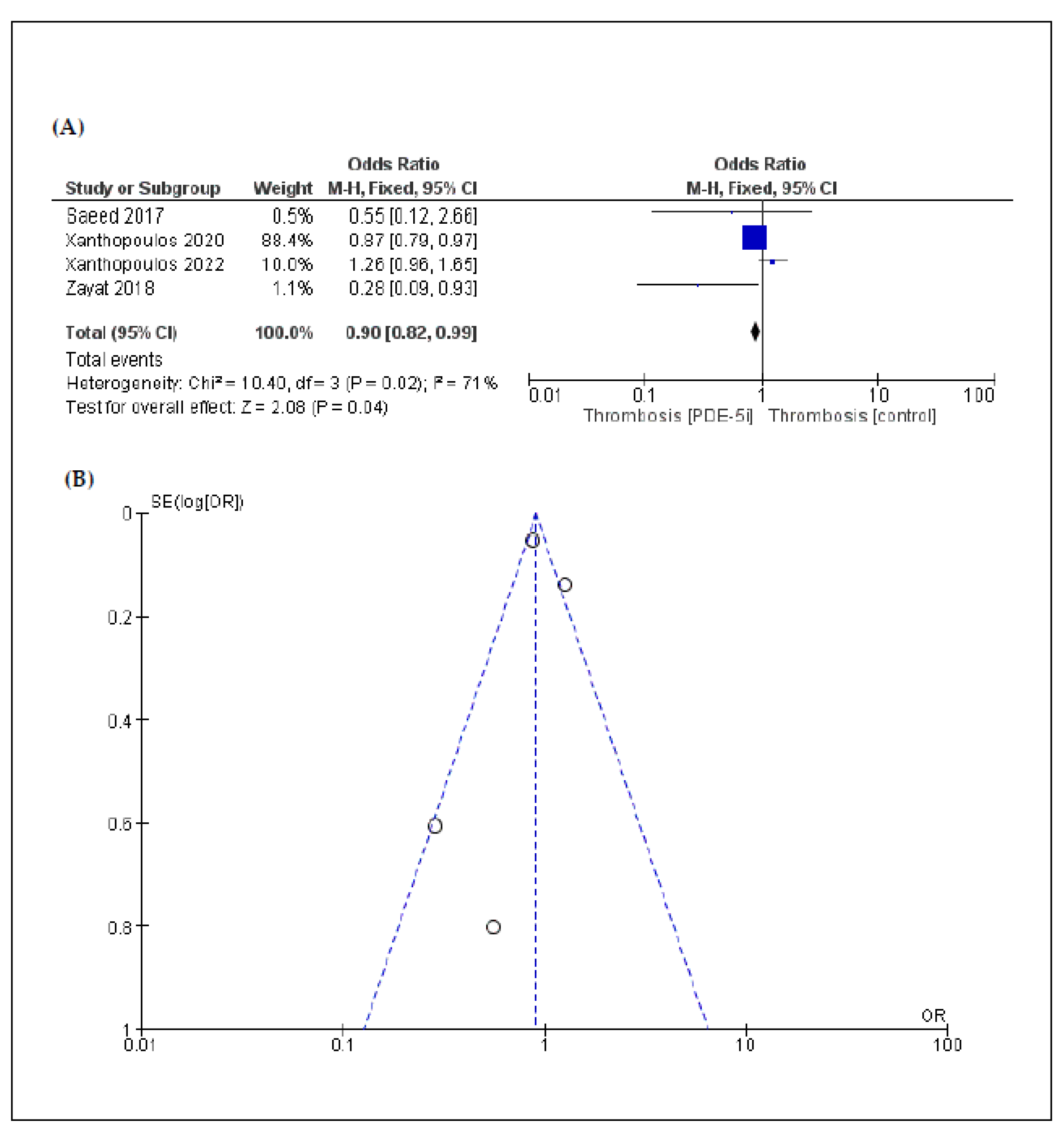
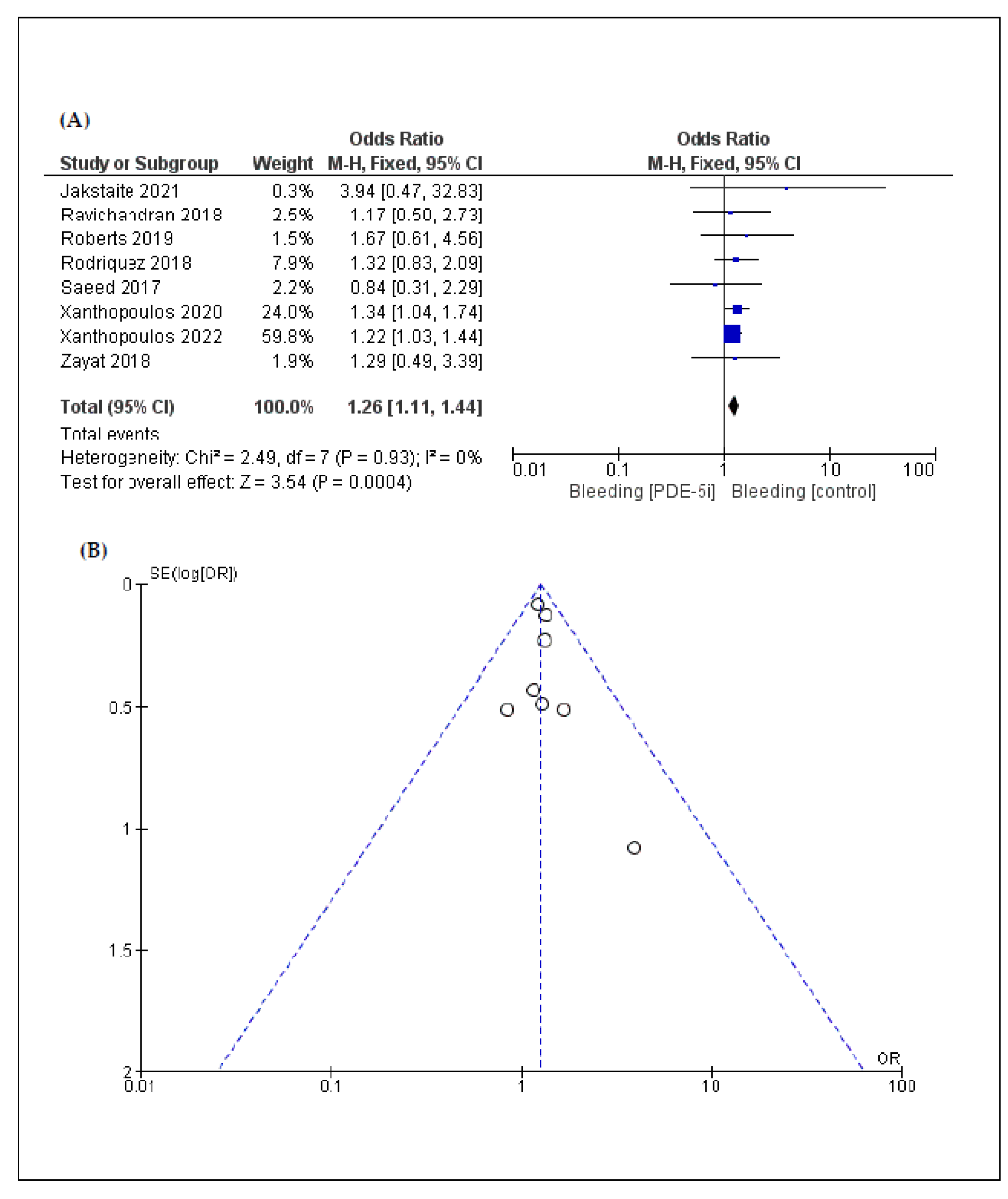
3.3. Secondary Endpoints: Complications
3.4. Sensitivity Analysis
3.5. Publication Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Starling, R.C.; Moazami, N.; Silvestry, S.C.; Ewald, G.; Rogers, J.G.; Milano, C.A.; Rame, J.E.; Acker, M.A.; Blackstone, E.H.; Ehrlinger, J.; et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N. Engl. J. Med. 2014, 370, 33–40. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef]
- Colombo, P.C.; Mehra, M.R.; Goldstein, D.J.; Estep, J.D.; Salerno, C.; Jorde, U.P.; Cowger, J.; ClevelandJr, J.C.; Uriel, N.; Sayer, G.; et al. Comprehensive Analysis of Stroke in the Long-Term Cohort of the MOMENTUM 3 Study. Circulation 2019, 139, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Milano, C.A.; Rogers, J.G.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Mokadam, N.A.; Mahr, C.; Miller, J.S.; Markham, D.W.; et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018, 6, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef]
- Hollis, I.B.; Doligalski, C.T.; Jennings, D.J. Pharmacotherapy for durable left ventricular assist devices. Pharmacotherapy 2021, 41, 14–27. [Google Scholar] [CrossRef]
- Monzo, L.; Reichenbach, A.; Al-Hiti, H.; Borlaug, B.A.; Havlenova, T.; Solar, N.; Tupy, M.; Ters, J.; Kautzner, J.; Melenovsky, V. Acute Unloading Effects of Sildenafil Enhance Right Ventricular-Pulmonary Artery Coupling in Heart Failure. J. Card. Fail. 2021, 27, 224–232. [Google Scholar] [CrossRef]
- Hutchings, D.; Anderson, S.; Caldwell, J.L.; Trafford, A.W. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart 2018, 104, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Tryposkiadis, K.; Triposkiadis, F.; Fukamachi, K.; Soltesz, E.G.; Young, J.B.; Wolski, K.; Blackstone, E.H.; Starling, R.C. Postimplant Phosphodiesterase Type 5 Inhibitors Use Is Associated With Lower Rates of Thrombotic Events After Left Ventricular Assist Device Implantation. J. Am. Heart Assoc. 2020, 9, e015897. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Wolski, K.; Wang, Q.; Blackstone, E.H.; Randhawa, V.K.; Soltesz, E.G.; Young, J.B.; Nissen, S.E.; Estep, J.D.; Triposkiadis, F.; et al. Postimplant Phosphodiesterase-5 Inhibitor Use in Centrifugal Flow Left Ventricular Assist Devices. JACC Heart Fail. 2022, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Grandin, E.W.; Gulati, G.; Nunez, J.I.; Kennedy, K.; Rame, J.E.; Atluri, P.; Pagani, F.D.; Kirklin, J.K.; Kormos, R.L.; Teuteberg, J.; et al. Outcomes With Phosphodiesterase-5 Inhibitor Use After Left Ventricular Assist Device: A STS-INTERMACS Analysis. Circ. Heart Fail. 2022, 15, e008613. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R. The burden of haemocompatibility with left ventricular assist systems: A complex weave. Eur. Heart J. 2019, 40, 673–677. [Google Scholar] [CrossRef]
- Uriel, N.; Colombo, P.C.; Cleveland, J.C.; Long, J.W.; Salerno, C.; Goldstein, D.J.; Patel, C.B.; Ewald, G.A.; Tatooles, A.J.; Silvestry, S.C.; et al. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation 2017, 135, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Crandall, D.L.; Gustafsson, F.; Jorde, U.P.; Katz, J.N.; Netuka, I.; Uriel, N.; Connors, J.M.; Sood, P.; Heatley, G.; et al. Aspirin and left ventricular assist devices: Rationale and design for the international randomized, placebo-controlled, non-inferiority ARIES HM3 trial. Eur. J. Heart Fail. 2021, 23, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Antonides, C.F.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J. Heart Lung Transplant. 2020, 39, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org (accessed on 1 March 2022).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Jakstaite, A.; Luedike, P.; Schmack, B.; Pizanis, N.; Riebisch, M.; Weymann, A.; Kamler, M.; Ruhparwar, A.; Rassaf, T.; Papathanasiou, M. Increased bleeding risk with phosphodiesterase-5 inhibitors after left ventricular assist device implantation. ESC Heart Fail. 2021, 8, 2419–2427. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.K.; LaRue, S.J.; Novak, E.; Joseph, S.A.; Schilling, J.D. Sildenafil in Left Ventricular Assist Device Is Safe and Well-Tolerated. ASAIO J. 2018, 64, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Shuster, J.E.; Britt, N.S.; Balsara, K.R.; Graetz, T.J.; Helwani, M.; Itoh, A.; Tellor, B.R. Evaluation of Clinical Outcomes with Phosphodiesterase-5 Inhibitor Therapy for Right Ventricular Dysfunction After Left Ventricular Assist Device Implantation. ASAIO J. 2019, 65, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.C.; Lawrecki, T.M.; Graney, N.; Siemeck, R.; Bhat, G.; Gavrilos, G. Evaluation of gastrointestinal bleeds in left ventricular assist device patients receiving phosphodiesterase 5 inhibitors. ASAIO J. 2018, 64, 62. [Google Scholar] [CrossRef]
- Saeed, O.; Rangasamy, S.; Selevany, I.; Madan, S.; Fertel, J.; Eisenberg, R.; Aljoudi, M.; Patel, S.R.; Shin, J.; Sims, D.B.; et al. Sildenafil Is Associated With Reduced Device Thrombosis and Ischemic Stroke Despite Low-Level Hemolysis on Heart Mate II Support. Circ. Heart Fail. 2017, 10, e004222. [Google Scholar] [CrossRef]
- Zayat, R.; Ahmad, U.; Stoppe, C.; Khattab, M.A.; Arab, F.; Moza, A.; Tewarie, L.; Goetzenich, A.; Autschbach, R.; Schnoering, H. Sildenafil Reduces the Risk of Thromboembolic Events in HeartMate II Patients with Low-Level Hemolysis and Significantly Improves the Pulmonary Circulation. Int. Heart J. 2018, 59, 1227–1236. [Google Scholar] [CrossRef]
- Critoph, C.; Green, G.; Hayes, H.; Baumwol, J.; Lam, K.; Larbalestier, R.; Chih, S. Clinical Outcomes of Patients Treated With Pulmonary Vasodilators Early and in High Dose After Left Ventricular Assist Device Implantation. Artif. Organs 2016, 40, 106–114. [Google Scholar] [CrossRef]
- Hamdan, R.; Mansour, H.; Nassar, P.; Saab, M. Prevention of right heart failure after left ventricular assist device implantation by phosphodiesterase 5 inhibitor. Artif. Organs 2014, 38, 963–967. [Google Scholar] [CrossRef]
- Hassanein, M.T.; Sheikh, F.H.; Boyce, S.W.; Mohammed, S.F.; Hofmeyer, M.; Majure, D.T. Phosphodiesterase type 5 inhibitor use does not affect heart failure hospitalizations in patients with left ventricular assist devices. J. Card. Fail. 2017, 23, S132. [Google Scholar] [CrossRef][Green Version]
- Sridhar, A.M.; Mukherjee, V.; Patel, D.; Ruiz, G.; Chawla, R.; Boyce, S.; Najjar, S. Single center experience in the use of sildenafil for right ventricular dysfunction after LVAD implantation. J. Heart Lung Transplant. 2011, 30, S209–S210. [Google Scholar] [CrossRef]
- Raina, A.; Kanwar, M.; Sokos, G.; Moraca, R.; Bailey, S.; Benza, R.L.; Murali, S. An aggressive, targeted perioperative management strategy results in low rates of postoperative right ventricular failure after left ventricular assist device implantation. J. Card. Fail. 2012, 18, S45. [Google Scholar] [CrossRef]
- Solomon, R.; Obi, C.; Sharma, S.; Smith, Z.; Gheewala, N.; Cabrera, R.; Lanfear, D.; Jennings, D.; Long, T. Hemodynamic effects of sildenafil on right heart function in left ventricular devices. J. Am. Coll. Cardiol. 2019, 73, 831. [Google Scholar] [CrossRef]
- Kittipibul, V.; Blumer, V.; Angsubhakorn, N.; Hernandez, G.A.; Chaparro, S.; Tedford, R.J.; Agarwal, R. Phosphodiesterase-5 Inhibitors and Outcomes during Left Ventricular Assist Device Support: A Systematic Review and Meta-Analysis. J. Card. Fail. 2021, 27, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, C.T.; LaRue, S.J.; Schilling, J.D. Intersection of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients on Left Ventricular Assist Device Support: Is There a Role for Pulmonary Vasodilators? Circ. Heart Fail. 2018, 11, e004255. [Google Scholar] [CrossRef]
- Feldman, D.; Pamboukian, S.V.; Teuteberg, J.J.; Birks, E.; Lietz, K.; Moore, S.A.; Morgan, J.A.; Arabia, F.; Bauman, M.E.; Buchholz, H.W.; et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J. Heart Lung Transplant. 2013, 32, 157–187. [Google Scholar] [CrossRef]
- Gulati, G.; Grandin, E.W.; Kennedy, K.; Cabezas, F.; DeNofrio, D.D.; Kociol, R.; Rame, J.E.; Pagani, F.D.; Kirklin, J.K.; Kormos, R.L.; et al. Preimplant Phosphodiesterase-5 Inhibitor Use Is Associated With Higher Rates of Severe Early Right Heart Failure After Left Ventricular Assist Device Implantation. Circ. Heart Fail. 2019, 12, e005537. [Google Scholar] [CrossRef] [PubMed]
- Jorde, U.P.; Saeed, O. Association of Improved Outcomes and Phosphodiesterase-5 Inhibition during Contemporary LVAD Support: End of the Beginning? JACC Heart Fail. 2022, 10, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Nour, K.R.; Zalos, G.; Mincemoyer, R.; Waclawiw, M.A.; Rivera, C.E.; Willie, G.; Ellahham, S.; Quyyumi, A.A. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J. Am. Coll. Cardiol. 2002, 40, 1232–1240. [Google Scholar] [CrossRef]
- Gudmundsdóttir, I.J.; McRobbie, S.J.; Robinson, S.D.; Newby, D.E.; Megson, I.L. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem. Biophys. Res. Commun. 2005, 337, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Kiernan, M.S. Phosphodiesterase-5 Inhibitor Therapy for Left Ventricular Assist Device Patients: More Data, More Questions. J. Am. Heart Assoc. 2020, 9, e017585. [Google Scholar] [CrossRef]
- Imamura, T.; Kinugawa, K.; Uriel, N. Therapeutic Strategy for Gastrointestinal Bleeding in Patients with Left Ventricular Assist Device. Circ. J. 2018, 82, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Klaeske, K.; Dieterlen, M.; Eifert, S.; Scholz, U.; Garbade, J.; Jawad, K.; Sieg, F.; Borger, M.A.; Meyer, A.L. Device-induced platelet dysfunction in patients after left ventricular assist device implantation. J. Thromb. Haemost. 2021, 19, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, C.T.; Nassif, M.E.; Raymer, D.S.; Novak, E.; LaRue, S.J.; Schilling, J.D. Pre-Operative Right Ventricular Dysfunction Is Associated with Gastrointestinal Bleeding in Patients Supported With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail. 2015, 3, 956–964. [Google Scholar] [CrossRef]
- Yin, M.Y.; Ruckel, S.; Kfoury, A.G.; McKellar, S.H.; Taleb, I.; Gilbert, E.M.; Nativi-Nicolau, J.; Stehlik, J.; Reid, B.B.; Koliopoulou, A.; et al. Novel Model to Predict Gastrointestinal Bleeding During Left Ventricular Assist Device Support. Circ. Heart Fail. 2018, 11, e005267. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Patarroyo-Aponte, M. Prevention and Treatment of Right Ventricular Failure During Left Ventricular Assist Device Therapy. Crit. Care Clin. 2018, 34, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Park, M.H.; Landzberg, M.J.; Lala, A.; Waxman, A.B. International Right Heart Failure Foundation Scientific Working G. Right heart failure: Toward a common language. J. Heart Lung Transplant. 2014, 33, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, M.; Mercier, O.; Kobayashi, Y.; Moneghetti, K.; Haddad, F. Forgotten No More: A Focused Update on the Right Ventricle in Cardiovascular Disease. JACC Heart Fail. 2018, 6, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Skoularigis, J.; Starling, R.C. Therapeutic augmentation of NO-sGC-cGMP signalling: Lessons learned from pulmonary arterial hypertension and heart failure. Heart Fail. Rev. 2022, 27, 1991–2003. [Google Scholar] [CrossRef]

| Study ID, Year | Country | Study Design | LVAD Model | Patients, n | Female, n (%) | Mean Age ± SD | Type of PDE5I | NOS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDE5i | Control | PDE5i | Control | PDE5i | Control | ||||||
| Critoph et al., 2016 [28] | Australia | R | 52% HVAD 11% HM2 | 4 | 61 | ND | ND | ND | ND | Sildeanfil | 5 |
| Grandin et al., 2022 [13] | United States of America | P | CF-LVAD | 1600 | 1600 | 334 (20.9) | 345 (21.6) | 56.3 ± 12.5 | 56.4 ± 12.7 | ND | 7 |
| Hamdan et al., 2014 [29] | Lebanon | R | 71% HM2 29% HVAD | 8 | 6 | 3 (37.5) | 1 (16.7) | 34.3 ± 14.1 | 36.5 ± 16.5 | Sildenafil | 6 |
| Hassanein et al., 2017 (abstract) [30] | United States of America | R | HM2/HVAD | 59 | 14 | 10 (17) | 4 (28.6) | 57.0 ± 11.6 | 56.4 ± 11.3 | Sildenafil/ Tadalafil | 6 |
| Jakstaite et al., 2021 [22] | Germany | R | 90% HVAD 10% HM3 | 75 | 34 | 9 (12) | 7 (21) | 53 ± 13 | 57 ± 10 | 7 Sildenafil 68 Tadalafil | 7 |
| Mahankali et al., 2011 (abstract) [31] | United States of America | R | CF-LVAD | 29 | 23 | ND | ND | ND | ND | Sildenafil | 5 |
| Raina et al., 2012 (abstract) [32] | United States of America | R | CF-LVAD | 80 | 16 | ND | ND | ND | ND | Sildenafil | 5 |
| Ravichandran et al., 2018 [23] | United States of America | R | 82% HM2 8% HVAD | 53 | 69 | 35 (51) | 19 (35) | 55.5 ± 12.3 | 54.4 ± 13.5 | Sildenafil | 6 |
| Roberts et al., 2019 [24] | United States of America | R | 86% HM 2 14% HVAD | 77 | 77 | 24 (31.2) | 19 (24.7) | 59.5 (51–64) | 60.5 (53–66) | Sildenafil/ Tadalafil | 7 |
| Rodriguez et al., 2018 (abstract) [25] | United States of America | R | 76.5% HM2 19.7% HVAD 3.8% HM3 | 119 | 199 | ND | ND | ND | ND | Sildenafil/ Tadalafil | 5 |
| Saeed et al., 2017 [26] | United States of America | R | HM2 | 37 | 107 | ND | ND | ND | ND | Sildenafil | 6 |
| Solomon et al., 2019 (abstract) [33] | United States of America | R | HM2 | 62 | 106 | ND | ND | ND | ND | Sildenafil | 5 |
| Xanthopoulos et al., 2020 [11] | United States of America | P | CF-LVAD * | 4950 | 8822 | 1063 (21.5) | 1875 (21.3) | 56 ± 13 | 58 ± 13 | ND | 8 |
| Xanthopoulos et al., 2022 [12] | United States of America | P | 64% HM3 36% HVAD | 2173 | 5056 | 514 (23.7) | 1142 (22.6) | 56.2 ± 13.0 | 57.6 ± 13.0 | ND | 8 |
| Zayat et al., 2018 [27] | Germany | R | HM2 | 56 | 27 | ND | ND | ND | ND | Sildenafil | 6 |
| Categorical Outcomes | n | OR (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p | ||||
| PDE5I vs. Control | |||||
| Female ratio | 6 | 1.03 [0.97, 1.10] | 0.34 | 74% | <0.01 |
| Gastrointestinal bleeding | 8 | 1.26 [1.11, 1.44] | <0.01 | 0% | 0.93 |
| Pump thrombosis | 4 | 0.90 [0.82, 0.99] | 0.04 | 71% | 0.02 |
| Ischemic stroke | 5 | 0.87 [0.78, 0.98] | 0.02 | 0% | 0.42 |
| Mortality | 6 | 0.92 [0.85, 0.98] | 0.02 | 0% | 0.42 |
| PDE5I vs. Control | |||||
| Continuous Outcomes | n | WMD (95% CI) | p | I2 | p |
| Mean age | 6 | −1.44 [−1.74, −1.13] | <0.01 | 74% | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulos, A.; Magouliotis, D.E.; Tryposkiadis, K.; Zotos, P.-A.; Spiliopoulos, K.; Athanasiou, T.; Giamouzis, G.; Skoularigis, J.; Starling, R.C.; Triposkiadis, F. Post-Implant Phosphodiesterase-5 Inhibitors in Patients with Left Ventricular Assist Device: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5988. https://doi.org/10.3390/jcm11205988
Xanthopoulos A, Magouliotis DE, Tryposkiadis K, Zotos P-A, Spiliopoulos K, Athanasiou T, Giamouzis G, Skoularigis J, Starling RC, Triposkiadis F. Post-Implant Phosphodiesterase-5 Inhibitors in Patients with Left Ventricular Assist Device: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(20):5988. https://doi.org/10.3390/jcm11205988
Chicago/Turabian StyleXanthopoulos, Andrew, Dimitrios E. Magouliotis, Konstantinos Tryposkiadis, Prokopis-Andreas Zotos, Kyriakos Spiliopoulos, Thanos Athanasiou, Grigorios Giamouzis, John Skoularigis, Randall C. Starling, and Filippos Triposkiadis. 2022. "Post-Implant Phosphodiesterase-5 Inhibitors in Patients with Left Ventricular Assist Device: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 20: 5988. https://doi.org/10.3390/jcm11205988
APA StyleXanthopoulos, A., Magouliotis, D. E., Tryposkiadis, K., Zotos, P.-A., Spiliopoulos, K., Athanasiou, T., Giamouzis, G., Skoularigis, J., Starling, R. C., & Triposkiadis, F. (2022). Post-Implant Phosphodiesterase-5 Inhibitors in Patients with Left Ventricular Assist Device: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(20), 5988. https://doi.org/10.3390/jcm11205988










