Awake Prone Positioning, High-Flow Nasal Oxygen and Non-Invasive Ventilation as Non-Invasive Respiratory Strategies in COVID-19 Acute Respiratory Failure: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
- Cochrane COVID-19 Study Register (COVID-19.cochrane.org), comprising Cochrane Central Register of Controlled Trials, MEDLINE (PubMed), Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, and medRxiv (last searched on 26 October 2021 for both topics);
- WHO COVID-19 Global literature on coronavirus disease (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov) (last searched on 26 October 2021 for both topics);
- Web of Science (Science Citation Index Expanded and Emerging Sources Citation Index) (last searched on 1 March 2021 for NIV and 7 June 2021 for APP);
- CINAHL (Cumulative Index to Nursing and Allied Health Literature) (last searched on 1 March 2021 for NIV and 7 June 2021 for APP).
- -
- All-cause mortality at day 28, day 60, and time-to-event, and at hospital discharge
- -
- Clinical status at day 28, day 60, and up to the longest follow-up, including:
- -
- worsening of clinical status: patients with clinical deterioration or death
- -
- improvement of clinical status: patients discharged alive without clinical deterioration or death
- -
- quality of life, including fatigue and neurological status, assessed with standardized scales at up to 7 days, up to 28 days and longest follow-up available
- -
- Serious adverse events during the study period, defined as number of patients with any event
- -
- Adverse events (any grade) during the study period, defined as the number of patients with any event
- -
- Clinical status at day 28, day 60 and up to longest follow up, including:
- -
- worsening of clinical status: new need for invasive mechanical ventilation
- -
- improvement of clinical status:
- -
- weaning or liberation from invasive mechanical ventilation
- -
- liberation from supplemental oxygen in surviving patients
- -
- ventilator-free days
- -
- duration to liberation from invasive mechanical ventilation
- -
- duration to liberation from supplemental oxygen
- -
- Admission to intensive care unit at day 28
- -
- Duration of hospitalization
- -
- Skin lesions from proning measures
- -
- serious (−1) or very serious (−2) risk of bias
- -
- serious (−1) or very serious (−2) inconsistency
- -
- serious (−1) or very serious (−2) uncertainty about directness
- -
- serious (−1) or very serious (−2) imprecision of the data
- -
- serious (−1) or very serious (−2) probability of reporting bias
3. Results
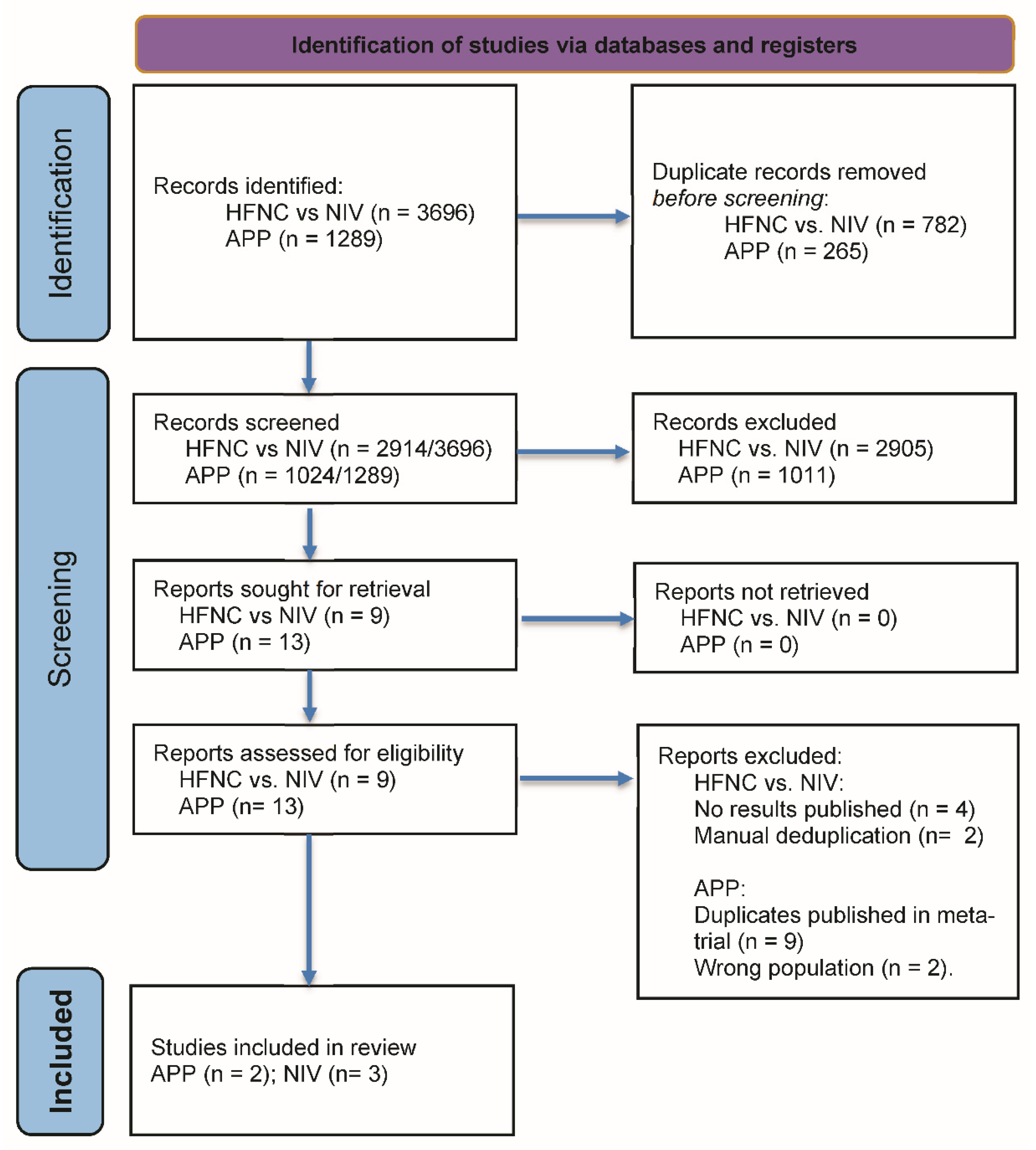
3.1. Study Selection
3.2. Study Characteristics
3.2.1. HFNC vs. NIV
3.2.2. APP
3.3. Risk of Bias
3.3.1. HFNC vs. NIV
3.3.2. APP
3.4. Effects of the Intervention
3.4.1. HFNC vs. NIV in COVID-19 Acute Respiratory Failure
3.4.2. APP in COVID-19 Acute Respiratory Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy
Appendix A.1. Awake Prone Positioning (APP)
Appendix A.1.1. Cochrane COVID-19 Study Register
Appendix A.1.2. WHO COVID-19 Global Literature on Coronavirus Disease
Appendix A.1.3. Web of Science (Science Citation Index Expanded and Emerging Citation Index)
Appendix A.1.4. CINAHL (Cumulative Index to Nursing and Allied Health Literature)
Appendix A.2. High-Flow Nasal Oxygen or Non-Invasive Ventilation (NIV)
Appendix A.2.1. Cochrane COVID-19 Study Register
Appendix A.2.2. WHO COVID-19 Global Literature on Coronavirus Disease
Appendix A.2.3. Web of Science (Science Citation Index Expanded and Emerging Citation Index)
Appendix A.2.4. CINAHL (Cumulative Index to Nursing and Allied Health Literature)
References
- Word Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 24 November 2021).
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.B.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, 22 January–30 May 2020. Mmwr Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case Characteristics, Resource Use, and Outcomes of 10 021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M.; Pozzi, T.; Palumbo, M.M.; Cressoni, M.; Herrmann, P.; et al. Physiological and Quantitative CT-Scan Characterization of COVID-19 and Typical ARDS: A Matched Cohort Study. Intensiv. Care Med. 2020, 46, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical Features, Ventilatory Management, and Outcome of ARDS Caused by COVID-19 Are Similar to Other Causes of ARDS. Intensiv. Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-Associated Acute Respiratory Distress Syndrome: A Multicentre Prospective Observational Study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Botta, M.; Tsonas, A.M.; Pillay, J.; Boers, L.S.; Algera, A.G.; Bos, L.D.J.; Dongelmans, D.A.; Hollmann, M.W.; Horn, J.; Vlaar, A.P.J.; et al. Ventilation Management and Clinical Outcomes in Invasively Ventilated Patients with COVID-19 (PRoVENT-COVID): A National, Multicentre, Observational Cohort Study. Lancet Respir Med. 2020, 9, 139–148. [Google Scholar] [CrossRef]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- Rochwerg, B.; Einav, S.; Chaudhuri, D.; Mancebo, J.; Mauri, T.; Helviz, Y.; Goligher, E.C.; Jaber, S.; Ricard, J.-D.; Rittayamai, N.; et al. The Role for High Flow Nasal Cannula as a Respiratory Support Strategy in Adults: A Clinical Practice Guideline. Intensiv. Care Med. 2020, 46, 2226–2237. [Google Scholar] [CrossRef]
- Carter, C.; Aedy, H.; Notter, J. COVID-19 Disease: Non-Invasive Ventilation and High Frequency Nasal Oxygenation. Clin. Integr. Care 2020, 1, 100006. [Google Scholar] [CrossRef]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Sorbo, L.D.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure. JAMA 2020, 324, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Crichton, M.L.; Goeminne, P.C.; Cao, B.; Humbert, M.; Shteinberg, M.; Antoniou, K.M.; Ulrik, C.S.; Parks, H.; Wang, C.; et al. Management of Hospitalised Adults with Coronavirus Disease 2019 (COVID-19): A European Respiratory Society Living Guideline. Eur. Respir. J. 2021, 57, 2100048. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Hubmayr, R.D. The Prone Position Eliminates Compression of the Lungs by the Heart. Am. J. Respir. Crit. Care 2000, 161, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Rehder, K.; Knopp, T.J.; Sessler, A.D. Regional Intrapulmonary Gas Distribution in Awake and Anesthetized-Paralyzed Prone Man. J. Appl. Physiol. 1978, 45, 528–535. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Porter, B.M.; Elder, P.J.; Evans, S.B.; Turnbull, C.D.; Hynes, G.; Lardner, R.; Archer, K.; Bettinson, H.V.; Nickol, A.H.; et al. Successful Awake Proning Is Associated with Improved Clinical Outcomes in Patients with COVID-19: Single-Centre High-Dependency Unit Experience. BMJ Open Respir. Res. 2020, 7, e000678. [Google Scholar] [CrossRef]
- Paternoster, G.; Sartini, C.; Pennacchio, E.; Lisanti, F.; Landoni, G.; Cabrini, L. Awake Pronation with Helmet Continuous Positive Airway Pressure for COVID-19 Acute Respiratory Distress Syndrome Patients Outside the ICU: A Case Series. Med. Intensiv. 2020. [CrossRef]
- Winearls, S.; Swingwood, E.L.; Hardaker, C.L.; Smith, A.M.; Easton, F.M.; Millington, K.J.; Hall, R.S.; Smith, A.; Curtis, K.J. Early Conscious Prone Positioning in Patients with COVID-19 Receiving Continuous Positive Airway Pressure: A Retrospective Analysis. BMJ Open Respir. Res. 2020, 7, e000711. [Google Scholar] [CrossRef]
- Schmid, B.; Grummich, K.; Romero, C.S.; Popp, M.; Meybohm, P.; Kranke, P.; Weibel, S. Risk and Harm of Prone Positioning vs. Supine or Lateral Positioning in Hospitalised COVID-19 Patients with Severe Respiratory Insufficiency (PROSPERO Protocol). Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021261862 (accessed on 29 November 2021).
- Stroehlein, J.; Griesel, M.; Popp, M.; Fichtner, F.; Skoetz, N.; Metzendorf, M.-I.; Wedekind, L. Patients with Severe/Critical COVID-19 and Respiratory Failure: Non-Invasive Ventilation Versus Intubation and Invasive Mechanical Ventilation. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021230825 (accessed on 1 December 2021).
- WHO; Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; et al. Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; Soyza, A.D.; et al. An Adaptive Randomized Controlled Trial of Non-Invasive Respiratory Strategies in Acute Respiratory Failure Patients with COVID-19. Medrxiv 2021, 58. [Google Scholar] [CrossRef]
- Grieco, D.L.; Menga, L.S.; Raggi, V.; Bongiovanni, F.; Anzellotti, G.M.; Tanzarella, E.S.; Bocci, M.G.; Mercurio, G.; Dell’Anna, A.M.; Eleuteri, D.; et al. Physiological Comparison of High-Flow Nasal Cannula and Helmet Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care 2019, 201, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.R.; Haritha, D.; Behera, S.; Kayina, C.A.; Maitra, S.; Anand, R.K.; Ray, B.R.; Soneja, M.; Subramaniam, R.; Baidya, D.K. Comparison of High-Flow Nasal Cannula and Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure Due to Severe COVID-19 Pneumonia. Respir. Care 2021, 66, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Li, J.; Ibarra-Estrada, M.; Perez, Y.; Pavlov, I.; McNicholas, B.; Roca, O.; Mirza, S.; Vines, D.; Garcia-Salcido, R.; et al. Awake Prone Positioning for COVID-19 Acute Hypoxaemic Respiratory Failure: A Randomised, Controlled, Multinational, Open-Label Meta-Trial. Lancet Respir. Med. 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
- Rosén, J.; von Oelreich, E.; Fors, D.; Fagerlund, M.J.; Taxbro, K.; Skorup, P.; Eby, L.; Jalde, F.C.; Johansson, N.; Bergström, G.; et al. Awake Prone Positioning in Patients with Hypoxemic Respiratory Failure Due to COVID-19: The PROFLO Multicenter Randomized Clinical Trial. Crit. Care 2021, 25, 209. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS Clinical Practice Guidelines: Noninvasive Ventilation for Acute Respiratory Failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.-M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal Guidelines: Management of Acute Respiratory Distress Syndrome. Ann. Intensiv. Care 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- The World Bank World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 2 December 2021).
| Authors | Population | Intervention | Comparator | Outcomes | Results per Endpoint |
|---|---|---|---|---|---|
| High-flow nasal cannula vs. non-invasive ventilation | |||||
| Perkins et al. [25] Standard care in both arms: Dexamethasone and Tocilizumab added as standard care in June 2020 and January 2021, respectively Recruitment: from April 2020 to May 2021 with ca. 90% of patients recruited between September 2020 and March 2021 | n = 797 (allocated) age (years): mean 57.6 (SD 13.0) in HFNC, 56.7 (SD 12.5) in CPAP sex: 65.2% male vs. 68.4% male comorbidities: none in 34% vs. 39%, hypertension, morbid obesity, type 2 diabetes requiring medication, chronic lung disease clinical status at enrolment: inpatients not intubated with hypoxemic respiratory failure PaO2/FiO2 ratio 139 vs. 132, resp. rate 25 vs. 26 | n = 417 HFNC Local policies, and clinical discretion informed decisions regarding choice of device, set-up, titration, and discontinuation of treatment, mean flow 51 L/min adherence to intended treatment protocol: 92.1% | n = 380 NIV Local policies, and clinical discretion informed decisions regarding choice of device, set-up, titration, and discontinuation of treatment, mean PEEP 9.5 (SD 8.4) adherence to intended treatment protocol: 91.6% | Endotracheal intubation or death within 30 days In-hospital mortality 30-day mortality Endotracheal intubation within 30 days Mean length of stay in hospital (SD) Serious adverse event (as no. of patients with at least one) Adverse events (as no. of patients with at least one) Adverse events (as total no. of events per patients) | HFNC 184/414 vs. NIV 137/377: RR 1.22 (1.03 to 1.45) 88/404 vs. 72/364: RR 1.10 (0.83 to 1.45) 78/415 vs. 63/378: RR 1.13 (0.83 to 1.52) 170/414 vs. 126/377: RR 1.23 (1.02 to 1.48) 18.3 (20.0) vs. 16.4 (17.5) 0/417 vs. 7/380: RR 0.06 (0.00 to 1.06) 86/417 vs. 130/380: RR 0.60 (0.48 to 0.76) 157/417 vs. 200/380 |
| Grieco et al. [26] Standard care in both arms: Dexamethasone 100%, Remdesivir 81%. Sedation allowed but discouraged (continuous infusion 18%HFNC group vs. 37% in the helmet group). Awake proning allowed, 60% in HFNC vs. 0% in NIV underwent proning. Other aspects according to the clinical practice in each institution. Recruitment: from October 2020 to December 2020 | n = 109 (allocated) age (years): 63 (IQR 55–69) (intervention) 66 (IQR 57–72) (control) sex: male 84% vs. 77% comorbidities: hypertension, diabetes mell., smoking, immunocompromised state, history of cancer clinical status at enrolment: inpatients not intubated with hypoxemic respiratory failure with PaO2/FiO2 ratio equal or below 200: PaO2/FiO2 ratio 102 vs. 105, PaCO2 34 vs. 34 mmHg, resp. rate 28 vs. 28 | n = 55 HFNC for at least 48 h with 60 L/min and FiO2 titrated to SpO2 goal 92%. Stepwise weaning allowed when FiO2 was equal to or lower than 40% and respiratory rate lower than 25/min adherence to intended treatment protocol: received by 87% for 48 h or until intubation, one participant crossed over, other participants were weaned early according to the trial protocol | n = 55 Helmet NIV for at least 48 h with initial PS 10–12 mbar and PEEP of 10–12 mbar and FiO2 titrated to SpO2 goal 92%. Stepwise weaning allowed when FiO2 was equal to or lower than 40% and respiratory rate lower than 25/min adherence to intended treatment protocol: received by 91% for 48 h or until intubation, 4% received it at least 16 h per day, 4% interrupted treatment, 2% did not receive the allocated treatment | Days free of respiratory support within 28 days Endotracheal intubation within 28 days 28-day mortality 60-day mortality In-hospital mortality Adverse events (predefined selection of AEs relevant in respiratory and intensive care medicine, as total no. of events per patients) | Mean (SD) in HFNC vs. NIV: 15 (11) vs. 13 (11) with MD 2 days (95% CI, −2 to 6) 28/55 vs. 16/54: RR 1.72 (1.06 to 2.79) 10/55 vs. 8/54: RR 1.23 (0.52 to 2.87) 12/55 vs. 13/54: RR 0.91 (0.46 to 1.80) 14/55 vs. 13/54: RR 1.06 (0.55 to 2.04) 70/55 vs. 37/54 |
| Nair et al. [27] Standard care in both arms: clinical management and drug therapy as per institutional protocols without further specification except: awake proning was encouraged, but only in almost all HFNC participants regularly performed. Recruitment: from August 2020 to December 2020 | n = 109 (allocated) age (years): HFNC 57 (IQR 48–65) vs. NIV 57.5 (IQR 47–64) sex: male 80% vs. 64.8% comorbidities: Diabetes mell., hypertension, coronary heart disease, chronic kidney disease clinical status at enrolment: hospitalised, not intubated with hypoxemic respiratory failure despite O2 insufflation: PaO2/FiO2 ratio 105 vs. 111, PaCO2 34 vs. 32 mmHg, resp. rate 30 vs. 31 | n = 55 HFNC through large-bore binasal prongs with a high-flow heated humidifier device, initial gas flow 50 L/min and FIO2 of 1.0, flow and FIO2 were subsequently adjusted between 30–60 L/min and 0.5–1.0, to maintain SpO2 of 94% or more. adherence to intended treatment protocol: Not quantified, but “Increased subject compliance and ease with which awake proning could be facilitated in HFNC group could have influenced the outcome in favor of the latter.” | n = 54 NIV with either mask/helmet device connected to an ICU ventilator, PS of 10–20 cm H2O (aim of obtaining an expired tidal volume of 7–10 mL per kg of predicted body weight), PEEP 5–10 cm H2O, FIO2 0.5–1.0 titrated to target SpO2 > 94%. adherence to intended treatment protocol: Not quantified, but “Although NIV was initiated with PS 10–20 cm H2O, most of the subjects required PS) 5 cm H2O, [...]. Subjects on NIV usually felt claustrophobic and frequently complained of dry mouth, leading to repeated detachments of the oxygenation interface. Moreover, most of the subjects on NIV were not compliant.” The incompliance with NIV could have been even higher than expected due to aggressive initial ventilator settings. | Intubation or death Intubation within 7 days In-hospital mortality Ventilator-free days at 28 d | HR 0.51 (95% CI 0.28–0.94, p = 0.03) 15/55 vs. 25/54: RR 0.59 (0.35 to 0.99) 16/55 vs. 25/54: RR 0.63 (0.38 to 1.04); HR 0.54 (0.29 to 1.01) median 28.0 (IQR 27–28) vs. 27.5 (27–28) These data are implausible to us, given that 27% vs. 46% of patients were intubated within 7 days and the respective Kaplan-Meier estimator in the publication. This might be due to an unreported particular definition of this complex endpoint prone to misinterpretation. |
| awake prone positioning | |||||
| Ehrmann et al. [28] Recruitment: 4 April 2020–26 January 2021 | n = 1121 | n = 564 | n = 557 | intubation or death | RR 0.86 (95% CI 0.75 to 0.98) |
| age (years) 61.5 ± 13.3 (intervention) 60.7 ± 13.3 (control) | (awake) prone positioning for as long as possible | unrestricted (self) positioning except prone | 28-day mortality | RR 0.87 (0.71 to 1.07) | |
| comorbidities diabetes mellitus, chronic heart disease, obesity | intubation within 28 days | RR 0.83 (0.71 to 0.96) | |||
| clinical status inpatient treatment, need for HFNC or NIV, no mechanical ventilation (i.e., WHO clinical progression scale 6) | hospitalization: length of hospital stay (censored at 28 days) | difference of means −0.2 days (−1.35 to 0.96) | |||
| skin lesions | RR 0.5 (0.16 to 1.56) | ||||
| weaning from HFNC (time to event in days) | difference of means -0.9 days (0.35 to 1.45) | ||||
| Rosén et al. [29] Recruitment: 7 October 2020–7 February 2021 | n = 75 | n = 36 | n = 39 | intubation within 30 days | RR 1.00 (0.53 to 1.90) |
| age (years) 65 (IQR 53–74; intervention) 65 (IQR 55–70; control) | (awake) prone positioning ≥16 h/day | unrestricted (self) positioning except prone | 30-day mortality | RR 2.17 (0.58 to 8.03) | |
| comorbidities diabetes mellitus, arterial hypertension, obesity | hospitalization: length of hospital stay (censored after 30 days) | difference of means −2.0 days (−7.16 to 3.16) | |||
| clinical status inpatient treatment, need for HFNC or NIV, no mechanical ventilation (i.e., WHO clinical progression scale 6) | skin lesions | RR 0.24 (0.06 to 1.04) | |||
| days free of HFNC | difference of means 2.0 days (0.13 to 3.87) | ||||
| Study | Outcome | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|
| APP | |||||||
| Ehrmann et al. [28] | Intubation or death at 28 days |  |  |  |  |  |  |
| Ehrmann et al. | Mortality rate at 28 days |  |  |  |  |  |  |
| Ehrmann et al. | Need for intubation within 28 days |  |  |  |  |  |  |
| Ehrmann et al. | Hospital length of stay (censored at 28 days) |  |  |  |  |  |  |
| Ehrmann et al. | Occurrence of skin lesions |  |  |  |  |  |  |
| Ehrmann et al. | Weaning of HFNC (time to event) |  |  |  |  |  |  |
| Rosén et al. [29] | Mortality rate at day 30 |  |  |  |  |  |  |
| Rosén et al. | Need for intubation within 30 days |  |  |  |  |  |  |
| Rosén et al. | Occurrence of skin lesions |  |  |  |  |  |  |
| Rosén et al. | Hospital length of stay (censored at 30 days) |  |  |  |  |  |  |
| Rosén et al. | Days free of HFNC |  |  |  |  |  |  |
| HFNC vs. NIV | |||||||
| Grieco et al. [26] | Mortality rate at 28 days/60 days |  |  |  |  |  |  |
| Grieco et al. | In-hospital mortality |  |  |  |  |  |  |
| Grieco et al. | Intubation up to day 28 |  |  |  |  |  |  |
| Grieco et al. | Respirator-free days at 30 days |  |  |  |  |  |  |
| Grieco et al. | Hospital length of stay |  |  |  |  |  |  |
| Grieco et al. | Adverse events |  |  |  |  |  |  |
| Perkins et al. [25] | Intubation or death at 30 days |  |  |  |  |  |  |
| Perkins et al. | In-hospital mortality |  |  |  |  |  |  |
| Perkins et al. | Mortality rate at 30 days |  |  |  |  |  |  |
| Perkins et al. | Hospital length of stay |  |  |  |  |  |  |
| Perkins et al. | Intubation up to day 30 |  |  |  |  |  |  |
| Perkins et al. | Serious adverse events |  |  |  |  |  |  |
| Perkins et al. | Adverse events |  |  |  |  |  |  |
| Nair et al. [27] | In-hospital mortality |  |  |  |  |  |  |
| Nair et al. | Intubation rate at 7 days |  |  |  |  |  |  |
| Nair et al. | Intubation or death |  |  |  |  |  |  |
| Nair et al. | Hospital length of stay |  |  |  |  |  |  |
 low risk,
low risk,  some concerns,
some concerns,  high risk.
high risk.| Outcome | Results | Absolute Effect Estimates | Certainty of Evidence | |
|---|---|---|---|---|
| NIV | HFNC | |||
| Mortality: in-hospital (up to longest follow-up) | RR: 0.92 (95% CI 0.65–1.33) 986 patients from 3 studies | 233 per 1000 | 214 per 1000 | very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 19 less per 1000 (95% CI 82 less to 77 more) | ||||
| Mortality: up to day 30 (follow-up 28 to 30 days) | RR: 1.14 (95% CI 0.86–1.51) 902 patients from 2 studies | 164 per 1000 | 187 per 1000 | very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 23 more per 1000 (95% CI 23 less to 84 more) | ||||
| Intubation or death (follow-up 30 days) | RR 1.22 (95% CI 1.03–1.45) 791 patients from 1 study | 363 per 1000 | 443 per 1000 | low due to serious risk of bias and indirectness |
| difference: 80 more per 1000 (95% CI 11 more to 164 more) | ||||
| Intubation (follow-up 28 to 30 days) | RR 1.34 (95% CI 1.00–1.80) 900 patients from 2 studies | 329 per 1000 | 441 per 1000 | very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 112 more per 1000 (95% CI 0 more to 263 more) | ||||
| Serious adverse events (follow-up 30 days) | RR 0.06 (95% CI 0.00–1.06) 797 patients from 1 study | 18 per 1000 | 1 per 1000 | very low due to serious imprecision, serious risk of bias and indirectness |
| difference 17 less per 1000 (95% CI 18 less to 2 more) | ||||
| Adverse events (follow-up 30 days) | 906 patients from 2 studies | Perkins: NIV 200/380 vs. HFNC 157/417 (as no. events in total) Grieco: 37/54 vs. 70/55 | very low due to serious risk of bias, inconsistency and indirectness | |
| Length of hospital stay (censored at 30 days) | difference of means +1.90 days 768 patients from 1 study | 16.4 days | 18.3 days | low due to serious risk of bias, serious imprecision and indirectness |
| difference: 1.90 days more (95% CI 0.75 less to 4.55 more) | ||||
| Respiratory-support-free days: no invasive ventilation, HFNC, or NIV (follow-up 28 days) | difference of means 2 days 109 patients from 1 study | 15 (11) | 13 (11) | low due very serious imprecision |
| difference: 2.0 days more (95% CI 6.16 less to 2.13 more) | ||||
| Outcome | Results | Absolute Effect Estimates | Certainty of Evidence | |
|---|---|---|---|---|
| SoC | APP | |||
| All-cause mortality (28 days) | RR: 1.08 (95% CI 0.51–2.31) 1196 patients from 2 studies | 227 per 1000 | 245 per 1000 | low due to serious risk of bias and serious imprecision |
| difference: 18 more per 1000 (95% CI 111 less to 297 more) | ||||
| Intubation or death at 28 days | RR 0.86 (95% CI 0.75–0.98) 1121 patients from 1 study | 461 per 1000 | 396 per 1000 | moderate due to serious risk of bias |
| difference: 65 less per 1000 (95% CI 115 less to 9 less) | ||||
| Intubation within 28 days | RR 0.83 (95% CI 0.71–0.96) 1196 patients from 2 studies | 396 per 1000 | 329 per 1000 | moderate due to serious risk of bias |
| difference: 67 less per 1000 (95% CI 115 less to 16 less) | ||||
| Hospital length of stay (censored at 28 days) | difference of means −0.2 days 1196 patients from 2 studies | 16.6 days | 16.4 days | moderate due to serious risk of bias |
| difference: 0.2 days less (95% CI 1.35 less to 0.96 more) | ||||
| Days free of HFNC within 30 days | difference of means 2 days 50 patients from 1 study | 24 days | 26 days | low due to serious risk of bias and serious imprecision |
| difference: 2.0 days more (95% CI 0.13 more to 3.87 more) | ||||
| Weaning of HFNC (time to event within 28 days) | difference of means 0.9 days 1121 patients from 1 study | 6.0 days | 6.9 days | moderate due to serious risk of bias |
| difference: 0.9 days more (95% CI 0.35 more to 1.45 more) | ||||
| Skin lesions within 28 days | RR 0.5 (95% CI 0.16–1.56) 1196 patients from 2 studies | 32 per 1000 | 16 per 1000 | very low due to serious risk of bias and very serious imprecision |
| difference: 16 less per 1000 (95% CI 27 less to 18 more) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, B.; Griesel, M.; Fischer, A.-L.; Romero, C.S.; Metzendorf, M.-I.; Weibel, S.; Fichtner, F. Awake Prone Positioning, High-Flow Nasal Oxygen and Non-Invasive Ventilation as Non-Invasive Respiratory Strategies in COVID-19 Acute Respiratory Failure: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 391. https://doi.org/10.3390/jcm11020391
Schmid B, Griesel M, Fischer A-L, Romero CS, Metzendorf M-I, Weibel S, Fichtner F. Awake Prone Positioning, High-Flow Nasal Oxygen and Non-Invasive Ventilation as Non-Invasive Respiratory Strategies in COVID-19 Acute Respiratory Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(2):391. https://doi.org/10.3390/jcm11020391
Chicago/Turabian StyleSchmid, Benedikt, Mirko Griesel, Anna-Lena Fischer, Carolina S. Romero, Maria-Inti Metzendorf, Stephanie Weibel, and Falk Fichtner. 2022. "Awake Prone Positioning, High-Flow Nasal Oxygen and Non-Invasive Ventilation as Non-Invasive Respiratory Strategies in COVID-19 Acute Respiratory Failure: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 2: 391. https://doi.org/10.3390/jcm11020391
APA StyleSchmid, B., Griesel, M., Fischer, A.-L., Romero, C. S., Metzendorf, M.-I., Weibel, S., & Fichtner, F. (2022). Awake Prone Positioning, High-Flow Nasal Oxygen and Non-Invasive Ventilation as Non-Invasive Respiratory Strategies in COVID-19 Acute Respiratory Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(2), 391. https://doi.org/10.3390/jcm11020391







