Clinical Trial Evaluating Quality of Life in Patients with Intra-Oral Halitosis
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Data Collection
2.4. Halitosis Assessment
2.5. Questionnaire
2.6. Statistical Methods
3. Results
3.1. Adverse Events and Safety Monitoring
3.2. Study Population
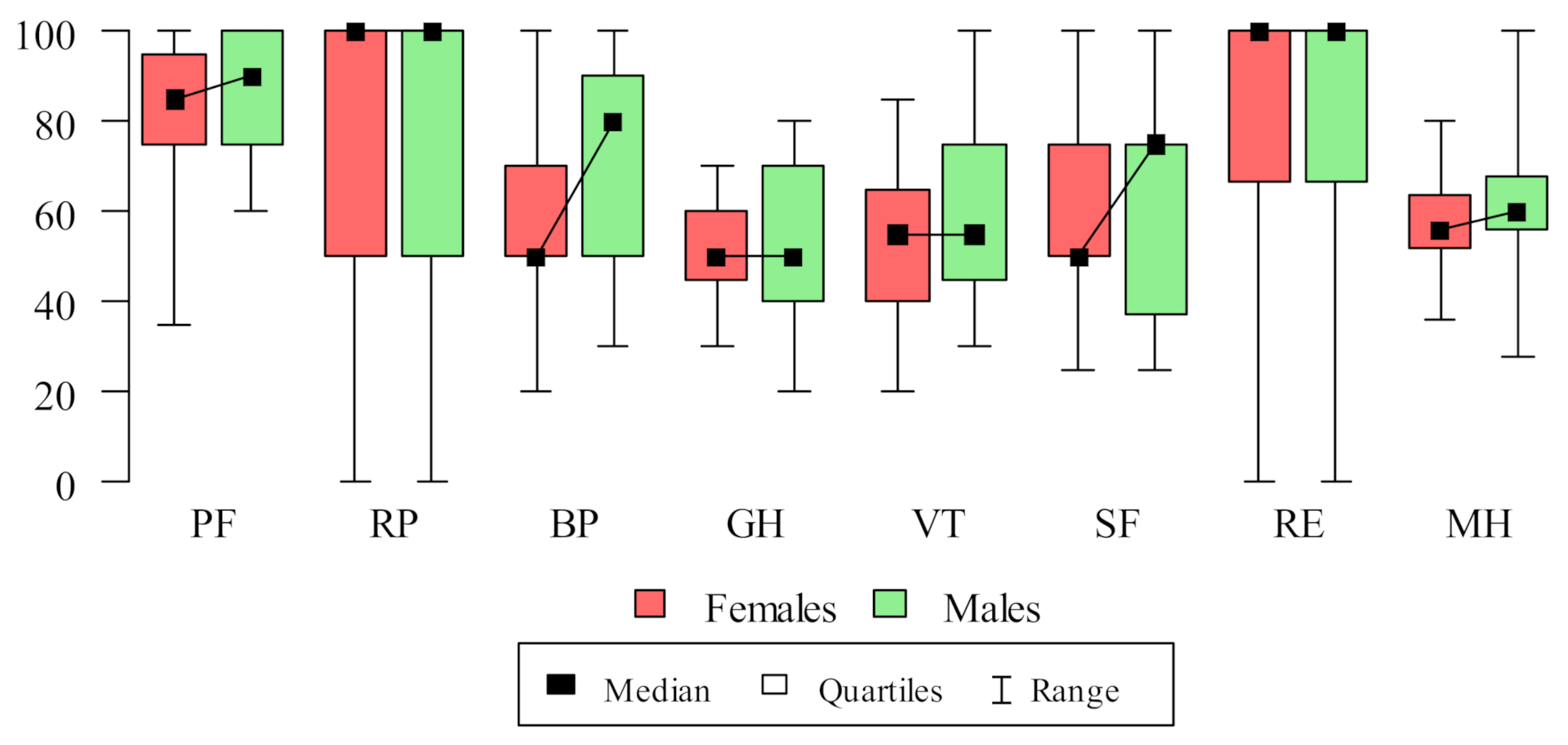
3.3. SF-36 Domains’ Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Domain | N | Mean | SD | Median | Min | Max | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|
| PF (physical functioning) | 100 | 83.8 | 15.96 | 90 | 35 | 100 | 75 | 95 |
| RP (activity limitations due to physical problems) | 100 | 70 | 38.8 | 100 | 0 | 100 | 50 | 100 |
| BP (pain complaints) | 100 | 64 | 24.33 | 60 | 20 | 100 | 50 | 90 |
| GH (general health perception) | 100 | 51.5 | 13.49 | 50 | 20 | 80 | 41.25 | 60 |
| VT (vitality) | 100 | 56 | 17.11 | 55 | 20 | 100 | 45 | 65 |
| SF (social functioning) | 100 | 61.5 | 22.28 | 56.25 | 25 | 100 | 40.62 | 75 |
| RE (activity limitations caused by emotional problems) | 100 | 75.33 | 34.21 | 100 | 0 | 100 | 66.67 | 100 |
| MH (emotional wellbeing) | 100 | 59.84 | 13.64 | 60 | 28 | 100 | 52 | 67 |
References
- Kazor, C.E.; Mitchell, P.M.; Lee, A.M.; Stokes, L.N.; Loesche, W.J.; Dewhirst, F.E.; Paster, B.J. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 2003, 41, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renvert, S.; Noack, M.J.; Lequart, C.; Roldán, S.; Laine, M.L. The Underestimated Problem of Intra-Oral Halitosis in Dental Practice: An Expert Consensus Review. Clin. Cosmet. Investig. Dent. 2020, 12, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, M.D.D.; Giudice, F.S.; Carvalho, L.F. The Halitosis Consequences Inventory: Psychometric properties and relationship with social anxiety disorder. BDJ Open 2018, 4, 18002. [Google Scholar] [CrossRef]
- Aydin, M.; Harvey-Woodworth, C.N. Halitosis: A new definition and classification. Br. Dent. J. 2014, 217, E1. [Google Scholar] [CrossRef]
- Miyazaki, H.; Arao, M.; Okamura, K.; Kawaguchi, Y.; Toyofuku, A.; Hoshi, K.; Yaegaki, K. Tentative classifcation of halitosis and its treatment needs. Niigata Dent. J. 1999, 32, 7–11. [Google Scholar]
- Yaegaki, K.; Coil, J.M. Examination, classification, and treatment of halitosis; clinical perspectives. J. Can. Dent. Assoc. 2000, 66, 257–261. [Google Scholar]
- Chen, X.; Zhang, Y.; Lu, H.X.; Feng, X.P. Factors associated with halitosis in white-collar employees in Shanghai, China. PLoS ONE 2016, 11, e0155592. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, M.; Takahashi, T.; Tokunaga, M.; Iwasaki, M.; Kataoka, S.; Kakuta, S.; Soh, I.; Awano, S.; Hirata, H.; Kagawa, M.; et al. Relationships between pathologic subjective halitosis, olfactory reference syndrome, and social anxiety in young Japanese women. BMC Psychol. 2017, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitsu, T.; Ueno, M.; Shinada, K.; Wright, F.A.; Kawaguchi, Y. Social anxiety disorder in genuine halitosis patients. Health Qual. Life Outcomes 2011, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Eldarrat, A.H. Influence of oral health and lifestyle on oral malodour. Int. Dent. J. 2011, 61, 47–51. [Google Scholar] [CrossRef]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontology 2002, 28, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Kizhner, V.; Xu, D.; Krespi, Y.P. A new tool measuring oral malodor quality of life. Eur. Arch. Otorhinolaryngol. 2011, 268, 1227–1232. [Google Scholar] [CrossRef]
- Tanaka, M.; Anguri, H.; Nishida, N.; Ojima, M.; Nagata, H.; Shizukuishi, S. Reliability of clinical parameters for predicting the outcome of oral malodor treatment. J. Dent. Res. 2003, 82, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.S.; Cunningham, W.E.; Hays, R.D. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual. Life Outcomes 2007, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, H.; Sakao, S.; Katoh, Y.; Takehara, T. Correlation Between Volatile Sulphur Compounds and Certain Oral Health Measurements in the General Population. J. Periodontol. 1995, 66, 679–684. [Google Scholar] [CrossRef]
- Zawisza, K.; Tobiasz-Adamczyk, B.; Zapała, J.; Marecik, T. Validity and reliability of the SF-36 health questionnaire in patients with cancer of the head and neck. Czas. Stomatol. 2009, 62, 751–763. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Haraldstad, K.; Wahl, A.; Andenæs, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Staquet, M.; Berzon, R.; Osoba, D.; Machin, D. Guidelines for reporting results of quality of life assessments in clinical trials. Qual. Life Res. 1996, 5, 496–502. [Google Scholar] [CrossRef]
- The World Health Organization Quality of Life assessment (WHOQOL). Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Mayo, N. Dictionary of Quality of Life and Health Outcomes Measurement; International Society for Quality of Life Research: Milwaukee, WI, USA, 2015. [Google Scholar]
- Ruta, D.A.; Abdalla, M.I.; Garratt, A.M.; Coutts, A.; Russell, I.T. SF 36 health survey questionnaire: I. Reliability in two patient based studies. Qual. Health Care 1994, 3, 180–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, J.E., Jr. SF-36 health survey update. Spine (Phila Pa 1976). Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Kantz, M.E.; Harris, W.J.; Levitsky, K.; Ware, J.E.; Davies, A.R. Methods for Assessing Condition-Specific and Generic Functional Status Outcomes after Total Knee Replacement. Med. Care 1992, 30, MS240–MS252. [Google Scholar] [CrossRef]
- Phillips, R.C.; Lansky, D.J. Outcomes management in heart valve replacement surgery: Early experience. J. Heart Valve Dis. 1992, 1, 42–50. [Google Scholar]
- Ware, J.E., Jr.; Kosinski, M.; Bayliss, M.S.; McHorney, C.A.; Rogers, W.H.; Raczek, A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med. Care 1995, 33 (Suppl. 4), AS264–AS279. [Google Scholar]
- Coulehan, J.L.; Schulberg, H.C.; Block, M.R.; Madonia, M.J.; Rodriguez, E. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch. Intern. Med. 1997, 157, 1113–1120. [Google Scholar] [CrossRef]
- Silveira, J.O.D.; Cota, L.O.M.; Bendo, C.B.; Faria, S.F.S.; Costa, F.O. Validation of the Brazilian version of the Halitosis Associated Life-Quality Test (HALT). Braz. Oral Res. 2020, 34, e098. [Google Scholar] [CrossRef]
- Lu, H.X.; Chen, X.L.; Wong, M.; Zhu, C.; Ye, W. Oral health impact of halitosis in Chinese adults. Int. J. Dent. Hyg. 2017, 15, e85–e92. [Google Scholar] [CrossRef] [PubMed]
- Buunk-Werkhoven, Y.; Dijkstra-le Clercq, M.; Verheggen-Udding, E.; de Jong, N.; Spreen, M. Halitosis and oral health-related quality of life: A case report. Int. J. Dent. Hyg. 2012, 10, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Ist. Super Sanita. 2016, 52, 184–189. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [Green Version]
- Paller, C.J.; Campbell, C.M.; Edwards, R.R.; Dobs, A.S. Sex-based differences in pain perception and treatment. Pain Med. 2009, 10, 289–299. [Google Scholar] [CrossRef]
- Case, A.; Deaton, A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc. Nat. Acad. Sci. USA 2015, 112, 15078–15083. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, M.F.; Grimley, R.; Lannin, N.A. Quality of life and age following stroke. Aging 2019, 11, 845–846. [Google Scholar] [CrossRef]
- Lannin, N.A.; Anderson, C.S.; Kim, J.; Kilkenny, M.; Bernhardt, J.; Levi, C.; Dewey, H.M.; Bladin, C.; Hand, P.; Castley, H.; et al. Treatment and Outcomes of Working Aged Adults with Stroke: Results from a National Prospective Registry. Neuroepidemiology 2017, 49, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.Y.; Löckenhoff, C.; Lee, C.Y.; Yu, S.H.; Wu, I.C.; Chang, H.Y.; Chiu, Y.F.; Hsiung, C.A. The paradox of aging and health-related quality of life in Asian Chinese: Results from the Healthy Aging Longitudinal Study in Taiwan. BMC Geriatr. 2020, 20, 91. [Google Scholar] [CrossRef]
- He, M.; Lu, H.; Cao, J.; Zhang, Y.; Wong, M.C.M.; Fan, J.; Ye, W. Psychological characteristics of Chinese patients with genuine halitosis. Oral Dis. 2020, 26, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Aylıkcı, B.U.; Colak, H. Halitosis: From diagnosis to management. J. Nat. Sci. Biol. Med. 2013, 4, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaorska, E.; Konop, M.; Ostaszewski, R.; Koszelewski, D.; Ufnal, M. Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe. Molecules 2018, 23, 2241. [Google Scholar] [CrossRef] [Green Version]
- Sopapornamorn, P.; Ueno MShinada, K.; Yanagishita, M.; Kawaguchi, Y. Relationship between total salivary protein content and volatile sulfur compounds levels in malodor patients. Oral Surg. Oral. Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Santaella, N.G.; Maciel, A.P.; Simpione, G.; Santos, P.S. Halitosis, reduced salivary flow and the quality of life in pre-kidney transplantation patients. J. Clin. Exp. Dent. 2020, 12, e1045–e1049. [Google Scholar] [CrossRef]
- Troger, B.; Almeida, H.L., Jr.; Duquia, R.P. Emotional impact of halitosis. Trends Psychiatry Psychother. 2014, 36, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Nadanovsky, P.; Carvalho, L.B.; Ponce de Leon, A. Oral malodour and its association with age and sex in a general population in Brazil. Oral Dis. 2007, 13, 105–109. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoneda, M.; Naito, T.; Iwamoto, T.; Hirofuji, T. Relationship between halitosis and psychologic status. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 542–547. [Google Scholar] [CrossRef]
- Azodo, C.C.; Ogbebor, O.G. Social distance towards halitosis sufferers. Swiss. Dent. J. 2019, 129, 1026–1030. [Google Scholar]
- Wang, J.; He, L. Comparison of the Psychological Condition of Chinese Patients with or without Halitosis Complaints. Chin. J. Dent. Res. 2018, 21, 69–76. [Google Scholar] [CrossRef]
- Veeresha, K.L.; Bansal, M.; Bansal, V. Halitosis: A frequently ignored social condition. J. Int. Soc. Prev. Community Dent. 2011, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Schertel Cassiano, L.; Abdullahi, F.; Leite, F.R.M.; López, R.; Peres, M.A.; Nascimento, G.G. The association between halitosis and oral-health-related quality of life: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 1458–1469. [Google Scholar] [CrossRef]
| Mean (SD) | Median (Quartiles) | ||
|---|---|---|---|
| H2S | 496.22 (441.19) | 412 (132.75–724.25) | |
| CH3SH | 205.66 (329.18) | 53 (26–265.25) | |
| (CH3)2S | 147.36 (231.83) | 40 (8–214) | |
| Total VSC | 849.24 (600.36) | 761 (405.5–1246.75) | |
| Age | 43.5 (15.17) | 41.5 (30–56.5) | |
| N | % | ||
| Sex | Female | 58 | 58% |
| Male | 42 | 42% | |
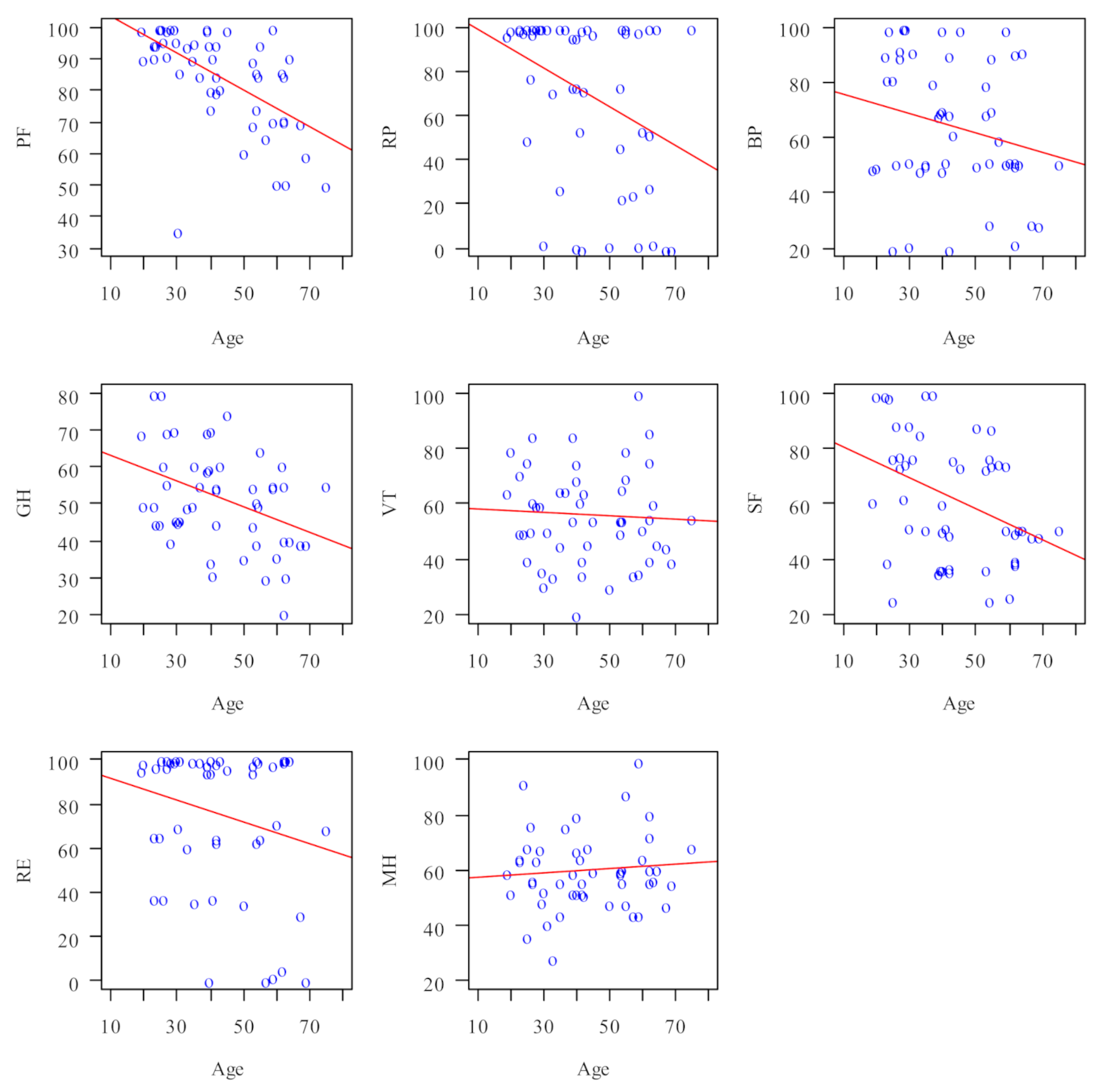
| Domain | Correlation with Age | |||
|---|---|---|---|---|
| Correlation Coefficient | p | Direction | Strength [19] | |
| PF (physical functioning) | −0.611 | <0.001 | Negative | Moderate |
| RP (activity limitations due to physical problems) | −0.341 | 0.015 | Negative | Weak |
| BP (pain complaints) | −0.219 | 0.126 | --- | --- |
| GH (general health perception) | −0.37 | 0.008 | Negative | Weak |
| VT (vitality) | −0.105 | 0.469 | --- | --- |
| SF (social functioning) | −0.368 | 0.009 | Negative | Weak |
| RE (activity limitations caused by emotional problems) | −0.175 | 0.225 | --- | --- |
| MH (emotional wellbeing) | 0.028 | 0.849 | --- | --- |
| Domain | * Correlation with H2S | |||
|---|---|---|---|---|
| Correlation Coefficient | p | Direction | Strength [19] | |
| PF | −0.332 | 0.015 | Negative | Weak |
| RP | −0.613 | 0.511 | --- | --- |
| BP | −0.352 | 0.008 | Negative | Weak |
| GH | −0.386 | 0.009 | Negative | Weak |
| VT | −0.601 | 0.003 | Negative | Moderate |
| SF | −0.646 | 0.005 | Negative | Moderate |
| RE | −0.312 | 0.015 | Negative | Weak |
| MH | −0.512 | 0.04 | Negative | Weak |
| Domain | * Correlation with CH3SH | |||
|---|---|---|---|---|
| Correlation Coefficient | p | Direction | Strength [19] | |
| PF | 0.048 | 0.739 | --- | --- |
| RP | 0.047 | 0.747 | --- | --- |
| BP | −0.032 | 0.827 | --- | --- |
| GH | −0.067 | 0.643 | --- | --- |
| VT | −0.244 | 0.087 | --- | --- |
| SF | 0.107 | 0.459 | --- | --- |
| RE | −0.06 | 0.679 | --- | --- |
| MH | −0.027 | 0.853 | --- | --- |
| Domain | * Correlation with (CH3)2S | |||
|---|---|---|---|---|
| Correlation Coefficient | p | Direction | Strength [19] | |
| PF | 0.094 | 0.518 | --- | --- |
| RP | −0.159 | 0.269 | --- | --- |
| BP | −0.01 | 0.942 | --- | --- |
| GH | −0.062 | 0.667 | --- | --- |
| VT | −0.023 | 0.876 | --- | --- |
| SF | −0.065 | 0.655 | --- | --- |
| RE | −0.206 | 0.151 | --- | --- |
| MH | −0.268 | 0.06 | --- | --- |
| Domain | * Correlation with Total VSC | |||
|---|---|---|---|---|
| Correlation Coefficient | p | Direction | Strength [19] | |
| PF | −0.256 | 0.016 | Negative | Very weak |
| RP | −0.289 | 0.001 | Negative | Very weak |
| BP | −0.376 | 0.005 | Negative | Weak |
| GH | −0.378 | 0.009 | Negative | Weak |
| VT | −0.631 | 0.008 | Negative | Moderate |
| SF | −0.642 | 0.015 | Negative | Moderate |
| RE | −0.359 | 0.015 | Negative | Weak |
| MH | −0.386 | 0.009 | Negative | Weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Czyz, I.; Sozkes, S.; Dudzik, A. Clinical Trial Evaluating Quality of Life in Patients with Intra-Oral Halitosis. J. Clin. Med. 2022, 11, 326. https://doi.org/10.3390/jcm11020326
Olszewska-Czyz I, Sozkes S, Dudzik A. Clinical Trial Evaluating Quality of Life in Patients with Intra-Oral Halitosis. Journal of Clinical Medicine. 2022; 11(2):326. https://doi.org/10.3390/jcm11020326
Chicago/Turabian StyleOlszewska-Czyz, Iwona, Sarkis Sozkes, and Agata Dudzik. 2022. "Clinical Trial Evaluating Quality of Life in Patients with Intra-Oral Halitosis" Journal of Clinical Medicine 11, no. 2: 326. https://doi.org/10.3390/jcm11020326
APA StyleOlszewska-Czyz, I., Sozkes, S., & Dudzik, A. (2022). Clinical Trial Evaluating Quality of Life in Patients with Intra-Oral Halitosis. Journal of Clinical Medicine, 11(2), 326. https://doi.org/10.3390/jcm11020326






