Clinical Characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
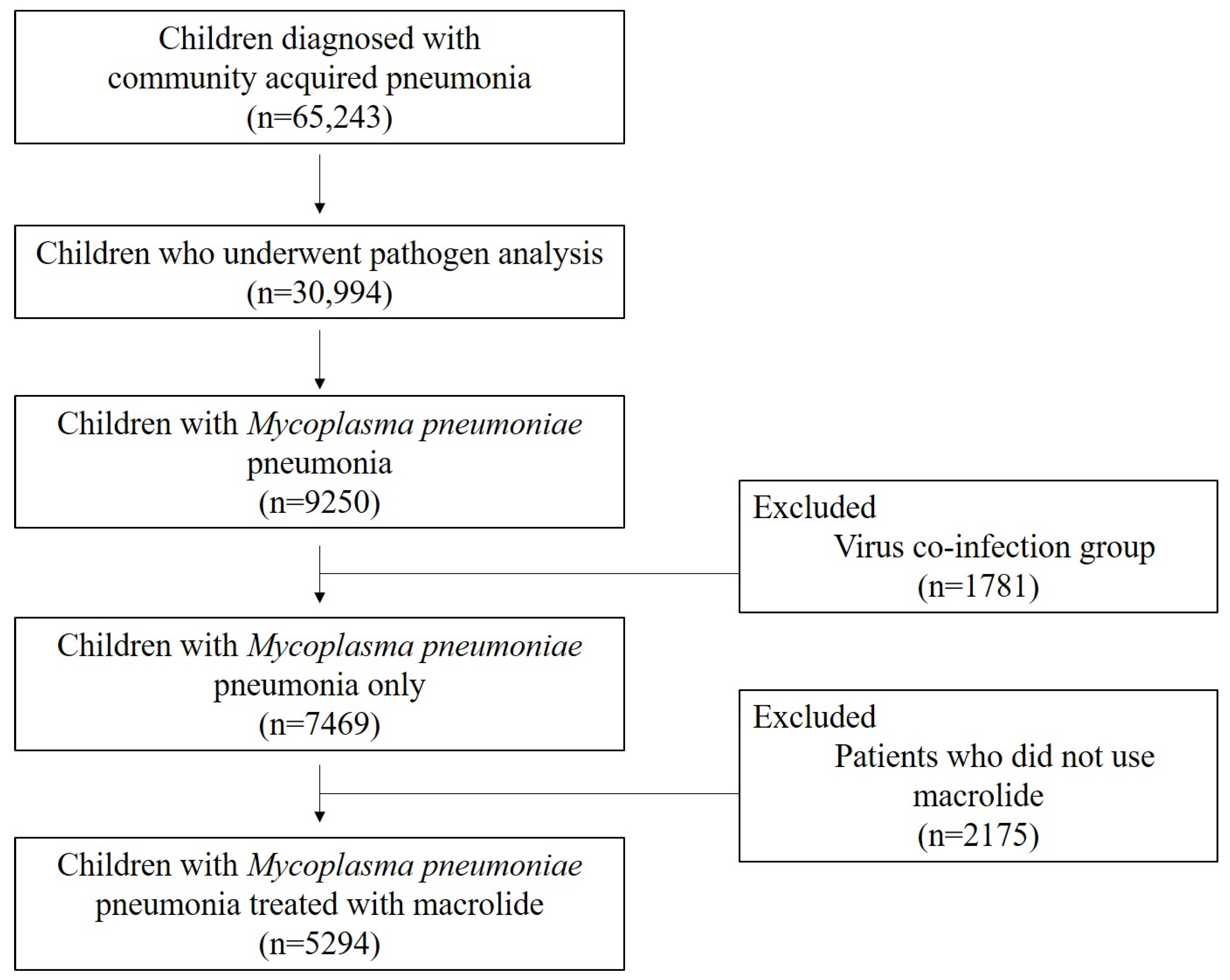
2.1. Study Subjects
2.2. Laboratory Tests and Image Study
2.3. Statistical Analyses
3. Results
3.1. Subject’s Demographics and Comparison of the Patient’s Clinical Characteristics
3.2. Laboratory Findings
3.3. Chest Imaging Findings
3.4. Extrapulmonary Manifestations and Postinfectious Sequalae
3.5. Patient’s Pre-Existing Conditions
3.6. Prediction Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef]
- Radisic, M.; Torn, A.; Gutierrez, P.; Defranchi, H.A.; Pardo, P. Severe Acute Lung Injury Caused by Mycoplasma pneumoniae: Potential Role for Steroid Pulses in Treatment. Clin. Infect. Dis. 2000, 31, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, Y.; Shikama, N.; Aotsuka, N.; Koseki, H.; Terano, T.; Hirai, A. Fulminant Mycoplasma pneumoniae Pneumonia. Intern. Med. 2001, 40, 345–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Li, S.; Yang, D.; Wu, X.; Chen, Z. The Clinical Characteristics and Predictors of Refractory Mycoplasma pneumoniae Pneumonia in Children. PLoS ONE 2016, 11, e0156465. [Google Scholar] [CrossRef]
- Cho, H.-K. Consideration in treatment decisions for refractory Mycoplasma pneumoniae pneumonia. Clin. Exp. Pediatr. 2021, 64, 459–467. [Google Scholar] [CrossRef]
- Waites, K.B.; Ratliff, A.; Crabb, D.M.; Xiao, L.; Qin, X.; Selvarangan, R.; Tang, Y.-W.; Zheng, X.; Bard, J.D.; Hong, T.; et al. Macrolide-Resistant Mycoplasma pneumoniae in the United States as Determined from a National Surveillance Program. J. Clin. Microbiol. 2019, 57, 00968-19. [Google Scholar] [CrossRef] [PubMed]
- Schönwald, S.; Kuzman, I.; Orešković, K.; Burek, V.; Škerk, V.; Car, V.; Božinović, D.; Čulig, J.; Radošević, S. Azithromycin: Single 1.5g dose in the treatment of patients with atypical pneumonia syndrome—A randomized study. Infection 1999, 27, 198–202. [Google Scholar] [CrossRef]
- Schönwald, S.; Skerk, V.; Petričevic, I.; Car, V.; Majerus-Mišic, L.; Gunjača, M. Comparison of three-day and five-day courses of azithromycin in the treatment of atypical pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 877–880. [Google Scholar] [CrossRef]
- Lu, A.; Wang, C.; Zhang, X.; Wang, L.; Qian, L. Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir. Care 2015, 60, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Yan, Y.; Zhu, C.; Huang, L.; Shao, X.; Xu, J.; Zhu, H.; Sun, X.; Ji, W.; et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int. J. Infect. Dis. 2014, 29, 18–23. [Google Scholar] [CrossRef]
- Inamura, N.; Miyashita, N.; Hasegawa, S.; Kato, A.; Fukuda, Y.; Saitoh, A.; Kondo, E.; Teranishi, H.; Wakabayashi, T.; Akaike, H.; et al. Management of refractory Mycoplasma pneumoniae pneumonia: Utility of measuring serum lactate dehydrogenase level. J. Infect. Chemother. 2014, 20, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, C.H.; Lee, Y.J.; Kim, H.B.; Kim, B.S.; Kim, H.Y.; Kim, Y.; Kim, S.; Park, C.; Seo, J.-H.; et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis. 2020, 20, 132. [Google Scholar] [CrossRef]
- Kawai, Y.; Miyashita, N.; Kubo, M.; Akaike, H.; Kato, A.; Nishizawa, Y.; Saito, A.; Kondo, E.; Teranishi, H.; Ogita, S.; et al. Therapeutic Efficacy of Macrolides, Minocycline, and Tosufloxacin against Macrolide-Resistant Mycoplasma pneumoniae Pneumonia in Pediatric Patients. Antimicrob. Agents Chemother. 2013, 57, 2252–2258. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, Y.; Jiang, W.; Hao, C. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Sci. Rep. 2016, 6, 39929. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Sunakawa, K.; Eguchi, H.; Ouchi, K.; Okada, K.; Kurosaki, T. Japanese Guidelines for the Management of Respiratory Infectious Diseases in Children 2007 with focus on pneumonia. Pediatr. Int. 2011, 53, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zhang, T.; Guo, W.; Zhu, Z.; Tian, J.; Cai, C.; Xu, Y. Identify clinical factors related to Mycoplasma pneumoniae pneumonia with hypoxia in children. BMC Infect. Dis. 2020, 20, 534. [Google Scholar] [CrossRef]
- Izumikawa, K. Clinical Features of Severe or Fatal Mycoplasma pneumoniae Pneumonia. Front. Microbiol. 2016, 7, 800. [Google Scholar] [CrossRef]
- Miyashita, N.; Akaike, H.; Teranishi, H.; Ouchi, K.; Okimoto, N. Macrolide-Resistant Mycoplasma pneumoniae Pneumonia in Adolescents and Adults: Clinical Findings, Drug Susceptibility, and Therapeutic Efficacy. Antimicrob. Agents Chemother. 2013, 57, 5181–5185. [Google Scholar] [CrossRef]
- Wu, H.-M.; Wong, K.-S.; Huang, Y.-C.; Lai, S.-H.; Tsao, K.-C.; Lin, Y.-J.; Lin, T.-Y. Macrolide-resistant Mycoplasma pneumoniae in children in Taiwan. J. Infect. Chemother. 2013, 19, 782–786. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, J.; Zheng, X.; Yan, L.; Zhang, Y.; Zeng, Q.; Tian, D.; Fu, Z.; Dai, J. Development and validation of a simple-to-use nomogram for predicting refractory Mycoplasma pneumoniae pneumonia in children. Pediatr. Pulmonol. 2020, 55, 968–974. [Google Scholar] [CrossRef]
- Matsuda, K.; Narita, M.; Sera, N.; Maeda, E.; Yoshitomi, H.; Ohya, H.; Araki, Y.; Kakuma, T.; Fukuoh, A.; Matsumoto, K. Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC Infect. Dis. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Chen, W.; Shen, N.; Tao, Y.; Zhao, R.; Luo, L.; Li, B.; Cao, Q. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, S.; Zhou, Y.; Huang, M.; Dong, G.; Chen, Z. Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci. Rep. 2016, 6, 37037. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Cheon, B.R.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Increased risk of refractory Mycoplasma pneumoniae pneumonia in children with atopic sensitization and asthma. Korean J. Pediatr. 2014, 57, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-A.; Tsai, C.-K.; Kuo, K.-C.; Yu, H.-R. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J. Microbiol. Immunol. Infect. 2021, 54, 557–565. [Google Scholar] [CrossRef]
- Dai, F.F.; Liu, F.Q.; Chen, X.; Yang, J.; Wang, K.; Guo, C.Y. The treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. J. Clin. Pharm. Ther. 2021, 46, 705–710. [Google Scholar] [CrossRef]
- Korean Academy of Pediatric Allergy and Respiratory Disease and the Korean Society of Pediatric Infectious Diseases. Treatment Guideline for Pediatric Macrolide-Refractory Severe Mycoplasma pneumoniae Pneumonia 2019. Available online: https://www.kapard.or.kr/community/guide.php (accessed on 3 January 2019).
- Narita, M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front. Microbiol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vargas, F.M.; Gómez-Duarte, O.G. Mycoplasma pneumoniae—An emerging extra-pulmonary pathogen. Clin. Microbiol. Infect. 2008, 14, 105–115. [Google Scholar] [CrossRef]
- Yiş, U.; Kurul, S.H.; Çakmakçı, H.; Dirik, E. Mycoplasma pneumoniae: Nervous system complications in childhood and review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2008, 167, 973–978. [Google Scholar] [CrossRef]
- Narita, M. Pathogenesis of Neurologic Manifestations of Mycoplasma pneumoniae Infection. Pediatr. Neurol. 2009, 41, 159–166. [Google Scholar] [CrossRef]
- D’Alonzo, R.; Mencaroni, E.; Di, G.L.; Laino, D.; Principi, N.; Esposito, S. Pathogenesis and treatment of neurologic diseases associated with Mycoplasma pneumoniae infection. Front. Microbiol. 2018, 9, 2751. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, S.; Lorrot, M.; Marc, E.; Moulin, F.; Boutonnat-Faucher, B.; Heilbronner, C.; Iniguez, J.-L.; Chaussain, M.; Nicand, E.; Raymond, J.; et al. Mycoplasma pneumoniae and Asthma in Children. Clin. Infect. Dis. 2004, 38, 1341–1346. [Google Scholar] [CrossRef]
- Hong, S.-J. The Role of Mycoplasma pneumoniae infection in asthma. Allergy Asthma Immunol. Res. 2012, 4, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-S.; Ma, F.-L.; Gao, X. Association of Mycoplasma pneumoniae infection with increased risk of asthma in children. Exp. Ther. Med. 2017, 13, 1813–1819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Li, Y.-C.; Zhou, X.-J.; Wu, J.-Y. Prediction of Refractory Mycoplasma Pneumoniae Pneumonia in Pediatric Patients. Pediatr. Allergy Immunol. Pulmonol. 2017, 30, 92–96. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Lee, W.-J.; Tsai, C.-M.; Kuo, K.-C.; Lee, C.-H.; Hsieh, K.-S.; Chang, C.-H.; Su, Y.-T.; Niu, C.-K.; Yu, H.-R. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr. Neonatol. 2018, 59, 501–506. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Jeon, J.-H.; Oh, J.-W. Critical combination of initial markers for predicting refractory Mycoplasma pneumoniae pneumonia in children: A case control study. Respir. Res. 2019, 20, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Zong, Z.; Liu, Y.-B.; Ye, H.; Lv, X.-J. PCR versus serology for diagnosing Mycoplasma pneumoniae infection: A systematic review & meta-analysis. Indian J. Med. Res. 2011, 134, 270–280. [Google Scholar]
- Chang, H.-Y.; Chang, L.-Y.; Shao, P.-L.; Lee, P.-I.; Chen, J.-M.; Lee, C.-Y.; Lu, C.-Y.; Huang, L.-M. Comparison of real-time polymerase chain reaction and serological tests for the confirmation of Mycoplasma pneumoniae infection in children with clinical diagnosis of atypical pneumonia. J. Microbiol. Immunol. Infect. 2014, 47, 137–144. [Google Scholar] [CrossRef]
| Variables | No. (%) or Mean ± SD |
|---|---|
| Total number of patients | 5294 |
| Age, years | 5.6 (±3.3) |
| Male sex | 2596 (49.0) |
| Allergy | 714 (13.5) |
| Bronchopulmonary dysplasia | 14 (0.3) |
| MrMP/MLMP/MSMP | 240 (4.5)/925 (17.5)/4129 (78.0) |
| Total duration of fever (from the initial onset of fever), days | 6.4 (±4.2) |
| Respiratory distress | 744 (14.1) |
| Mild/moderate to severe | 668 (12.6)/76 (1.4) |
| Oxygen support | 231 (4.4) |
| ICU admission | 19 (0.4) |
| Hospital days (days) | 6.7 (±4.5) |
| Category | MrMP | MLMP | MSMP | p -Value | |||
|---|---|---|---|---|---|---|---|
| Number of Subjects | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| Male sex, n (%) | 117 (48.8) | 240 | 444 (48.0) | 925 | 2035 (49.3) | 4129 | 0.78 |
| Age (years), mean (SD) | 6.4 (3.1) | 240 | 6.1 (3.2) | 925 | 5.4 (3.3) | 4129 | <0.01 † |
| Hospital stay (days), median (IQR) | 11.0 (8.0–14.0) | 240 | 8.0 (6.0–9.0) | 925 | 5.0 (4.0–7.0) | 4129 | <0.01 * |
| Fever duration (days) | |||||||
| Total, median (IQR) | 13.0 (11.0–16.0) | 237 | 9.0 (7.0–11.0) | 913 | 5.0 (3.0–7.0) | 3695 | <0.01 * |
| Before admission, median (IQR) | 5.0 (3.0–7.0) | 237 | 5.0 (3.0–7.0) | 913 | 4.0 (2.0–7.0) | 3696 | <0.01 † |
| After macrolide administration, median (IQR) | 8.0 (7.0–9.0) | 240 | 4.0 (3.0–5.0) | 925 | 0.0 (0.0–2.0) | 4129 | <0.01 * |
| During hospitalization, median (IQR) | 8.0 (6.0–11.0) | 240 | 4.0 (3.0–6.0) | 925 | 1.0 (0–2.0) | 4035 | <0.01 * |
| Respiratory rate on admission (/min), median (IQR) | 26.0 (24.0–28.0) | 240 | 24.0 (23.0–28.0) | 924 | 24.0 (23.0–28.0) | 3814 | 0.63 |
| Respiratory distress, n (%) | 52 (21.7) | 240 | 174 (18.9) | 922 | 518 (13.6) | 3810 | <0.01 |
| Mild, n (%) | 44 (84.6) | 150 (86.2) | 474 (91.5) | ||||
| Moderate to severe, n (%) | 8 (15.4) | 24 (13.8) | 44 (8.5) | ||||
| Oxygen saturation ≤ 90% or cyanosis, n (%) | 16 (6.9) | 233 | 34 (3.8) | 897 | 47 (1.3) | 3732 | <0.01 |
| Oxygen support during hospitalization, n (%) | 29 (12.1) | 240 | 52 (5.6) | 925 | 150 (3.6) | 4129 | <0.01 |
| Oxygen support (days), median (IQR) | 4.0 (2.0–6.0) | 240 | 2.0 (1.0–6.0) | 925 | 2.0 (1.0–4.0) | 4129 | 0.01 ‡ |
| Admission to the ICU, n (%) | 2 (0.8) | 240 | 4 (0.4) | 925 | 13 (0.3) | 4129 | 0.1 |
| Mechanical ventilation, n (%) | 0 (0) | 240 | 4 (0.4) | 925 | 5 (0.1) | 4129 | |
| Categories | Total Population | MrMP | MLMP | MSMP | p -Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 5294 | Number of Actual Responses | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| WBC, 109/L | 9.4 (4.7) | 5213 | 8.1 (5.7) | 231 | 8.2 (3.7) | 905 | 9.7 (4.8) | 4077 | <0.01 § |
| Neutrophil, % | 58.3 (16.9) | 63.9 (15.3) | 61.3 (16.6) | 57.3 (16.9) | |||||
| Lymphocyte, % | 30.4 (14.1) | 26.1 (12.4) | 26.7 (11.9) | 31.5 (14.4) | |||||
| Eosinophil, % | 2.8 (3.1) | 2.3 (3.0) | 2.6 (3.2) | 2.9 (31.0) | |||||
| Aspartate aminotransferase (AST), IU/L | 41.9 (88.4) | 5129 | 73.4 (213.9) | 234 | 51.2 (100.2) | 892 | 38.0 (70.9) | 4003 | <0.01 * |
| Alanine aminotransferase, (ALT), IU/L | 25.9 (72.6) | 4954 | 54.1 (185.5) | 226 | 32.6 (82.7) | 863 | 22.8 (56.3) | 3865 | <0.01 * |
| Lactate dehydrogenase (LDH), IU/L | 529.9 (333.7) | 2266 | 746.1 (635.1) | 121 | 627.6 (451.8) | 484 | 485.8 (235) | 1661 | <0.01 * |
| C-reactive protein (CRP), g/L | 8.1 (19.2) | 5018 | 15 (37.9) | 223 | 11.2 (21.1) | 880 | 7 (16.9) | 3915 | <0.01 * |
| Category | Total Population | MrMP | MLMP | MSMP | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 5294 | Number of Actual Responses | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| Bronchopneumonia | 3339 (67.2) | 4972 | 131 (54.6) | 240 | 515 (55.7) | 925 | 2693 (70.7) | 3807 | <0.01 |
| Lobar pneumonia | 2378 (45.4) | 5235 | 156 (65.0) | 240 | 555 (60.0) | 925 | 1667 (41.0) | 4070 | <0.01 |
| Bilateral | 197 (3.8) | 5213 | 12 (5.1) | 237 | 48 (5.2) | 921 | 137 (3.4) | 4055 | 0.02 |
| Unilateral | 2159 (41.4) | 5215 | 141 (59.5) | 237 | 503 (54.6) | 921 | 1515 (37.4) | 4051 | <0.01 |
| Both bronchial and lobar involvement | 861 (17.3) | 4967 | 52 (21.7) | 240 | 167 (18.1) | 925 | 642 (16.9) | 3802 | 0.14 |
| Atelectasis | 273 (5.5) | 4967 | 26 (10.8) | 240 | 59 (6.4) | 923 | 188 (4.9) | 3804 | 0.01 |
| Category | Total Population | MrMP | MLMP | MSMP | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 5294 | Number of Actual Responses | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| Pleural effusion | 402 (7.7) | 5240 | 40 (16.7) | 240 | 125 (13.5) | 925 | 237 (5.8) | 4075 | <0.01 |
| <1/4 of the thorax ‖ | 290 (72.1) | 30 (75.0) | 99 (79.2) | 161 (68.5) | <0.01 | ||||
| 1/4-1/2 of the thorax ‖ | 5 (1.2) | 2 (5.0) | 0 (0) | 3 (1.3) | |||||
| ≥1/2 of the thorax ‖ | 2 (0.5) | 1 (2.5) | 1 (0.8) | 0 (0) | |||||
| Missing/unknown | 105 (26.2) | 7 (17.5) | 25 (0.2) | 73 (31.1) | |||||
| Intervention | |||||||||
| Thoracentesis | 12 (0.2) | 5240 | 4 (1.7) | 240 | 4 (0.4) | 925 | 4 (0.1) | 4129 | 0.01 |
| Chest tube insertion | 39 (0.7) | 5240 | 16 (6.7) | 240 | 12 (1.3) | 925 | 11 (0.3) | 4129 | <0.01 |
| Category | Total Population | MrMP | MLMP | MSMP | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 5294 | Number of Actual Responses | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| Any manifestation | 1091 (22.2) | 4910 | 99 (41.4) | 239 | 284 (31.1) | 912 | 708 (18.8) | 3759 | <0.01 |
| Digestive system | |||||||||
| Liver enzyme elevation | 857 (17.2) | 4990 | 78 (32.5) | 240 | 238 (25.8) | 921 | 541 (14.1) | 3829 | <0.01 |
| Urinary system | |||||||||
| Proteinuria | 208 (4.2) | 4928 | 21 (8.8) | 239 | 51 (5.7) | 916 | 135 (3.6) | 3773 | <0.01 |
| Skin and mucosa | 216 (4.1) | 5294 | 19 (7.9) | 240 | 54 (5.8) | 143 (3.5) | 4129 | <0.01 | |
| Rash | 212 (4.0) | 19 (7.9) | 53 (5.7) | 140 (3.4) | <0.01 | ||||
| Erythema multiforme | 11 (0.2) | 3 (1.3) | 1 (0.1) | 7 (0.2) | 0.01 | ||||
| Mucositis | 6 (0.1) | 0 (0) | 1 (0.1) | 5 (0.1) | |||||
| Cardiovascular system | 21 (0.4) | 4999 | 3 (1.3) | 240 | 2 (0.2) | 923 | 16 (0.4) | 3836 | 0.09 |
| Myocarditis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Kawasaki disease | 17 (0.3) | 1 (0.4) | 2 (0.2) | 14 (0.4) | 0.08 | ||||
| DIC | 4 (0) | 2 (0.8) | 0 (0) | 2 (0.05) | |||||
| Nervous system | 22 (0.4) | 4999 | 5 (2.1) | 240 | 4 (0.4) | 924 | 13 (0.3) | 3835 | 0.01 |
| Encephalitis | 6 (0.1) | 1 (0.4) | 0 (0) | 5 (0.1) | 0.22 | ||||
| Meningitis | 14 (0.3) | 2 (0.8) | 4 (0.4) | 8 (0.2) | 0.10 | ||||
| Peripheral neuropathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Musculoskeletal system | |||||||||
| Arthritis | 10 (0.2) | 5002 | 3 (1.3) | 240 | 2 (0.2) | 924 | 5 (0.1) | 3838 | <0.01 |
| Category | Total Population | MrMP | MLMP | MSMP | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 5294 | Number of Actual Responses | 240 | Number of Actual Responses | 925 | Number of Actual Responses | 4129 | Number of Actual Responses | |
| Persistent atelectasis | 48 (1.6) | 2956 | 5 (3.2) | 154 | 16 (3.2) | 506 | 27 (1.2) | 2296 | <0.01 |
| Bronchiolitis obliterans | 22 (0.4) | 5240 | 1 (0.4) | 236 | 3 (0.3) | 911 | 18 (0.4) | 4093 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Chung, E.H.; Lee, E.; Kim, C.-H.; Lee, Y.J.; Kim, H.-B.; Kim, B.-S.; Kim, H.Y.; Cho, Y.; Seo, J.-H.; et al. Clinical Characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: A Multicenter Retrospective Study. J. Clin. Med. 2022, 11, 306. https://doi.org/10.3390/jcm11020306
Choi YJ, Chung EH, Lee E, Kim C-H, Lee YJ, Kim H-B, Kim B-S, Kim HY, Cho Y, Seo J-H, et al. Clinical Characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: A Multicenter Retrospective Study. Journal of Clinical Medicine. 2022; 11(2):306. https://doi.org/10.3390/jcm11020306
Chicago/Turabian StyleChoi, Yun Jung, Eun Hee Chung, Eun Lee, Chul-Hong Kim, Yong Ju Lee, Hyo-Bin Kim, Bong-Seong Kim, Hyung Young Kim, Yoojung Cho, Ju-Hee Seo, and et al. 2022. "Clinical Characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: A Multicenter Retrospective Study" Journal of Clinical Medicine 11, no. 2: 306. https://doi.org/10.3390/jcm11020306
APA StyleChoi, Y. J., Chung, E. H., Lee, E., Kim, C.-H., Lee, Y. J., Kim, H.-B., Kim, B.-S., Kim, H. Y., Cho, Y., Seo, J.-H., Sol, I. S., Sung, M., Song, D. J., Ahn, Y. M., Oh, H. L., Yu, J., Jung, S., Lee, K. S., Lee, J. S., ... Suh, D. I. (2022). Clinical Characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: A Multicenter Retrospective Study. Journal of Clinical Medicine, 11(2), 306. https://doi.org/10.3390/jcm11020306






