Abstract
Background: In glomerular disease, the degree of proteinuria is closely related to the progression of chronic kidney disease, and its reduction is associated with a slower decline in the glomerular filtration rate (eGFR) and consequent improvement in the renal prognosis. The aim of this study was to evaluate the impact of proteinuria reduction on the decline of the eGFR in patients with glomerular disease, during the first year after the diagnosis. Methods: This was a retrospective analysis of patients with primary glomerular disease, followed at the Nephrology Department of Centro Hospitalar Universitário Lisboa Norte, during 2019. We analyzed demographic, clinical and laboratorial characteristics (creatinine, GFR, urine analysis and quantification of proteinuria determined by the proteinuria/creatinuria ratio, in the first morning urine or a 24 h urine sample). The outcome assessed was the decline in renal function, defined as a reduction in the GFR ≥ 25%, during the follow-up period. Results: We analyzed 197 patients with glomerular disease, with a mean age of 41.7 ± 19.7 years and follow-up time of 6.5 ± 5.3 years. At the time of the diagnosis, the eGFR was 81.5 ± 49.8 mL/min/1.73 m2 and proteinuria was 3.5 g/24 h (IQR 5.8). At one-year follow-up, median proteinuria was 0.9 g/24 h (IQR 2.4). At the end of the follow-up, mean eGFR was 72.1 ± 43.3 mL/min/1.73 m2. Proteinuria (p = 0.435) and the eGFR (p = 0.880) at the time of diagnosis did not correlate with long-term decline in the eGFR. Proteinuria < 1 g/24 h (HR 0.45 (95% CI 0.25–0.83) p = 0.011) after the first year was protective against long-term decline in the eGFR. It maintained this association with the long-term eGFR decline, independently of the duration of the follow-up (HR 0.30 (95% CI 0.17–0.52) p < 0.001). Conclusions: Proteinuria reduction to lower than 1 g/24 h, during the first year after diagnosis, was a protective factor for the long-term decline of kidney function, having a more important role than proteinuria or the GFR at the time of the diagnosis.
1. Background
Chronic kidney disease (CKD) is a common disease, with an estimated prevalence of 8 to 16% worldwide [1], and its progression is associated with poor outcomes such as increased cardiovascular morbidity, poorer quality of life and reduced survival [2,3]. In the adult population, diabetes and arterial hypertension account for more than half of the cases of CKD [4]; however, in young adults, glomerular diseases are the main cause of CKD, mainly in male patients [5]. Glomerular diseases are the third leading cause of end-stage renal disease in Europe and United States, accounting for approximately 10,000 cases of end-stage renal disease annually [6,7].
Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy are the primary glomerular diseases associated with greater decline in renal function [8].
The decline in renal function is intimately related to the degree of proteinuria, the presence of arterial hypertension and the glomerular filtration rate (GFR) at the time of the diagnosis. The proteins that are filtrated by the glomerular capillaries exert direct toxic damage to the tubulointerstitial structures, by activating several genes responsible for codifying vasoactive and proinflammatory molecules, which, associated with other risk factors such as hypertension, accelerate the decline of the kidney function [9].
Therefore, proteinuria is a major risk factor for an accelerated decline of the GFR. Ruggenenti et al. studied 352 patients with nondiabetic renal disease and concluded that patients with proteinuria superior to 3.9 g/24 h at the time of the diagnosis had an association with a greater decline of the GFR, compared with patients with proteinuria lower than 1.9 g/24 h, and more frequently reached end-stage kidney disease (ESKD) [10]. Another study showed that the risk of progression to ESKD progressively increased with increasing proteinuria, becoming significant for a proteinuria concentration of 0.5–1 g/24 h (HR 1.8 and 1.85 in diabetic and nondiabetic patients, respectively) and > 1 g (HR 2.70 and 2.69 in diabetic and nondiabetic patients, respectively) [11]. A recent meta-analysis that included 28 studies showed that a reduction in proteinuria of 30%, during a 2-year follow-up period, was associated with a 22% reduction in the risk of developing ESKD [12]. Therefore, the reduction in proteinuria in patients with glomerular disease was associated with a better renal prognosis. Proteinuria is also associated with a greater risk for coronary arterial disease, cerebrovascular disease, gastrointestinal bleeding, hypercoagulability, thromboembolic events and mortality [13].
The aim of this single center study was to evaluate the impact of proteinuria reduction on the decline of the GFR in patients with glomerular disease, during the first year after the diagnosis.
2. Materials and Methods
This was a retrospective analysis that included adult patients with glomerular diseases, followed at the Nephrology and Renal Transplantation Department of Centro Hospitalar Universitário Lisboa Norte (CHULN) during 2019. The study included patients that were diagnosed from 2005 to 2017. This study was approved by the Ethical Committee in agreement with institutional guidelines. Due to the retrospective and noninterventional nature of the study, informed consent was waived by the Ethical Committee.
2.1. Participants
Eligible patients were selected as adult patients (≥18 years of age) with a diagnosis of primary glomerular disease, confirmed by kidney biopsy, with at least 2 years of follow-up.
The included glomerular diseases were minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, IgA nephropathy and membranoproliferative glomerulonephritis.
Exclusion criteria comprised patients with an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 at the time of clinical presentation and patients lost in the follow-up. We excluded patients with pauci-immune glomerulonephritis, neoplasia and other secondary causes of glomerulonephritis.
2.2. Variables and Outcomes
Patient variables were collected from individual clinical records. We analyzed several clinical variables including patient demographic characteristics (age, gender, ethnicity), laboratory values at the time of the diagnosis (serum creatinine (SCr), 24 h proteinuria measurement or urinary protein to creatine ratio in first morning urine), the histopathology result of the kidney biopsy and immunosuppressive regiment used.
The glomerular filtration rate was estimated based on the CKD-EPI formula [13]. We evaluated the decline of the eGFR during the follow-up.
Complete remission was defined as proteinuria < 0.3 g/24 h, normal SCr and serum albumin > 3.5 g/dL. Partial remission was defined as proteinuria between 0.3 g/24 h and 3.5 g/24 h, with a decline of at least 50% from the highest measurement, and stable SCr (variation < 25%).
CKD was defined as an eGFR < 60 mL/min/1.73 m2, persistent for at least 3 months [14].
The primary outcome was the decline of kidney function, defined as a reduction in the eGFR ≥ 25% during the follow-up. We also analyzed the reduction in the eGFR ≥ 50%, the variation of the eGFR during the follow-up and the annual decline of the eGFR.
2.3. Statistical Methods
Normal distribution of the variables was evaluated with a Kolmogorov–Smirnov test. Categorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean ± standard deviation. Continuous variables were compared with the Student’s t-test and categorical variables were compared with a chi-square test. Variables that did not follow a normal distribution were compared using the Mann-Whitney test and described as the median and interquartile range (IQR). To calculate the probability of the decline of the eGFR dependent on proteinuria reduction, we used a Kaplan–Meier analysis as a log-rank.
We used the Cox logistic regression method to determine the variables with a significant statistical difference for the decline of the eGFR. Data were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value < 0.05. Statistical analysis was performed with the statistical software package, SPSS for Windows (version 21.0, SPSS Inc., Chicago, IL, USA).
3. Results
We identified 197 patients with glomerular disease. One hundred and nine (55.3%) were male, with a mean age of 41.7 ± 19.7 years (Table 1). Nearly one third of patients (31.9%, n = 63) were diagnosed with focal segmental glomerulosclerosis (FSGS), 28.4% (n = 56) with IgA nephropathy, 18.3% (n = 36) with minimal change disease, 10.7% (n = 21) with membranoproliferative glomerulonephritis and 10.7% (n = 21) with membranous nephropathy (MN) (Table 2).

Table 1.
Patients’ characteristics.

Table 2.
Patients’ characteristics according to glomerular disease.
At the time of the diagnosis, the mean eGFR was 81.5 ± 49.8 mL/min/1.73 m2, with 41.6% (n = 82) of patients having an eGFR < 60 mL/min/1.73 m2. Median proteinuria was 3.5 g/g or g/24 h (IQR 5.8), with 21.3% (n = 42) having proteinuria < 1 g/24 h, 24.4% (n = 54) with proteinuria of 1–3 g/24 h, 19.3% (n = 38) with 3–5 g/24 h and 35.0% (n = 69) having proteinuria ≥ 5 g/24 h (Figure 1). Patients with FSGS had a lower eGFR (52.3 ± 20.3 mL/min/1.73 m2) and patients with MN had higher baseline proteinuria (6.1 g/g or g/24 h (IQR 4.5)) (Table 2). At the time of the diagnosis, 71.1% of patients (n = 140) had hematuria. Immunosuppressive therapy was used in 53.8% of patients (n = 106). All patients were under renin–angiotensin–aldosterone system (RAAS) inhibitors (Table 1).
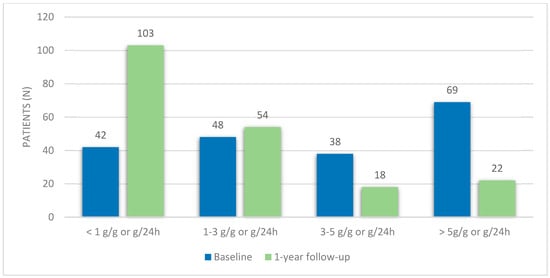
Figure 1.
Proteinuria variation during the first-year follow-up.
At a one-year follow-up, median proteinuria was 0.9 g/g or g/24 h (IQR 2.4). The majority of patients (64.0%, n = 126) had a proteinuria reduction ≥ 25%, and 49.7% (n = 98) had a reduction ≥ 50%. Concerning the level of proteinuria, most patients had proteinuria lower than 1 g/24 h (52.3%, n = 103), 27.4% (n = 54) had 1–3 g/24 h, 9.1% (n = 18) had 3–5 g/24 h and 11.2% (n = 22) had proteinuria ≥ 5 g/24 h. Accordingly, at a one-year follow-up, 27.4% of patients (n = 54) were in complete remission and 48.2% (n = 95) were in partial remission (Table 1). There were no significant differences in proteinuria at the 1-year follow-up between patients with different glomerular diseases (Table 2).
The mean time of observation was 6.5 ± 5.3 years. The eGFR at the end of the follow-up was 72.1 ± 43.3 mL/min/1.73 m2, which corresponds to a variation of 9.3 mL/min/1.73 m2 (IQR 26.6). The annual decline of the eGFR was 1.4 mL/min/1.73 m2 (IQR 5.2). Almost one third of patients had at least a 25% decline in the eGFR (32.5%, n = 64), and 13.2% (n = 26) had a decline ≥ 50%. At the end of the follow-up, 42.6% (n = 84) had an eGFR < 60 mL/min/1.73 m2, as shown in Table 1.
3.1. Decline of Kidney Function
In this study, 32.5% of patients (n = 64) presented an eGFR reduction ≥ 25% during follow-up. There was no significant difference in age, gender, the presence of hematuria, baseline eGFR or proteinuria (Table 3).

Table 3.
Patients’ characteristics according to eGFR decline ≥ 25%.
There were no significant differences in eGFR decline between patients with different glomerular diseases (Table 2).
Patients who experienced kidney function decline less frequently had a reduction in proteinuria ≥ 50% (40.6% vs. 54.1%, p = 0.076) and less frequently presented a complete remission of proteinuria (21.9% vs. 30.1%, p = 0.149) during the first year of follow-up, nearly reaching statistical significance. Concerning the amount of proteinuria, they less frequently had proteinuria < 1 g/24 h (39.1% vs. 58.6%, p = 0.010) at the first-year follow-up (Table 3).
At the long-term follow-up, patients who had an eGFR decline ≥ 25% had a significantly lower eGFR (43.4 ± 37.5 mL/min/1.73 m2 vs. 85.6 ± 39.1 mL/min/1.73 m2, p < 0.001) and more frequently had an eGFR < 60 mL/min/1.73 m2 (56.3% vs. 30.1%, p < 0.001). These patients had a longer follow-up time (7.4 ± 5.3 vs. 6.1 ± 5.2, p < 0.001), and the annual decline of the eGFR was significantly higher (5.1 mL/min/1.73 m2 (IQR 5.1) vs. 0.4 mL/min/1.73 m2 (IQR 3.3), p < 0.001) (Table 3).
The reduction in proteinuria to values of < 1 g/24 h, during the first year of diagnosis, was associated with a lesser eGFR decline in the long-term follow-up (log-rank 0.003) (Figure 2).
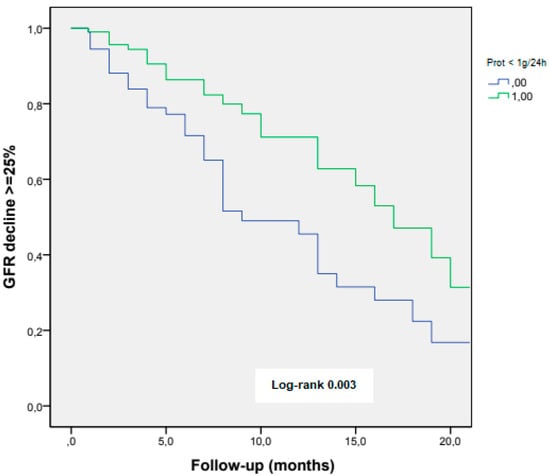
Figure 2.
eGFR decline ≥ 25% according to achieving proteinuria < 1 g/24 h at the first-year follow-up.
3.2. Predictors of Kidney Function Decline
We analyzed factors which could predict eGFR decline ≥ 25%. Proteinuria reduction ≥ 50% at the first-year follow-up was associated with lower risk for long-term decline of the eGFR (HR 0.58 (95% CI 0.32–1.06), p = 0.077). Proteinuria < 1 g/24 h (HR 0.45 (95% CI 0.25–0.83) p = 0.011) at the first-year follow-up was a predictor of long-term decline of the eGFR (Table 4).

Table 4.
Predictors of eGFR decline ≥ 25%—univariate analysis.
A subanalysis was performed to analyze the impact of proteinuria on eGFR decline, independently of the duration of follow-up, and proteinuria < 1 g/24 h maintained a significant association as protective against eGFR decline in the long term (HR 0.30 (95% CI 0.17–0.52) p < 0.001).
4. Discussion
In this study, we demonstrated that proteinuria reduction during the first-year follow-up was protective against the long-term decline in renal function. A reduction in proteinuria ≥ 50% was associated with a slower decline in renal function. A reduction to levels lower than 1 g/24 h, or reaching complete remission in the first year, was protective against the long-term decline of the GFR.
Several studies showed an association between the degree of proteinuria and a decline in renal function. Berhane et al. studied a population of 2420 diabetic patients and concluded that patients with microalbuminuria at the time of the diagnosis had a risk 2.1 times greater for developing ESKD, and patients with macroalbuminuria were 9.1 times more likely to develop ESKD, when compared with patients without proteinuria [15].
A similar association between proteinuria and decline of the kidney function was demonstrated in patients with nondiabetic kidney disease. Ali et al. studied a population of 336 patients with CKD and concluded that patients with a faster decline in the GFR (Δ eTFG ≤ −4 mL/min/1.73 m2/year) had proteinuria > 50 g/mol more frequently at the time of the diagnosis (64% vs. 17%, p < 0.001) [16]. In fact, most of the studies analyzed the impact of proteinuria at the time of diagnosis on the long-term decline of the GFR.
Reich et al. analyzed 542 patients with primary IgA nephropathy and found that those with sustained proteinuria > 3 g/24 h, during the six-and-a-half-year follow-up, presented a decline in the kidney function 25 times faster than patients with proteinuria < 1 g/24 h (−0.719 ± 0.61 mL/min/1.73 m2/month vs. −0.030 ± 0.46 mL/min/1.73 m2/month). All patients that reached proteinuria < 1 g/24 h during the follow-up, regardless of the proteinuria concentration at the diagnosis, presented a similar decline in the GFR [17]. As with our study, proteinuria at the time of the diagnosis was not a marker of an accelerated decline of the GFR, in contrast to the proteinuria level at the first-year follow-up, which was independently associated with a greater decline of the GFR.
Proteinuria is a marker of glomerular disease, being the result of filtration through a damaged glomerular basement membrane, but it also has an impact on the decline of the kidney function. It seems that the filtrated proteins exert a direct toxic effect on the tubulointerstitial structure, causing tubular atrophy, inflammation and interstitial fibrosis [12]. Filtrated albumin is responsible for the activation of several proinflammatory cytokines, complement activation and, consequently, tubulointerstitial damage [18,19]. In fact, in patients with diabetic nephropathy, the severity of tubulointerstitial damage is associated with a greater decline of the renal function than in other glomerular diseases [20].
However, we know that the level of proteinuria alone, as a biomarker of CKD, inadequately explains the variability of GFR decline, apart from it being a late marker of GFR decline. Moreover, proteinuria alone does not explain the complex physiopathology of tubular damage [21]. Recent advances in molecular analysis revealed that CKD was closely associated with the dysregulation of numerous metabolites, such as amino acids, lipids, nucleotides and glycoses [22]. Following insult, the kidney tubular cells undergo a cascade of cellular responses that result in the production and accumulation of low-molecular-weight proteins in the urine and systemic circulation that might be exploited as potential biomarkers [23]. Some of these recently identified biomarkers include kidney injury molecule 1 and monocyte chemoattractant protein 1 that represent markers of tubule cell injury, and α1-microglobulin and uromodulin that are associated with tubule cell dysfunction [24]. Persistent, low-grade inflammation also seems to have a determinant role in CKD progression [25]. Proinflammatory proteins such as transforming growth factor-β, insulin-like growth factor-1, tumor necrosis factor-α and interleukin-6 were found significantly higher in CKD patients, indicating acute phase response signaling [26]. Therefore, complicated pathomechanisms of CKD development and progression require not a single marker but a combination of markers in order to mirror all the types of alterations occurring in the course of this disease [27,28].
Although these recent studies identified several promising serum and urine biomarkers, which allowed a better understanding of the physiopathology of tubular damage and CKD progression, their cost is still prohibitive, which limits their routine use in clinical practice. Therefore, we continue to rely on classic biomarkers of glomerular and tubular damage, such as proteinuria, which is strongly associated with the decline of kidney function. Therefore, it is essential to outline strategies for the management of patients with proteinuria, in addition to immunosuppressive therapy aimed at the glomerular pathology, and to define the appropriate timing to apply these therapies. Our study showed that proteinuria reduction during the first year after the diagnosis was a predictor of long-term slower decline of the GFR, independently of the therapeutical strategy used for that reduction.
Apart from immunosuppressive therapy used for the treatment of glomerular diseases, there are several antiproteinuric therapies that must be used in order to prevent the decline of the renal function. Renin–angiotensin–aldosterone system (RAAS) inhibitors are the most studied and simultaneously the most effective drugs used for the reduction in proteinuria. By inducing vasodilatation of the efferent arteriola, they reduce intraglomerular pressure, which results in lower proteinuria [29]. In addition, they interfere directly with glomerular basement membrane pores, reducing their permeability to albumin and IgG [30]. In one study of nondiabetic patients, the use of Benazepril, an angiotensin-converting enzyme inhibitor, was associated with a 52% reduction in proteinuria (p < 0.001) and a 23% reduction in the rate of decline of the kidney function [31]. According to the REIN study, the use of Ramipril, another ACEI, was associated with a 15% reduction in proteinuria, followed by a significant reduction in the progression to ESKD [32].
Mineralocorticoid receptor antagonists have also been demonstrated to be effective in reducing proteinuria, reducing the inflammatory and profibrotic effect of proteinuria. However, their use is limited by the risk of hypercalemia. A recent meta-analysis that included 1646 patients with CKD showed that adding a mineralocorticoid receptor antagonist to the classic RAAS blockade produced an additional reduction in proteinuria of 38.7%; however patients were three times more likely to develop hypercalemia [33].
More recently, sodium-glucose transport protein 2 (SGLT2) inhibitors have been studied as antiproteinuric drugs. These drugs are currently the first line for the treatment of type 2 diabetes mellitus, and have been associated with a proteinuria reduction of 30–50% [34,35,36]. The renoprotective effect of these drugs seems to be the result of hemodynamic changes, in this case, the reduction in intraglomerular pressure, which suggests that they can also be used in nondiabetic nephropathies. A recently published prespecified analysis from the DAPA-CKD trial, which included 4304 patients with diabetic and nondiabetic CKD, revealed that dapagliflozin significantly reduced albuminuria in CKD patients with and without type 2 diabetes, with a larger relative reduction in patients with type 2 diabetes [37].
In our study, 63% of the patients were submitted to immunosuppressive therapy, and 54% presented a proteinuria reduction > 50% at the first-year follow-up. Although not all antiproteinuric drugs were evaluated, all the patients were using RAAS inhibitors, highlighting the importance of these drugs in the treatment of patients with glomerular disease.
This study has some limitations. First, it is a retrospective study, with a relatively small number of patients, which may affect the extrapolation to the general population. The limited number of patients may also affect the statistical significance of some variables, which may have reached significance with a larger population. Secondly, the method for the quantification of proteinuria was not uniform among the different patients, with some patients having a quantification of proteinuria in a 24 h urine sample and others having a proteinuria to creatinuria ratio based on the first morning urine. We also did not consider the characteristics of kidney biopsies, such as the degree of interstitial fibrosis and tubular atrophy, which may influence the prognosis; instead, we focused on clinical characteristics. However, the analysis of these patients as a whole allowed us to demonstrate the importance of the degree of proteinuria and renal prognosis, regardless of the cause of the glomerular disease. Finally, we did not analyze the immunosuppression used, which may have influenced the rate of reduction in proteinuria and the long-term reduction in the renal function.
Overall, our study was the first to demonstrate the impact of proteinuria reduction during the first year after diagnosis on the long-term decline of the eGFR, in patients with glomerular disease, regardless of the degree of proteinuria at the time of the diagnosis.
5. Conclusions
In this retrospective study of patients with glomerular disease, a proteinuria reduction to lower than 1 g/24 h, during the first year after diagnosis, was a protective factor against the long-term decline of kidney function. Therefore, the first year after the diagnosis of glomerular disease is of utmost importance for the renal prognosis. It is vital to invest in therapeutical strategies to reduce proteinuria in order to delay the decline of the renal function.
Author Contributions
Conceptualization, J.G.; methodology, J.G.; software, J.G. and F.M.; validation, I.G., M.P., P.F., S.J., J.A.L. and J.G.; formal analysis, F.M. and J.G; investigation, J.R. and F.M.; resources, F.M., J.R. and J.G.; data curation, F.M., J.R. and J.G; writing—original draft preparation, F.M. and J.R.; writing—review and editing, J.G.; supervision, J.A.L. and J.G.; project administration, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Centro Hospitalar Universitário Lisboa Norte, EPE with protocol approval in 4 February 2020.
Informed Consent Statement
Patient consent was waived due to the retrospective and non-interventional nature of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, T.K.; Sperati, C.J.; Thavarajah, S.; Grams, M.E. Reducing Kidney Function Decline in Patients With CKD: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 77, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, D.; Kunwar, R.; Shrestha, B.; Amatya, R.; Risal, A. Depression and Quality of Life among the Chronic Kidney Disease Patients. J. Nepal. Health Res. Counc. 2020, 18, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Powe, N.R.; McCulloch, C.E.; Saran, R.; Gillespie, B.W.; Saydah, S.; Crews, D.C. National Trends in the Prevalence of Chronic Kidney Disease Among Racial/Ethnic and Socioeconomic Status Groups, 1988–2016. JAMA Netw. Open 2020, 3, e207932. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Amann, K. Primary glomerulonephritides. Lancet 2016, 387, 2036–2048. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Lawrence, Y.C.; Albertus, P.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Cao, J.; Chen, J.L.T.; et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2017, 69 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef]
- Remuzzi, G.; Ruggenenti, P.; Benigni, A. Understanding the nature of renal disease progression. Kidney Int. 1997, 51, 2–15. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Perna, A.; Mosconi, L.; Pisoni, R.; Remuzzi, G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int. 1998, 53, 1209–1216. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Sasso, F.C.; Santoro, D.; Bellizzi, V.; Conte, G.; et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: Pooled analysis of four cohort studies. Nephrol. Dial. Transplant. 2018, 33, 1942–1949. [Google Scholar] [CrossRef]
- Coresh, J.; Heerspink, H.J.L.; Sang, Y.; Matsushita, K.; Arnlov, J.; Astor, B.C.; Black, C.; Brunskill, N.J.; Carrero, J.-J.; Feldman, H.I.; et al. Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019, 7, 115–127. [Google Scholar] [CrossRef]
- Haider, M.Z.; Aslam, A. Proteinuria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- CKD-EPI Creatinine Equation. 2021. Available online: https://www.kidney.org/content/ckd-epi-creatinine-equation-2021 (accessed on 6 February 2022).
- Cattran, D.C.; Feehally, J.; Cook, H.T.; Liu, Z.H.; Fervenza, F.C.; Mezzano, S.A.; Floege, J.; Nachman, P.H.; Gipson, D.S.; Praga, M.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter. Suppl. 2012, 2, 139–274. [Google Scholar]
- Berhane, A.M.; Weil, E.J.; Knowler, W.C.; Nelson, R.G.; Hanson, R.L. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin. J. Am. Soc. Nephrol. 2011, 6, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Chinnadurai, R.; Ibrahim, S.T.; Green, D.; Kalra, P.A. Predictive factors of rapid linear renal progression and mortality in patients with chronic kidney disease. BMC Nephrol. 2020, 21, 345. [Google Scholar] [CrossRef]
- Reich, H.N.; Troyanov, S.; Scholey, J.W.; Cattran, D.C.; Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3177–3183. [Google Scholar]
- Wang, Y.; Rangan, G.K.; Tay, Y.C.; Wang, Y.; Harris, D.C. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J. Am. Soc. Nephrol. 1999, 10, 1204–1213. [Google Scholar] [CrossRef]
- Nakajima, H.; Takenaka, M.; Kaimori, J.-Y.; Hamano, T.; Iwatani, H.; Sugaya, T.; Ito, T.; Hori, M.; Imai, E. Activation of the signal transducer and activator of transcription signaling pathway in renal proximal tubular cells by albumin. J. Am. Soc. Nephrol. 2004, 15, 276–285. [Google Scholar] [CrossRef]
- Okada, T.; Nagao, T.; Matsumoto, H.; Nagaoka, Y.; Wada, T.; Nakao, T. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology 2012, 17, 68–75. [Google Scholar] [CrossRef]
- Sandokji, I.; Greenberg, J.H. Plasma and Urine Biomarkers of CKD: A Review of Findings in the CKiD Study. Semin. Nephrol. 2021, 41, 416–426. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Ma, S.-X.; Chen, Y.-Y.; Chen, L.; Liu, B.-L.; Liu, Q.-Q.; Zhao, Y.-Y. Chronic kidney disease: Biomarker diagnosis to therapeutic targets. Clin. Chim. Acta 2019, 499, 54–63. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Shlipak, M.G. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am. J. Kidney Dis. 2021, 78, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.; Sanford, B.; Renner, B.; Eyck, P.T.; Laskowski, J.; Cooper, J.; Sun, M.; Zakharia, Y.; Spitz, D.; Dokun, A.; et al. Detection of pro angiogenic and inflammatory biomarkers in patients with CKD. Sci. Rep. 2021, 11, 8786. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef]
- Pontillo, C.; Mischak, H. Urinary biomarkers to predict CKD: Is the future in multi-marker panels? Nephrol. Dial. Transplant. 2016, 31, 1373–1375. [Google Scholar] [CrossRef]
- Woo, K.T.; Lau, Y.K.; Wong, K.S.; Chiang, G.S. ACEI/ATRA therapy decreases proteinuria by improving glomerular permselectivity in IgA nephritis. Kidney Int. 2000, 58, 2485–2491. [Google Scholar] [CrossRef]
- Morelli, E.; Loon, N.; Meyer, T.; Peters, W.; Myers, B.D. Effects of converting-enzyme inhibition on barrier function in diabetic glomerulopathy. Diabetes 1990, 39, 76–82. [Google Scholar] [CrossRef]
- Hou, F.F.; Zhang, X.; Zhang, G.H.; Xie, D.; Chen, P.Y.; Zhang, W.R.; Jiang, J.P.; Liang, M.; Wang, G.B.; Liu, Z.R.; et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 2006, 354, 131–140. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Perna, A.; Gherardi, G.; Garini, G.; Zoccali, C.; Salvadori, M.; Scolari, F.; Schena, F.P.; Remuzzi, G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999, 354, 359–364. [Google Scholar] [CrossRef]
- Currie, G.; Taylor, A.H.M.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.; Townend, J.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: An exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 610–621. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Johnsson, E.; Gause-Nilsson, I.; Cain, V.A.; Sjöström, C.D. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016, 18, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Petrykiv, S.I.; Laverman, G.D.; de Zeeuw, D.; Heerspink, H.J.L. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017, 19, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Jongs, N.; Greene, T.; Chertow, G.M.; McMurray, J.J.V.; Langkilde, A.M.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; Stefansson, B.V.; Toto, R.D.; et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 755–766. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).