Angioside: The role of Angiogenesis and Hypoxia in Lung Neuroendocrine Tumours According to Primary Tumour Location in Left or Right Parenchyma
Abstract
1. Introduction
1.1. Lung Neuroendocrine Tumours: Classification, Features and Prognostic Factors
1.2. Angiogenesis, Hypoxia and NET
2. Materials and Methods
2.1. Patient Population
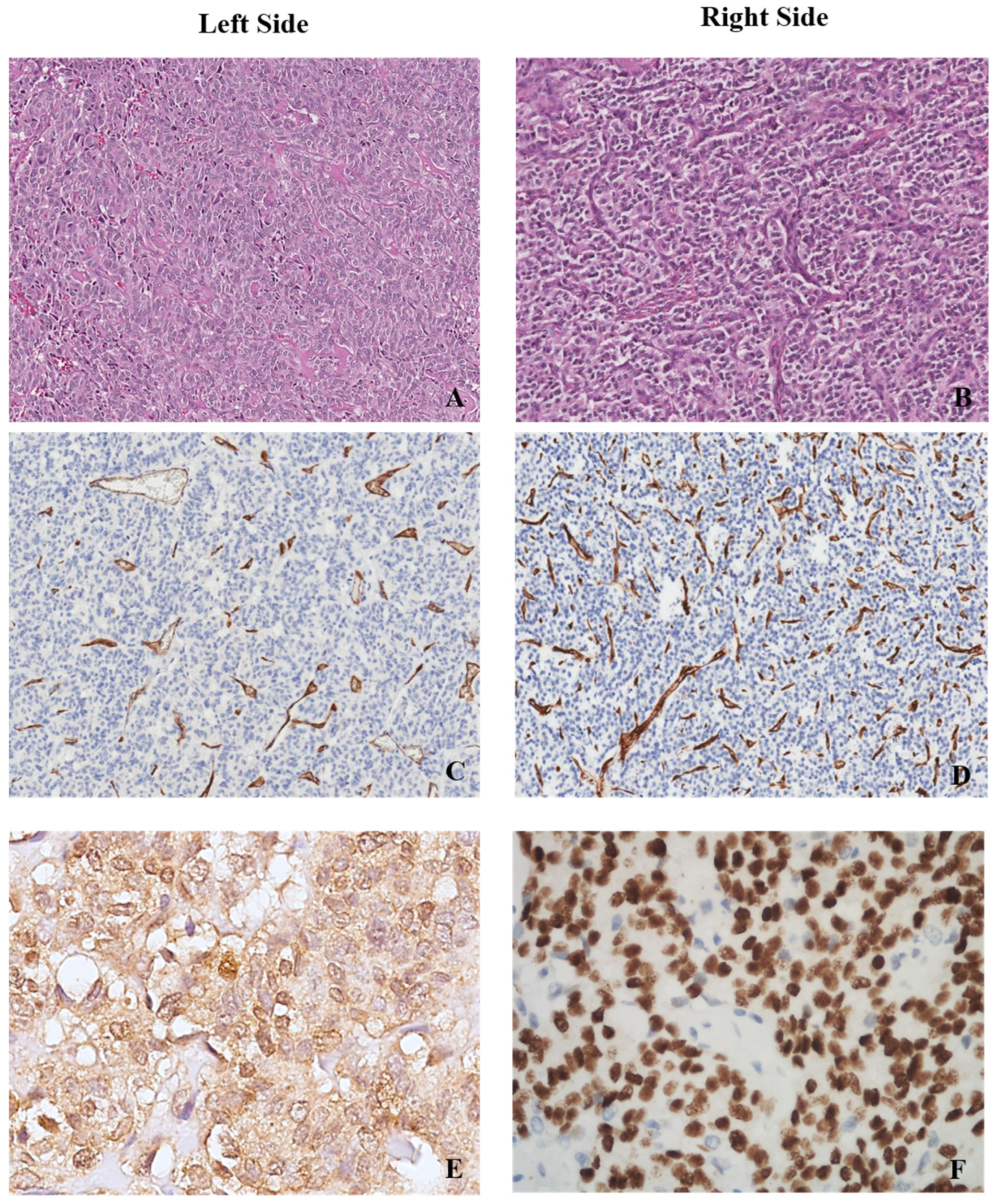
2.2. Histopathological and Immunohistochemical Examination
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Angiogenesis and Hypoxia in Left vs. Right Parenchyma in the Analysed Cases
3.3. Prognostic Impact of Side, Angiogenesis, Necrosis, TTF-1 and Hypoxia
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumours in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.I.; Kidd, M.; Chan, A. Bronchopulmonary neuroendocrine tumours. Cancer 2008, 113, 5–21. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumors Editorial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer World Health Organisation: Lyon, France, 2021. [Google Scholar]
- Kim, J.Y.; Hong, S.-M.; Ro, J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017, 29, 11–16. [Google Scholar] [CrossRef]
- Metovic, J.; Barella, M.; Bianchi, F.; Hofman, P.; Hofman, V.; Remmelink, M.; Kern, I.; Carvalho, L.; Pattini, L.; Sonzogni, A.; et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021, 478, 5–19. [Google Scholar] [CrossRef]
- Modlin, I.; Lye, K.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumours. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- Öberg, K.; Hellman, P.; Ferolla, P.; Papotti, M. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii120–vii123. [Google Scholar] [CrossRef]
- Travis, W.D.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Brambilla, E.; Jett, J.; Kennedy, C.; Rami-Porta, R.; Rusch, V.W.; et al. The IASLC Lung Cancer Staging Project: Proposals for the inclusion of broncho-pulmonary carcinoid tumours in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2008, 3, 1213–1223. [Google Scholar] [CrossRef]
- Wang, J.; Ye, L.; Cai, H.; Jin, M. Comparative study of large cell neuroendocrine carcinoma and small cell lung carcinoma in high-grade neuroendocrine tumours of the lung: A large population-based study. J. Cancer 2019, 10, 4226–4236. [Google Scholar] [CrossRef]
- Righi, L.; Gatti, G.; Volante, M.; Papotti, M. Lung neuroendocrine tumours: Pathological characteristics. J. Thorac. Dis. 2017, 9 (Suppl. 15), S1442-–S1447. [Google Scholar] [CrossRef]
- Thakur, S.; Florisson, D.; Telianidis, S.; Yaftian, N.; Lee, J.; Knight, S.; Barnett, S.; Seevanayagam, S.; Antippa, P.; Alam, N.; et al. Pulmonary carcinoid tumours: A multi-centre analysis of survival and predictors of outcome following sublobar, lobar, and extended pulmonary resections. Asian Cardiovasc. Thorac. Ann. 2021, 29, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumours: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Uppin, S.G.; Pasala, U.J.S.; Hui, M.; Kumar, N.N.; Bhaskar, K.; Paramjyothi, G. Clinicopathological and immunohistochemical study of pulmonary neuroendocrine tumors—A single-institute experience. Lung India 2021, 38, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Oka, N.; Kasajima, A.; Konukiewitz, B.; Sakurada, A.; Okada, Y.; Kameya, T.; Weichert, W.; Ishikawa, Y.; Suzuki, H.; Sasano, H.; et al. Classification and Prognostic Stratification of Bronchopulmonary Neuroendocrine Neoplasms. Neuroendocrinology 2020, 110, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, A.; Konukiewitz, B.; Oka, N.; Suzuki, H.; Sakurada, A.; Okada, Y.; Kameya, T.; Ishikawa, Y.; Sasano, H.; Weichert, W.; et al. Clinicopathological Profiling of Lung Carcinoids with a Ki67 Index > 20%. Neuroendocrinology 2019, 108, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K.; Kaltsas, G.; Öberg, K.; Tsolakis, A.V. Lung Carcinoids: Long-Term Surgical Results and the Lack of Prognostic Value of Somatostatin Receptors and Other Novel Immunohistochemical Markers. Neuroendocrinology 2018, 107, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Cattoni, M.; Vallières, E.; Brown, L.M.; Sarkeshik, A.A.; Margaritora, S.; Siciliani, A.; Filosso, P.L.; Guerrera, F.; Imperatori, A.; Rotolo, N.; et al. Improvement in TNM staging of pulmonary neuroendocrine tumours requires histology and regrouping of tumor size. J. Thorac. Cardiovasc. Surg. 2018, 155, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Rosenthal, A.; Cattoni, M.; Bograd, A.J.; Farivar, A.S.; Aye, R.W.; Vallières, E.; Louie, B.E. Staging System for Neuroendocrine Tumours of the Lung Needs to Incorporate Histologic Grade. Ann. Thorac. Surg. 2020, 109, 1009–1018. [Google Scholar] [CrossRef]
- Okereke, I.C.; Taber, A.M.; Griffith, R.C.; Ng, T.T. Outcomes after surgical resection of pulmonary carcinoid tumours. J. Cardiothorac. Surg. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Lee, P.C.; Osakwe, N.C.; Narula, N.; Port, J.L.; Paul, S.; Stiles, B.M.; Andrews, W.G.; Nasar, A.; Altorki, N.K. Predictors of diseasefree survival and recurrence in patients with resected bronchial carcinoid tumours. Thorac. Cardiovasc. Surg. 2015, 64, 159–165. [Google Scholar]
- Zhong, C.X.; Yao, F.; Zhao, H.; Shi, J.X.; Fan, L.M. Long-term outcomes of surgical treatment for pulmonary carcinoid tumours: 20 years’ experience with 131 patients. Chin. Med. J. 2012, 125, 3022–3026. [Google Scholar] [PubMed]
- Filosso, P.L.; Rena, O.; Donati, G.; Casadio, C.; Ruffini, E.; Papalia, E.; Oliaro, A.; Maggi, G. Bronchial carcinoid tumours: Surgical management and long-term outcome. J. Thorac. Cardiovasc. Surg. 2002, 123, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Yazici, U.; Gulgosteren, M.; Agackiran, Y.; Kaya, S.; Gulhan, E.; Tastepe, I.; Karaoglanoglu, N. Long-term outcomes and prognostic factors of patients with pulmonary carcinoid tumours. Neoplasma 2015, 62, 478–483. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- La Rosa, S.; Uccella, S.; Finzi, G.; Albarello, L.; Sessa, F.; Capella, C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumours: Correlation with microvessel density and clinicopathologic features. Hum. Pathol. 2003, 34, 18–27. [Google Scholar] [CrossRef]
- Cortez, E.; Gladh, H.; Braun, S.; Bocci, M.; Cordero, E.; Björkström, N.K.; Miyazaki, H.; Michael, I.P.; Eriksson, U.; Folestad, E.; et al. Functional malignant cell heterogeneity in pancreatic neuroendocrine tumors revealed by targeting of PDGF-DD. Proc. Natl. Acad. Sci. USA 2016, 113, E864–E873. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.N.; Xie, K.; Yao, J.C. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuro- endocrine tumours. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef]
- Puliani, G.; Sesti, F.; Anastasi, E.; Verrico, M.; Tarsitano, M.G.; Feola, T.; Campolo, F.; Di Gioia, C.R.T.; Venneri, M.A.; Angeloni, A.; et al. Angiogenic factors as prognostic markers in neuroendocrine neoplasms. Endocrine 2022, 76, 208–217. [Google Scholar] [CrossRef]
- Melen-Mucha, G.; Niedziela, A.; Mucha, S.; Motylewska, E.; Lawnicka, H.; Komorowski, J.; Stepien, H. Elevated Peripheral Blood Plasma Concentrations of Tie-2 and Angiopoietin 2 in Patients with Neuroendocrine Tumors. Int. J. Mol. Sci. 2012, 13, 1444–1460. [Google Scholar] [CrossRef]
- Detjen, K.M.; Rieke, S.; Deters, A.; Schulz, P.; Rexin, A.; Vollmer, S.; Hauff, P.; Wiedenmann, B.; Pavel, M.; Scholz, A. Angiopoietin-2 Promotes Disease Progression of Neuroendocrine Tumors. Clin. Cancer Res. 2010, 16, 420–429. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Dancey, G.; Hackshaw, A.; Luong, T.; Caplin, M.E.; Meyer, T. Circulating angiopoietin-2 is elevated in patients with neuroendocrine tumours and correlates with disease burden and prognosis. Endocrine-Relat. Cancer 2009, 16, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Garcia, P.; Castaño, A.; Martin, A.C.; Lopez-Rios, F.; Iglesias, J.; Muñoz-Galván, S.; Lopez-Calderero, I.; Molina-Pinelo, S.; Pastor, M.D.; Carnero, A.; et al. PDGFRα/β and VEGFR2 polymorphisms in colorectal cancer: Incidence and implications in clinical outcome. BMC Cancer 2012, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L. Sunitinib malate for the treatment of pancreatic neuroendocrine tumours. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Benavent, M.; Fonseca, P.J.; Castellano, D.; Alonso, T.; Teule, A.; Custodio, A.; Tafuto, S.; Munoa, A.L.C.; Spada, F.; et al. A phase II/III randomized double-blind study of octreotide acetate LAR with axitinib versus octreotide acetate LAR with placebo in patients with advanced G1-G2 NETs of non-pancreatic origin (AXINET trial-GETNE-1107). J. Clin. Oncol. 2021, 39, 360. [Google Scholar] [CrossRef]
- La Salvia, A.; Persano, I.; Siciliani, A.; Verrico, M.; Bassi, M.; Modica, R.; Audisio, A.; Zanata, I.; Marinucci, B.T.; Trevisi, E.; et al. Prognostic significance of laterality in lung neuroendocrine tumors. Endocrine 2022, 76, 733–746. [Google Scholar] [CrossRef]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.M.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; Van Diest, P.J.; Van Der Wall, E. Levels of Hypoxia-Inducible Factor-1 During Breast Carcinogenesis. JNCI J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef]
- Katoh, R. Angiogenesis in endocrine glands: Special reference to the expression of vascular endothelial growth factor. Microsc. Res. Tech. 2003, 60, 181–185. [Google Scholar] [CrossRef]
- Castellano, D.; Capdevila, J.; Sastre, J.; Alonso, V.; Llanos, M.; Garcia-Carbonero, R.; Mozo, J.L.M.; Sevilla, I.; Durán, I.; Salazar, R. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: A phase II study of Spanish Neuroendocrine Tumour Group (GETNE0801). Eur. J. Cancer 2013, 49, 3780–3787. [Google Scholar] [CrossRef]
- Berruti, A.; Fazio, N.; Ferrero, A.; Brizzi, M.P.; Volante, M.; Nobili, E.; Tozzi, L.; Bodei, L.; Torta, M.; D’Avolio, A.; et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-tomoderately differentiated neuroendocrine tumours: The xelbevoct study. BMC Cancer 2014, 14, 184. [Google Scholar] [CrossRef]
- Chan, J.A.; Faris, J.E.; Murphy, J.E.; Blaszkowsky, L.S.; Kwak, E.L.; McCleary, N.J.; Fuchs, C.S.; Meyerhardt, J.A.; Ng, K.; Zhu, A.X.; et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumours (pNET). J. Clin. Oncol. 2017, 35, 228. [Google Scholar] [CrossRef]
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; García-Donas, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumours: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumours (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Fazio, N.; Lopez, C.; Teule, A.; Valle, J.W.; Tafuto, S.; Custodio, A.; Reed, N.; Raderer, M.; Grande, E.; et al. Efficacy of lenvatinib in patients with advanced pancreatic (panNETs) and gastrointestinal (giNETs) grade 1/2 (G1/G2) neuroendocrine tumours: Results of the international phase II TALENT trial (GETNE 1509). Ann. Oncol. 2018, 29, 467. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Bai, C.; Xu, N.; Zhou, Z.; Li, Z.; Zhou, C.; Jia, R.; Lu, M.; Cheng, Y.; et al. Surufatinib in advanced well-differentiated neuroendocrine tumours: A multicenter, single-arm, open-label, phase Ib/II trial. Clin. Cancer Res. 2019, 25, 3486–3494. [Google Scholar] [CrossRef]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y.; et al. Nonclinical Antiangiogenesis and Antitumor Activities of Axitinib (AG-013736), an Oral, Potent, and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Couvelard, A.; O’Toole, D.; Turley, H.; Leek, R.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.; Gatter, K.; et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer 2005, 92, 94–101. [Google Scholar] [CrossRef]
- Hochwald, S.N.; Zee, S.; Conlon, K.C.; Colleoni, R.; Louie, O.; Brennan, M.; Klimstra, D.S. Prognostic Factors in Pancreatic Endocrine Neoplasms: An Analysis of 136 Cases With a Proposal for Low-Grade and Intermediate-Grade Groups. J. Clin. Oncol. 2002, 20, 2633–2642. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Gingras, A.-C.; Raught, B.; Sonenberg, N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001, 15, 807–826. [Google Scholar] [CrossRef]
- Thomas, G.V.; Tran, C.; Mellinghoff, I.K.; Welsbie, D.S.; Chan, E.; Fueger, B.; Czernin, J.; Sawyers, C.L. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 2006, 12, 122–127. [Google Scholar] [CrossRef]
- Kaufmann, O.; Dietel, M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology 2000, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sturm, N.; Rossi, A.; Lantuejoul, S.; Papotti, M.; Frachon, S.C.; Claraz, C.; Brichon, P.-Y.; Brambilla, C.; Brambilla, E. Expression of thyroid transcription factor-1 in the spectrum of neuroendocrine cell lung proliferations with special interest in carcinoids. Hum. Pathol. 2002, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Du, E.Z.; Goldstraw, P.; Zacharias, J.; Tiffet, O.; Craig, P.J.; Nicholson, A.G.; Weidner, N.; Yi, E.S. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum. Pathol. 2004, 35, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schmidt, L.A.; Hatanaka, K.; Thomas, D.; Lagstein, A.; Myers, J.L. Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumours. Am. J. Clin. Pathol. 2014, 142, 320–324. [Google Scholar] [CrossRef]
- Srivastava, A.; Hornick, J.L. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumours from pancreatic endocrine and pulmonary carcinoid tumours. Am. J. Surg. Pathol. 2009, 33, 626–632. [Google Scholar] [CrossRef]
- Vesterinen, T.; Mononen, S.; Salmenkivi, K.; Mustonen, H.; Räsänen, J.; Salo, J.A.; Ilonen, I.; Knuuttila, A.; Haglund, C.; Arola, J. Clinicopathological indicators of survival among patients with pulmonary carcinoid tumor. Acta Oncol. 2018, 57, 1109–1116. [Google Scholar] [CrossRef]
- Wood, L.W.; Cox, N.I.; Phelps, C.A.; Lai, S.-C.; Poddar, A.; Talbot, C., Jr.; Mu, D. Thyroid Transcription Factor 1 Reprograms Angiogenic Activities of Secretome. Sci. Rep. 2016, 6, 19857. [Google Scholar] [CrossRef]
| Feature | (n = 53) | % |
|---|---|---|
| Gender | ||
| Male | 30 | 56.6 |
| Female | 23 | 43.4 |
| Age | ||
| Median | 66 | |
| SD | 11.15 | |
| Min. | 39 | |
| Max | 81 | |
| Smoke | ||
| No | 15 | 28.3 |
| Yes | 14 | 26.4 |
| Unknown | 24 | 45.2 |
| Side | ||
| Left | 23 | 43.4 |
| Right | 30 | 56.6 |
| Diagnosis | ||
| TC | 40 (25 right-sided, 15 left-sided) | 75.5 |
| 13 (5 right-sided, 8 left-sided) | ||
| AC | 24.5 | |
| Nodal status | ||
| Negative | 46 | 86.8 |
| Positive | 5 | 9.4 |
| Unknown | 2 | 3.8 |
| TNM Stage | ||
| I | 37 | 69.8 |
| II | 9 | 17 |
| III | 3 | 5.7 |
| Unknown | 4 | 7.5 |
| Synaptophysin | ||
| Neg. | 1 | 1.9 |
| Pos. | 49 | 92.5 |
| Unknown | 3 | 5.7 |
| Chromogranin A | ||
| Neg. | 5 | 9.4 |
| Pos. | 40 | 75.5 |
| Unknown | 8 | 15.1 |
| TTF-1 | ||
| Neg. | 21 | 39.6 |
| Pos. | 25 | 47.2 |
| Unknown | 7 | 13.2 |
| Mitotic count < 2 per 2 mm2 | ||
| No | 42 | 79.2 |
| Yes | 11 | 20.7 |
| Necrosis | ||
| No | 43 | 81.1 |
| Yes | 10 | 18.8 |
| Ki-67 | ||
| 1–2% | 19 | 35.8 |
| 3–19% | 27 | 50.9 |
| ≥20% | 1 | 1.8 |
| Unknown | 6 | 11.3 |
| Left Primary Carcinoids (n = 19) | Right Primary Carcinoids (n = 22) | Left vs. Right (Median Value) | |||||
|---|---|---|---|---|---|---|---|
| Median | SD | Range | Median | SD | Range | p Value | |
| Number of vessels | 529.00 | 544.26 | 113.00–2214.00 | 759.00 | 461.11 | 192.00–1790.00 | 0.019 |
| MVD | 195.21 | 179.03 | 37.51–735.06 | 251.99 | 153.09 | 63.74–594.29 | 0.025 |
| Average vessel area | 182.53 | 118.17 | 106.25–549.00 | 202.11 | 66.80 | 82.55–330.34 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Salvia, A.; Carletti, R.; Verrico, M.; Feola, T.; Puliani, G.; Bassi, M.; Sesti, F.; Pernazza, A.; Mazzilli, R.; Lamberti, G.; et al. Angioside: The role of Angiogenesis and Hypoxia in Lung Neuroendocrine Tumours According to Primary Tumour Location in Left or Right Parenchyma. J. Clin. Med. 2022, 11, 5958. https://doi.org/10.3390/jcm11195958
La Salvia A, Carletti R, Verrico M, Feola T, Puliani G, Bassi M, Sesti F, Pernazza A, Mazzilli R, Lamberti G, et al. Angioside: The role of Angiogenesis and Hypoxia in Lung Neuroendocrine Tumours According to Primary Tumour Location in Left or Right Parenchyma. Journal of Clinical Medicine. 2022; 11(19):5958. https://doi.org/10.3390/jcm11195958
Chicago/Turabian StyleLa Salvia, Anna, Raffaella Carletti, Monica Verrico, Tiziana Feola, Giulia Puliani, Massimiliano Bassi, Franz Sesti, Angelina Pernazza, Rossella Mazzilli, Giuseppe Lamberti, and et al. 2022. "Angioside: The role of Angiogenesis and Hypoxia in Lung Neuroendocrine Tumours According to Primary Tumour Location in Left or Right Parenchyma" Journal of Clinical Medicine 11, no. 19: 5958. https://doi.org/10.3390/jcm11195958
APA StyleLa Salvia, A., Carletti, R., Verrico, M., Feola, T., Puliani, G., Bassi, M., Sesti, F., Pernazza, A., Mazzilli, R., Lamberti, G., Siciliani, A., Mancini, M., Manai, C., Venuta, F., Ibrahim, M., Tomao, S., D’Amati, G., Di Gioia, C., Giannetta, E., ... Faggiano, A. (2022). Angioside: The role of Angiogenesis and Hypoxia in Lung Neuroendocrine Tumours According to Primary Tumour Location in Left or Right Parenchyma. Journal of Clinical Medicine, 11(19), 5958. https://doi.org/10.3390/jcm11195958







