Mortality Prediction Model before Surgery for Acute Mesenteric Infarction: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
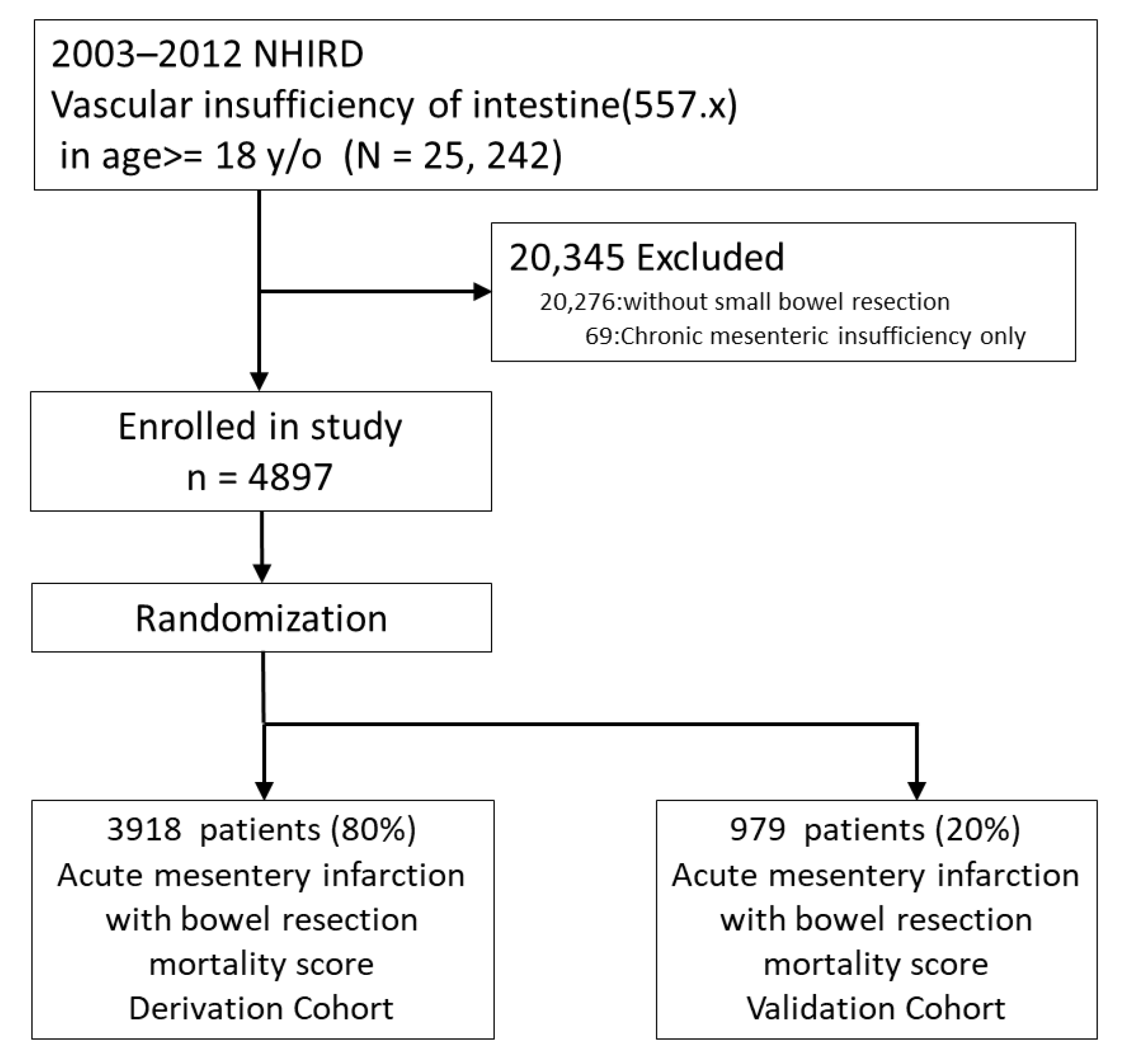
2.1. Database and Study Sample
2.2. Data Extraction and Inclusion/Exclusion Criteria
2.3. Primary Endpoint
2.4. Covariant Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Generating the Scoring System for the Prediction Model
3.3. The ‘Surgery for Acute Mesenteric Infarction Mortality Score’ (SAMIMS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clair, D.G.; Beach, J.M. Mesenteric Ischemia. N. Engl. J. Med. 2016, 374, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Berland, T.; Oldenburg, W.A. Acute mesenteric ischemia. Curr. Gastroenterol. Rep. 2008, 10, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Natarajan, B.; Gupta, H.; Fang, X.; Fitzgibbons, R.J. Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery 2011, 150, 779–787. [Google Scholar] [CrossRef]
- Acosta, S.; Ögren, M.; Sternby, N.-H.; Bergqvist, D.; Björck, M. Clinical Implications for the Management of Acute Thromboembolic Occlusion of the Superior Mesenteric Artery: Autopsy findings in 213 patients. Ann. Surg. 2005, 241, 516–522. [Google Scholar] [CrossRef]
- Adaba, F.; Askari, A.; Dastur, J.; Patel, A.; Gabe, S.; Vaizey, C.; Faiz, O.; Nightingale, J.; Warusavitarne, J. Mortality after acute primary mesenteric infarction. A systematic review and meta-analysis of observational studies. Color. Dis. 2015, 17, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Cudnik, M.T.; Darbha, S.; Jones, J.; Macedo, J.; Stockton, S.W.; Hiestand, B.C. The Diagnosis of Acute Mesenteric Ischemia: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2013, 20, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.H.; Rybin, D.; Doros, G.; McPhee, J.T.; Farber, A. Mortality of acute mesenteric ischemia remains unchanged despite significant increase in utilization of endovascular techniques. Vascular 2016, 24, 44–52. [Google Scholar] [CrossRef]
- Mamode, I.P.N. Failure to Improve Outcome in Acute Mesenteric Ischaemia: Seven Year Review. Eur. J. Surg. 1999, 165, 203–208. [Google Scholar] [CrossRef]
- Bala, M.; Kashuk, J.; Moore, E.E.; Kluger, Y.; Biffl, W.; Gomes, C.A.; Ben-Ishay, O.; Rubinstein, C.; Balogh, Z.J.; Civil, I.; et al. Acute mesenteric ischemia: Guidelines of the World Society of Emergency Surgery. World J. Emerg. Surg. 2017, 12, 38. [Google Scholar] [CrossRef]
- Emile, S.H.; Khan, S.M.; Barsoum, S.H. Predictors of bowel necrosis in patients with acute mesenteric ischemia: Systematic review and meta-analysis. Updat. Surg. 2021, 73, 47–57. [Google Scholar] [CrossRef]
- Otto, C.C.; Czigany, Z.; Heise, D.; Bruners, P.; Kotelis, D.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Klink, C.; Bednarsch, J. Prognostic Factors for Mortality in Acute Mesenteric Ischemia. J. Clin. Med. 2022, 11, 3619. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, J.; Zhou, Z. Preoperative Risk Factors for Short-Term Postoperative Mortality of Acute Mesenteric Ischemia after Laparotomy: A Systematic Review and Meta-Analysis. Emerg. Med. Int. 2020, 2020, 1382475. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.-L.; Ye, X.-N.; Lin, T.-T.; Qiu, Y.-H.; Huang, J.-Y.; Zheng, X.-W.; Chen, F.-F. The psoas muscle density as a predictor of postoperative complications and 30-day mortality for acute mesenteric ischemia patients. Abdom. Radiol. 2020, 47, 1644–1653. [Google Scholar] [CrossRef]
- Grotelüschen, R.; Bergmann, W.; Welte, M.; Reeh, M.; Izbicki, J.; Bachmann, K. What predicts the outcome in patients with intestinal ischemia? A single center experience. J. Visc. Surg. 2019, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Vural, V.; Ozozan, O.V. The Usefulness of Inflammation-based Prognostic Scores for the Prediction of Postoperative Mortality in Patients Who Underwent Intestinal Resection for Acute Intestinal Ischemia. Cureus 2019, 11, e6372. [Google Scholar] [CrossRef]
- Caluwaerts, M.; Castanares-Zapatero, D.; Laterre, P.-F.; Hantson, P. Prognostic factors of acute mesenteric ischemia in ICU patients. BMC Gastroenterol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Matthaei, H.; Klein, A.; Branchi, V.; Kalff, J.C.; Koscielny, A. Acute mesenteric ischemia (AMI): Absence of renal insufficiency and performance of early bowel resection may indicate improved outcomes. Int. J. Color. Dis. 2019, 34, 1781–1790. [Google Scholar] [CrossRef]
- Paladino, N.C.; Inviati, A.; Di Paola, V.; Busuito, G.; Amodio, E.; Bonventre, S.; Scerrino, G. Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study. Ann. Ital. Chir. 2014, 85, 265–270. [Google Scholar]
- Evennett, N.J.; Petrov, M.S.; Mittal, A.; Windsor, J.A. Systematic Review and Pooled Estimates for the Diagnostic Accuracy of Serological Markers for Intestinal Ischemia. World J. Surg. 2009, 33, 1374–1383. [Google Scholar] [CrossRef]
- Huang, H.-H.; Chang, Y.-C.; Yen, D.H.-T.; Kao, W.-F.; Chen, J.-D.; Wang, L.-M.; Huang, C.-I.; Lee, C.-H. Clinical Factors and Outcomes in Patients with Acute Mesenteric Ischemia in the Emergency Department. J. Chin. Med. Assoc. 2005, 68, 299–306. [Google Scholar] [CrossRef]
- Haga, Y.; Odo, M.; Homma, M.; Komiya, K.; Takeda, K.; Koike, S.; Takahashi, T.; Hiraka, K.; Yamashita, H.; Tanakaya, K. New Prediction Rule for Mortality in Acute Mesenteric Ischemia. Digestion 2009, 80, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Lepouse, C.; De Garine, A.; Frayssinet, N.; Leymarie, F.; Leon, A.; Jolly, D. Surgery for mesenteric infarction: Prognostic factors associated with early death within 72 hours. J. Cardiothorac. Vasc. Anesth. 2004, 18, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Akyıldız, H.Y.; Sözüer, E.; Uzer, H.; Baykan, M.; Oz, B. The length of necrosis and renal insufficiency predict the outcome of acute mesenteric ischemia. Asian J. Surg. 2015, 38, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Alhan, E.; Usta, A.; Çekiç, A.; Saglam, K.; Türkyılmaz, S.; Cinel, A. A study on 107 patients with acute mesenteric ischemia over 30 years. Int. J. Surg. 2012, 10, 510–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crawford, R.S.; Harris, D.G.; Klyushnenkova, E.N.; Tesoriero, R.B.; Rabin, J.; Chen, H.; Diaz, J.J. A Statewide Analysis of the Incidence and Outcomes of Acute Mesenteric Ischemia in Maryland from 2009 to 2013. Front. Surg. 2016, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.-C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Johnston, L.E.; Grimm, J.C.; Magruder, J.T.; Shah, A.S. Development of a Transplantation Risk Index in Patients With Mechanical Circulatory Support: A Decision Support Tool. JACC Heart Fail. 2016, 4, 277–286. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chen, Y.-J.; Ho, H.J.; Hsu, Y.-C.; Kuo, K.N.; Wu, M.-S.; Lin, J.-T. Association Between Nucleoside Analogues and Risk of Hepatitis B Virus–Related Hepatocellular Carcinoma Recurrence Following Liver Resection. JAMA 2012, 308, 1906–1914. [Google Scholar] [CrossRef]
- Tun, M.; Malik, A.K. Massive small bowel infarction and duodenal perforation due to abdominal polyarteritis nodosa: A case report. Malays. J. Pathol. 1994, 16, 75–78. [Google Scholar]
- Cheng, C.-Y.; Hsu, C.-Y.; Wang, T.-C.; Liu, C.-Y.; Yang, Y.-H.; Yang, W.-H. Risk of Cardiac Morbidities and Sudden Death in Patients With Epilepsy and No History of Cardiac Disease: A Population-Based Nationwide Study. Mayo. Clin. Proc. 2021, 96, 964–974. [Google Scholar] [CrossRef]
- Tamariz, L.; Harkins, T.; Nair, V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol. Drug Saf. 2012, 21, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Croft, J.B.; Giles, W.H.; Anda, R.F.; Mensah, G.A. Epidemiology of pacemaker procedures among Medicare enrollees in 1990, 1995 and 2000. Am. J. Cardiol. 2005, 95, 409–411. [Google Scholar] [CrossRef]
- DeLea, T.E.; Hagiwara, M.; Phatak, P.D. Retrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disorders. Curr. Med. Res. Opin. 2009, 25, 139–147. [Google Scholar] [CrossRef] [PubMed]
- D’Journo, X.B.; Boulate, D.; Fourdrain, A.; Loundou, A.; Henegouwen, M.I.V.B.; Gisbertz, S.S.; O’Neill, J.R.; Hoelscher, A.; Piessen, G.; van Lanschot, J.; et al. Risk Prediction Model of 90-Day Mortality After Esophagectomy for Cancer. JAMA Surg. 2021, 156, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Graviss, E.A. Development and validation of a prognostic score to predict tuberculosis mortality. J. Infect. 2018, 77, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Little, W.C.; Xavier, S.S.; Rassi, S.G.; Rassi, A.G.; Rassi, G.G.; Hasslochermoreno, A.M.; Sousa, A.S.; Scanavacca, M.I. Development and Validation of a Risk Score for Predicting Death in Chagas’ Heart Disease. N. Engl. J. Med. 2006, 355, 799–808. [Google Scholar] [CrossRef]
- Reilly, J.R.; Gabbe, B.J.; Brown, W.A.; Hodgson, C.L.; Myles, P.S. Systematic review of perioperative mortality risk prediction models for adults undergoing inpatient non-cardiac surgery. ANZ J. Surg. 2021, 91, 860–870. [Google Scholar] [CrossRef]
- Eugene, N.; Oliver, C.; Bassett, M.; Poulton, T.; Kuryba, A.; Johnston, C.; Anderson, I.; Moonesinghe, S.; Grocott, M.; Murray, D.; et al. Development and internal validation of a novel risk adjustment model for adult patients undergoing emergency laparotomy surgery: The National Emergency Laparotomy Audit risk model. Br. J. Anaesth. 2018, 121, 739–748. [Google Scholar] [CrossRef]
- Le Manach, Y.; Collins, G.; Rodseth, R.; LE Bihan-Benjamin, C.; Biccard, B.; Riou, B.; Devereaux, P.; Landais, P. Preoperative Score to Predict Postoperative Mortality (POSPOM): Derivation and Validation. Anesthesiology 2016, 124, 570–579. [Google Scholar] [CrossRef]
- Protopapa, K.L.; Simpson, J.C.; E Smith, N.C.; Moonesinghe, S.R. Development and validation of the Surgical Outcome Risk Tool (SORT). Br. J. Surg. 2014, 101, 1774–1783. [Google Scholar] [CrossRef]
- Glance, L.G.; Lustik, S.J.; Hannan, E.L.; Osler, T.M.; Mukamel, D.B.; Qian, F.; Dick, A.W. The Surgical Mortality Probability Model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Annals of surgery. Ann. Surg. 2012, 255, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Desserud, K.F.; Veen, T.; Søreide, K. Emergency general surgery in the geriatric patient. Br. J. Surg. 2016, 103, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Ter Borg, P.C.J. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.K.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Said, A.; Williams, J.; Holden, J.; Remington, P.; Gangnon, R.; Musat, A.; Lucey, M.R. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J. Hepatol. 2004, 40, 897–903. [Google Scholar] [CrossRef]
- Leise, M.D.; Kim, W.R.; Kremers, W.K.; Larson, J.J.; Benson, J.T.; Therneau, T.M. A Revised Model for End-Stage Liver Disease Optimizes Prediction of Mortality Among Patients Awaiting Liver Transplantation. Gastroenterology 2011, 140, 1952–1960. [Google Scholar] [CrossRef]
- Doyle, D.J.; Goyal, A.; Bansal, P. American Society of Anesthesiologists Classification; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mathew, A.; Devereaux, P.; O’Hare, A.; Tonelli, M.; Thiessen-Philbrook, H.; Nevis, I.; Iansavichus, A.; Garg, A. Chronic kidney disease and postoperative mortality: A systematic review and meta-analysis. Kidney Int. 2008, 73, 1069–1081. [Google Scholar] [CrossRef]
- Francoz, C.; Valla, D.; Durand, F. Portal vein thrombosis, cirrhosis, and liver transplantation. J. Hepatol. 2012, 57, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, N.; Caldwell, S.H.; Tripodi, A. Diagnosis, Development, and Treatment of Portal Vein Thrombosis in Patients With and Without Cirrhosis. Gastroenterology 2019, 156, 1582–1599.e1581. [Google Scholar] [CrossRef]

| Variable | Derivation Group | Validation Group | p-Value | ||
|---|---|---|---|---|---|
| n | 3918 | n | 979 | ||
| Age in years, median (IQR) | 71.92 | (22.33) | 72.00 | (23.33) | 0.838 |
| Sex | 0.067 | ||||
| Woman | 1809 | 46.17% | 420 | 42.90% | |
| Man | 2109 | 53.83% | 559 | 57.10% | |
| Major coexisting disease | |||||
| Myocardial infarction | 242 | 6.18% | 61 | 6.23% | 0.941 |
| Congestive heart failure | 687 | 17.53% | 162 | 16.55% | 0.479 |
| Vascular disease | 2283 | 58.27% | 559 | 57.10% | 0.515 |
| Cerebrovascular disease | 787 | 20.09% | 197 | 20.12% | 1.000 |
| Dementia | 80 | 2.04% | 30 | 3.06% | 0.069 |
| Chronic pulmonary disease | 685 | 17.48% | 169 | 17.26% | 0.888 |
| Rheumatic disease | 63 | 1.61% | 10 | 1.02% | 0.237 |
| Peptic ulcer disease | 1028 | 26.24% | 269 | 27.48% | 0.442 |
| Severe liver disease | 93 | 2.37% | 33 | 3.37% | 0.090 |
| Diabetes | 1039 | 26.52% | 255 | 26.05% | 0.777 |
| Hemiplegia | 154 | 3.93% | 38 | 3.88% | 1.000 |
| Under dialysis | 354 | 9.04% | 93 | 9.50% | 0.664 |
| Malignancy | 687 | 17.53% | 187 | 19.10% | 0.263 |
| Heart conduct disease | 801 | 20.44% | 206 | 21.04% | 0.691 |
| Hypertension | 1869 | 47.70% | 441 | 45.05% | 0.142 |
| Hyperlipidemia | 405 | 10.34% | 105 | 10.73% | 0.726 |
| Gout | 326 | 8.32% | 97 | 9.91% | 0.127 |
| Obesity | 8 | 0.20% | 2 | 0.20% | 1.000 |
| Laparoscope operation | 98 | 2.5% | 23 | 2.35% | 0.908 |
| Perioperative outcomes | |||||
| 30-day mortality | 684 | 17.46% | 168 | 17.16% | 0.851 |
| Median LOH (IQR) | 16 | (18) | 16 | (19) | 0.318 |
| Age | Score |
|---|---|
| Age > 62 | 3 |
| Comorbidities | Score |
| Severe liver disease | 4 |
| Hemodialysis | 2 |
| Congestive heart failure | 1 |
| Peptic ulcer disease | 1 |
| Cerebrovascular disease | 1 |
| Diabetes | 1 |
| Derivation Group | Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|
| Score | Mortality Rate | OR | p | Score | Mortality Rate | OR | p |
| 0 (Very Low) | 4.4% | 1 | 0 (Very Low) | 3.5% | 1 | ||
| 1–3 (Low risk) | 13.4% | 3.332 (2.287–4.857) | <0.001 | 1–3 (Low risk) | 12.9% | 4.117 (1.809–9.369) | 0.001 |
| 4–6 (Median risk) | 24.5% | 7.004 (4.902–10.008) | <0.001 | 4–6 (Median risk) | 24.7% | 9.082 (4.130–19.974) | <0.001 |
| 7–13 (High risk) | 32.5% | 10.410 (6.763–16.023) | <0.001 | 7–13 (High risk) | 33.8% | 14.165 (5.724–35.053) | <0.001 |
| ROC: AUC = 0.677, p < 0.001, 95% CI: 0.660–0.689, Calibration (Hosmer–Lemeshow goodness-of-fit test) χ2 = 19.887 (p = 0.001) | ROC: AUC = 0.696, p < 0.001, 95% CI: 0.666–0.725, Calibration (Hosmer–Lemeshow goodness-of-fit test) χ2 = 5.067 (p = 0.280) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-W.; Chen, C.-Y.; Su, Y.-C.; Wu, K.-T.; Yu, P.-C.; Yen, Y.-C.; Chen, J.-H. Mortality Prediction Model before Surgery for Acute Mesenteric Infarction: A Population-Based Study. J. Clin. Med. 2022, 11, 5937. https://doi.org/10.3390/jcm11195937
Lin S-W, Chen C-Y, Su Y-C, Wu K-T, Yu P-C, Yen Y-C, Chen J-H. Mortality Prediction Model before Surgery for Acute Mesenteric Infarction: A Population-Based Study. Journal of Clinical Medicine. 2022; 11(19):5937. https://doi.org/10.3390/jcm11195937
Chicago/Turabian StyleLin, Shang-Wei, Chung-Yen Chen, Yu-Chieh Su, Kun-Ta Wu, Po-Chin Yu, Yung-Chieh Yen, and Jian-Han Chen. 2022. "Mortality Prediction Model before Surgery for Acute Mesenteric Infarction: A Population-Based Study" Journal of Clinical Medicine 11, no. 19: 5937. https://doi.org/10.3390/jcm11195937
APA StyleLin, S.-W., Chen, C.-Y., Su, Y.-C., Wu, K.-T., Yu, P.-C., Yen, Y.-C., & Chen, J.-H. (2022). Mortality Prediction Model before Surgery for Acute Mesenteric Infarction: A Population-Based Study. Journal of Clinical Medicine, 11(19), 5937. https://doi.org/10.3390/jcm11195937






