Risk Factors of Infection in Relapsed/Refractory Multiple Myeloma Patients Treated with Lenalidomide and Dexamethasone (Rd) Regimen: Real-Life Results of a Large Single-Center Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients, Treatment, and Adverse Events
2.2. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. The Treatment Outcome
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Houlston, R.; Sud, A. Epidemiology, genetics and treatment of multiple myeloma and precursor diseases. Int. J. Cancer 2021, 149, 1980–1996. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Robak, P.; Robak, T. Novel Drugs for Multiple Myeloma. In Topics in Anti-Cancer Research; Atta-ur-Rahman, K.Z., Zaman, K.E., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2019; Volume 8, pp. 1–43. [Google Scholar] [CrossRef]
- Weber, D.M.; Chen, C.; Niesvizky, R.; Wang, M.; Belch, A.; Stadtmauer, E.A.; Siegel, D.; Borrello, I.; Rajkumar, S.V.; Chanan-Khan, A.A.; et al. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. N. Engl. J. Med. 2007, 357, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Spencer, A.; Attal, M.; Prince, H.M.; Harousseau, J.-L.; Dmoszynska, A.; Miguel, J.S.; Hellmann, A.; Facon, T.; Foà, R.; et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2007, 357, 2123–2132. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.A.; Miller, K.H.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.E.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Kang, J.; Havens, C.G.; Conklin, T.; Ning, Y.; Wu, L.; Ito, T.; Ando, H.; Waldman, M.F.; Thakurta, A.; et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors I karos and A iolos via modulation of the E 3 ubiquitin ligase complex CRL4CRBN. Br. J. Haematol. 2014, 164, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Blimark, C.; Holmberg, E.; Mellqvist, U.-H.; Landgren, O.; Björkholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 2015, 100, 107–113. [Google Scholar] [CrossRef]
- Balmaceda, N.; Aziz, M.; Chandrasekar, V.T.; McClune, B.; Kambhampati, S.; Shune, L.; Abdallah, A.-O.; Anwer, F.; Majeed, A.; Qazilbash, M.; et al. Infection risks in multiple myeloma: A systematic review and meta-analysis of randomized trials from 2015 to 2019. BMC Cancer 2021, 21, 730. [Google Scholar] [CrossRef]
- Al-Jasser, A.M. Infections in Patients with Multiple Myeloma in the Era of Novel Agents and Stem Cell Therapies. In Update on Multiple Myeloma; Al-Anazi, K.A.A.-A.E.-K.A., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. Ch.8. [Google Scholar] [CrossRef]
- Teh, B.W.; Harrison, S.J.; Worth, L.J.; Thursky, K.A.; Slavin, M.A. Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: A systematic review and meta-analysis. Eur. J. Cancer 2016, 67, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.P.G. Management of the adverse effects of lenalidomide in multiple myeloma. Adv. Ther. 2011, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.; Dechow, T.; Scheytt, M.; Schmidt, C.; Hackanson, B.; Knop, S. The clinical management of lenalidomide-based therapy in patients with newly diagnosed multiple myeloma. Ann. Hematol. 2020, 99, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Chen, C.; Spencer, A.; Niesvizky, R.; Attal, M.; Stadtmauer, E.A.; Petrucci, M.T.; Yu, Z.; Olesnyckyj, M.; Zeldis, J.B.; et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia 2009, 23, 2147–2152. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.-J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Hus, I.; Piekarska, A.; Roliński, J.; Brzeźniakiewicz-Janus, K.; Giannopoulos, K.; Jamroziak, K.; Piątkowska-Jakubas, B.; Wierzbowska, A.; Zaucha, J.M.; Giebel, S.; et al. Vaccination of adult patients with hematological malignancies and patients with asplenia—Guidelines of PTHiT and Infectious Diseases Working Group PALG. Acta Haematol. Pol. 2018, 49, 93–101. [Google Scholar] [CrossRef][Green Version]
- Ljungman, P.; Cordonnier, C.; Einsele, H.; Englund, J.; Machado, C.M.; Storek, J.; Small, T. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transpl. 2009, 44, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Boccadoro, M.; Moreau, P.; San-Miguel, J.; Cavo, M.; Pawlyn, C.; Zweegman, S.; Facon, T.; Driessen, C.; Hajek, R.; et al. Recommendations for vaccination in multiple myeloma: A consensus of the European Myeloma Network. Leukemia 2021, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Schütt, P.; Brandhorst, D.; Stellberg, W.; Poser, M.; Ebeling, P.; Müller, S.; Buttkereit, U.; Opalka, B.; Lindemann, M.; Grosse-Wilde, H.; et al. Immune parameters in multiple myeloma patients: Influence of treatment and correlation with opportunistic infections. Leuk. Lymphoma 2006, 47, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Nordøy, T.; Husebekk, A.; Aaberge, I.S.; Jenum, P.A.; Samdal, H.H.; Flugsrud, L.B.; Kolstad, A. IMMUNE RECONSTITUTION-Humoral immunity to viral and bacterial antigens in lymphoma patients 4–10 years after high-dose therapy with ABMT. Serological responses to revaccinations according to EBMT. Bone Marrow Transplant. 2001, 28, 681–688. [Google Scholar] [CrossRef]
- Renaud, L.; Schraen, S.; Fouquet, G.; Guidez, S.; Demarquette, H.; Nudel, M.; Cayssials, E.; Bories, C.; Herbaux, C.; Systchenko, T.; et al. Response to pneumococcal vaccination in multiple myeloma. Cancer Med. 2019, 8, 3822–3830. [Google Scholar] [CrossRef]
- Inazawa, N.; Hori, T.; Nojima, M.; Saito, M.; Igarashi, K.; Yamamoto, M.; Shimizu, N.; Yoto, Y.; Tsutsumi, H. Virus reactivations after autologous hematopoietic stem cell transplantation detected by multiplex PCR assay. J. Med. Virol. 2016, 89, 358–362. [Google Scholar] [CrossRef]
- Mittelman, M. The Implications of Anemia in Multiple Myeloma. Clin. Lymphoma 2003, 4, S23–S29. [Google Scholar] [CrossRef]
- Lin, C.; Shen, H.; Zhou, S.; Liu, M.; Xu, A.; Huang, S.; Shen, C.; Zhou, F. Assessment of infection in newly diagnosed multiple myeloma patients: Risk factors and main characteristics. BMC Infect. Dis. 2020, 20, 699. [Google Scholar] [CrossRef]
- Dumontet, C.; Hulin, C.; Dimopoulos, M.A.; Belch, A.; Dispenzieri, A.; Ludwig, H.; Rodon, P.; Van Droogenbroeck, J.; Qiu, L.; Cavo, M.; et al. A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: Analysis of the FIRST trial. Leukemia 2018, 32, 1404–1413. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lim, J.-Y.; Ryu, D.-B.; Kim, T.W.; Park, S.S.; Jeon, Y.-W.; Yoon, J.-H.; Cho, B.-S.; Eom, K.-S.; Kim, Y.-J.; et al. Low frequency of CD3+ CD4+ CD161+ T cells correlates with the occurrence of infections in refractory/relapsed multiple myeloma patients receiving lenalidomide plus low-dose dexamethasone treatment. Ann. Hematol. 2018, 97, 2163–2171. [Google Scholar] [CrossRef]
- Llibre, A.; Grudzinska, F.S.; O’Shea, M.K.; Duffy, D.; Thickett, D.R.; Mauro, C.; Scott, A. Lactate cross-talk in host–pathogen interactions. Biochem. J. 2021, 478, 3157–3178. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, R.; Long, H.; Guo, B.; Jia, Q.; Qin, D.; Liu, S.; Wang, Z.; Xiang, T.; Zhang, J.; et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 2018, 24, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Kim, J.E.; Yoo, C.; Lee, D.H.; Kim, S.-W.; Lee, J.-S.; Suh, C. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann. Hematol. 2010, 89, 391–397. [Google Scholar] [CrossRef]
- Encinas, C.; Hernandez-Rivas, J.; Oriol, A.; Rosiñol, L.; Blanchard, M.-J.; Bellón, J.-M.; García-Sanz, R.; de la Rubia, J.; de la Guía, A.L.; Jímenez-Ubieto, A.; et al. A simple score to predict early severe infections in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2022, 12, 68. [Google Scholar] [CrossRef]
- Tsai, C.-K.; Liu, Y.-C.; Kuan, A.S.; Lee, K.-L.; Yeh, C.-M.; Lee, Y.-T.; Hsiao, L.-T.; Ko, P.-S.; Wang, H.-Y.; Chen, P.-M.; et al. Risk and impact of invasive fungal infections in patients with multiple myeloma. Ann. Hematol. 2020, 99, 1813–1822. [Google Scholar] [CrossRef]
- Raje, N.S.; Anaissie, E.; Kumar, S.K.; Lonial, S.; Martin, T.; Gertz, M.A.; Krishnan, A.; Hari, P.; Ludwig, H.; O’Donnell, E.; et al. Consensus guidelines and recommendations for infection prevention in multiple myeloma: A report from the International Myeloma Working Group. Lancet Haematol. 2022, 9, e143–e161. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Hernandez-Ibarburu, G.; Alonso, R.; Sanchez-Pina, J.M.; Zamanillo, I.; Lopez-Muñoz, N.; Iñiguez, R.; Cuellar, C.; Calbacho, M.; Paciello, M.L.; et al. Impact of COVID-19 in patients with multiple myeloma based on a global data network. Blood Cancer J. 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Sonneveld, P.; Facon, T.; San-Miguel, J.; Avet-Loiseau, H.; Mohty, M.; Mateos, M.-V.; Moreau, P.; Cavo, M.; Pawlyn, C.; et al. COVID-19 vaccination in patients with multiple myeloma: A consensus of the European Myeloma Network. Lancet Haematol. 2021, 8, e934–e946. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2014, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Averitt, A.J.; Weng, C.; Ryan, P.; Perotte, A. Translating evidence into practice: Eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit. Med. 2020, 3, 67. [Google Scholar] [CrossRef]
- Mele, G.; Melpignano, A.; Quarta, G.; Palumbo, G.; Capalbo, S.; Falcone, A.; Cascavilla, N.; Palazzo, G.; Mazza, P.; Iannitto, E.; et al. “Real world” outcome of lenalidomide plus dexamethasone in the setting of recurrent and refractory multiple myeloma: Extended follow-up of a retrospective multicenter study by the “rete ematologica pugliese”. Leuk. Res. 2015, 39, 279–283. [Google Scholar] [CrossRef]

| Characteristics | Total |
|---|---|
| Number of patients | 174 (100%) |
| Sex | |
| M | 84 (48.3) |
| F | 90 (51.7) |
| Age at diagnosis | |
| Median (IQR) | 65 (59.0–71.0) |
| Age at Rd administration | |
| Median (IQR) | 68 (62.0–74.0) |
| Duration of Rd treatment (months) | |
| Median (IQR) | 8.8 (4.3–18.24) |
| Myeloma stage: | |
| ISS I | 37 (21.3) |
| ISS II | 44 (25.3) |
| ISS III | 72 (40.8) |
| Missing data | 21 (12.1) |
| Transplant eligibility: | |
| AHSCT before Rd | 57 (32.8) |
| AHSCT after Rd | 16 (9.2) |
| Without AHSCT | 109 (62.6) |
| Double AHSCT | 16 (9.2) |
| Lenalidomide administration: | |
| Second-line | 110 (63.2) |
| Third-line | 57 (32.8) |
| Fourth-line | 7 (4.0) |
| Adverse events: | |
| Infections | 54 (31.0) |
| Neutropenia grade III and IV | 50 (28.7) |
| Anemia grade III and IV | 41 (23.6) |
| Thrombocytopenia grade III and IV | 32 (18.4) |
| Pancytopenia | 20 (11.5) |
| Thrombosis | 20 (11.5) |
| Polyneuropathy | 15 (8.6) |
| Nephrotoxicity | 9 (5.2) |
| Cause of early ending of treatment | |
| Referral to AHSCT | 16 (9.2) |
| Patient’s resignation | 10 (5.7) |
| Hematological toxicity | 8 (4.6) |
| Other nonhematological toxicity | 4 (2.3) |
| Paraprotein type | |
| IgG | 115 (66.1) |
| IgA | 30 (17.2) |
| LCD kappa | 14 (8.0) |
| LCD lambda | 7 (4.0) |
| Biclonal | 3 (1.7) |
| Nonsecretory | 2 (1.1) |
| First-line treatment | |
| VCD | 92 (52.9) |
| MP/MPT | 16 (9.2) |
| CTD | 30 (17.2) |
| VTD | 8 (4.6) |
| Other | 28 (16.1) |
| CRAB symptoms at Rd administration | |
| Ca > 2.5 mmol/L | 24 (13.8) |
| Creatinine > 2 mg/dL | 14 (8.0) |
| HGB < 100 g/L | 39 (22.4) |
| Bone disease | 95 (54.6) |
| RTx | 85 (48.9%) |
| Cytogenetics * | 52 (100%) |
| 1q gain | 21 (40.4) |
| Trisomies | 11 (21.2) |
| del(13q) | 10 (19.2) |
| t(4;14) | 8 (15.4) |
| del(17p) | 7 (13.5) |
| t(11;14) | 2 (3.8) |
| t(14;16) | 1 (1.9) |
| Parameter | PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | HR | 95% CI | Coefficient | p-Value | HR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||||
| Sex (M) | 0.030 | 0.7453 | 1.061 | 0.742 | 1.517 | 0.113 | 0.2597 | 1.255 | 0.846 | 1.862 |
| Age > 70 | 0.094 | 0.3139 | 1.207 | 0.837 | 1.740 | 0.216 | 0.0357 | 1.541 | 1.029 | 2.306 |
| ISS 3 | 0.276 | 0.0052 | 1.737 | 1.179 | 2.560 | 0.295 | 0.0066 | 1.804 | 1.179 | 2.761 |
| AHSCT before Rd | −0.133 | 0.1828 | 0.767 | 0.519 | 1.133 | −0.195 | 0.0842 | 0.677 | 0.435 | 1.054 |
| AHSCT after Rd | −0.406 | 0.0270 | 0.444 | 0.216 | 0.912 | −0.617 | 0.0159 | 0.291 | 0.107 | 0.794 |
| Rd in II line | −0.162 | 0.0787 | 0.723 | 0.504 | 1.038 | −0.147 | 0.1499 | 0.746 | 0.500 | 1.112 |
| CR/VGPR after three cycles | −0.279 | 0.1514 | 0.572 | 0.266 | 1.227 | −0.503 | 0.0487 | 0.365 | 0.134 | 0.994 |
| CR/VGPR after six cycles | −0.503 | 0.0010 | 0.366 | 0.200 | 0.667 | −0.451 | 0.0101 | 0.405 | 0.204 | 0.806 |
| Best Response CR/VGPR | −0.451 | 0.0001 | 0.406 | 0.262 | 0.630 | −0.576 | 0.0000 | 0.316 | 0.185 | 0.542 |
| Ca > 2.5 mmol/L at Rd | −0.001 | 0.9918 | 0.997 | 0.586 | 1.697 | 0.071 | 0.6357 | 1.153 | 0.639 | 2.082 |
| HGB < 100 g/L at Rd | 0.188 | 0.0856 | 1.458 | 0.949 | 2.240 | 0.123 | 0.3069 | 1.278 | 0.798 | 2.045 |
| Creatinine > 2 mg/dL at Rd | 0.298 | 0.0605 | 1.816 | 0.974 | 3.387 | 0.240 | 0.1511 | 1.616 | 0.839 | 3.113 |
| Bone disease at Rd | −0.161 | 0.1200 | 0.724 | 0.482 | 1.088 | −0.060 | 0.6053 | 0.887 | 0.564 | 1.396 |
| M protein concentration (g/L) at Rd | 0.004 | 0.6602 | 1.004 | 0.987 | 1.021 | 0.008 | 0.3830 | 1.008 | 0.990 | 1.026 |
| B2M concentration (mg/L) at Rd | 0.022 | 0.3461 | 1.022 | 0.977 | 1.069 | 0.041 | 0.0534 | 1.042 | 0.999 | 1.086 |
| LDH concentration (U/L) at Rd | 0.001 | 0.1585 | 1.001 | 0.999 | 1.003 | 0.001 | 0.2613 | 1.001 | 0.999 | 1.004 |
| WBC (×103/μL) at Rd | 0.029 | 0.3246 | 1.029 | 0.972 | 1.090 | 0.026 | 0.3639 | 1.026 | 0.971 | 1.085 |
| PLT (×103/μL) at Rd | −0.003 | 0.0194 | 0.997 | 0.995 | 1.000 | −0.003 | 0.0359 | 0.997 | 0.995 | 1.000 |
| Cytogenetic risk group | ||||||||||
| Unknown | Reference | Reference | ||||||||
| High-risk | 0.520 | 0.0019 | 1.954 | 1.242 | 3.075 | 0.300 | 0.0987 | 1.498 | 0.908 | 2.471 |
| Standard-risk | −0.370 | 0.0574 | 0.803 | 0.456 | 1.414 | −0.196 | 0.3381 | 0.913 | 0.505 | 1.650 |
| Parameter | Coefficient | p-Value | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age > 70 | −0.184 | 0.5888 | 0.832 | 0.426 | 1.623 |
| ISS 3 | −0.156 | 0.6572 | 0.855 | 0.429 | 1.706 |
| AHSCT before Rd | 0.618 | 0.0708 | 1.855 | 0.949 | 3.625 |
| AHSCT after Rd | −0.529 | 0.4320 | 0.589 | 0.157 | 2.205 |
| Rd in II line | −0.195 | 0.5624 | 0.823 | 0.426 | 1.591 |
| CR/VGPR after three cycles | 0.221 | 0.7051 | 1.247 | 0.397 | 3.915 |
| CR/VGPR after six cycles | 0.113 | 0.8093 | 1.120 | 0.447 | 2.801 |
| Best Response CR/VGPR | 0.615 | 0.0870 | 1.850 | 0.915 | 3.741 |
| Ca > 2.5 mmol/L at Rd | −0.194 | 0.6893 | 0.824 | 0.318 | 2.133 |
| HGB < 100 g/L at Rd | 0.049 | 0.9022 | 1.050 | 0.482 | 2.287 |
| Creatinine > 2 mg/dL at 2 Rd | −0.145 | 0.8144 | 0.865 | 0.259 | 2.896 |
| Bone disease at Rd | −0.240 | 0.5224 | 0.787 | 0.377 | 1.641 |
| M protein concentration (g/L) at Rd | 0.003 | 0.8427 | 1.003 | 0.974 | 1.033 |
| B2M concentration (mg/L) at Rd | −0.009 | 0.8178 | 0.991 | 0.918 | 1.070 |
| LDH concentration (U/L) at Rd | 0.001 | 0.6299 | 1.001 | 0.996 | 1.006 |
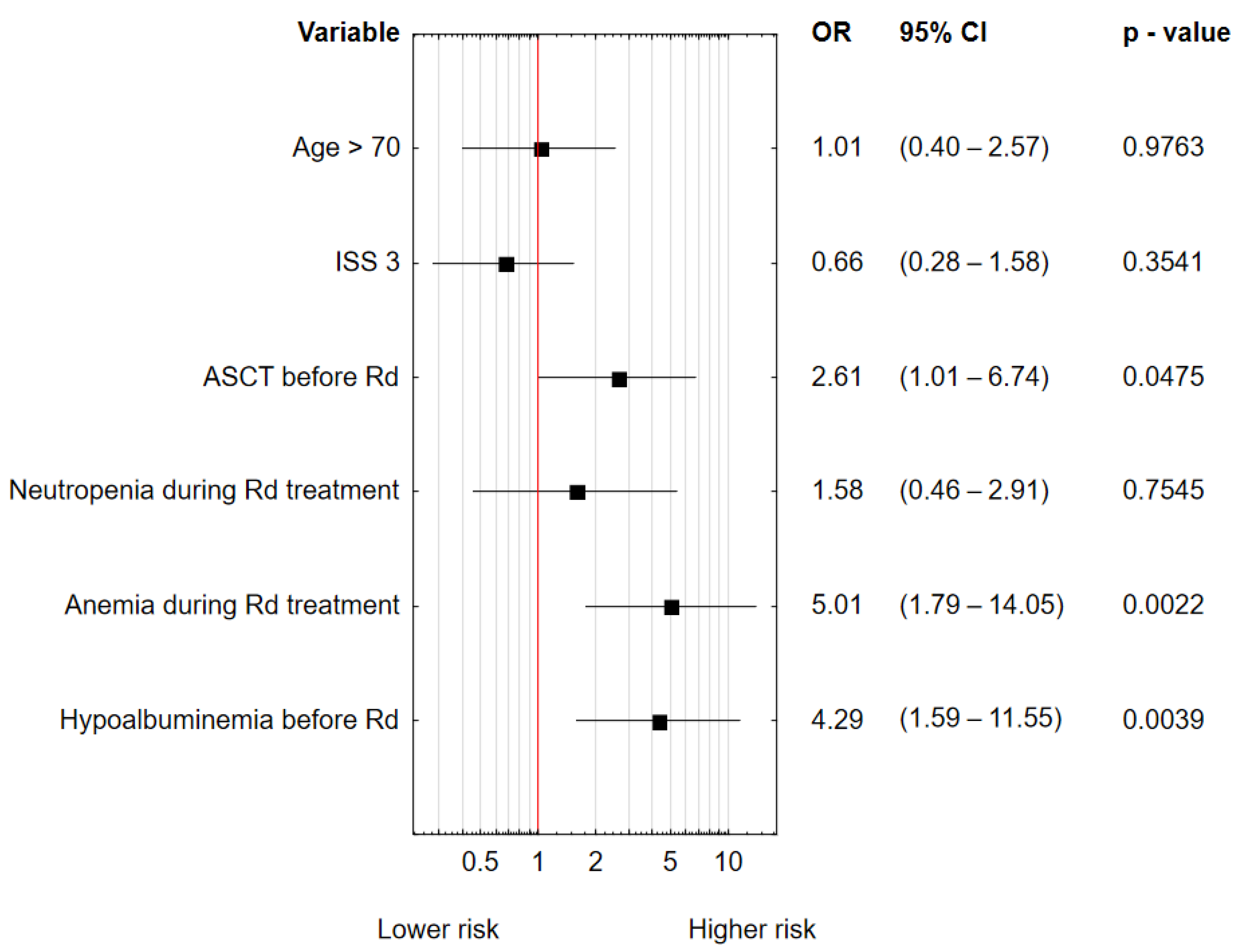
| Hypoalbuminemia | 0.916 | 0.0257 | 2.500 | 1.118 | 5.593 |
| Neutropenia grade 2 at Rd initiation | 0.186 | 0.7287 | 1.205 | 0.421 | 3.448 |
| Lymphocytopenia ≥ grade 2 at Rd initiation | −0.744 | 0.2012 | 0.475 | 0.152 | 1.487 |
| WBC (×103/μL) at Rd | −0.053 | 0.3148 | 0.949 | 0.856 | 1.051 |
| PLT (×103/μL) at Rd | 0.002 | 0.3253 | 1.002 | 0.998 | 1.006 |
| HGB (g/dL) at Rd | 0.109 | 0.1919 | 1.115 | 0.947 | 1.312 |
| Pancytopenia during Rd treatment | 0.194 | 0.6979 | 1.214 | 0.455 | 3.239 |
| Anemia grade ≥3 during Rd treatment | 1.286 | 0.0006 | 3.618 | 1.741 | 7.521 |
| Neutropenia grade ≥3 during Rd treatment | 0.804 | 0.0221 | 2.234 | 1.122 | 4.448 |
| Thrombocytopenia grade ≥3 during Rd treatment | 0.177 | 0.6693 | 1.194 | 0.530 | 2.691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulski, D.; Robak, P.; Ryżewska, W.; Stańczak, K.; Kościelny, K.; Góra-Tybor, J.; Robak, T. Risk Factors of Infection in Relapsed/Refractory Multiple Myeloma Patients Treated with Lenalidomide and Dexamethasone (Rd) Regimen: Real-Life Results of a Large Single-Center Study. J. Clin. Med. 2022, 11, 5908. https://doi.org/10.3390/jcm11195908
Mikulski D, Robak P, Ryżewska W, Stańczak K, Kościelny K, Góra-Tybor J, Robak T. Risk Factors of Infection in Relapsed/Refractory Multiple Myeloma Patients Treated with Lenalidomide and Dexamethasone (Rd) Regimen: Real-Life Results of a Large Single-Center Study. Journal of Clinical Medicine. 2022; 11(19):5908. https://doi.org/10.3390/jcm11195908
Chicago/Turabian StyleMikulski, Damian, Paweł Robak, Wiktoria Ryżewska, Kamila Stańczak, Kacper Kościelny, Joanna Góra-Tybor, and Tadeusz Robak. 2022. "Risk Factors of Infection in Relapsed/Refractory Multiple Myeloma Patients Treated with Lenalidomide and Dexamethasone (Rd) Regimen: Real-Life Results of a Large Single-Center Study" Journal of Clinical Medicine 11, no. 19: 5908. https://doi.org/10.3390/jcm11195908
APA StyleMikulski, D., Robak, P., Ryżewska, W., Stańczak, K., Kościelny, K., Góra-Tybor, J., & Robak, T. (2022). Risk Factors of Infection in Relapsed/Refractory Multiple Myeloma Patients Treated with Lenalidomide and Dexamethasone (Rd) Regimen: Real-Life Results of a Large Single-Center Study. Journal of Clinical Medicine, 11(19), 5908. https://doi.org/10.3390/jcm11195908







