A Novel Prediction Tool for Endoscopic Intervention in Patients with Acute Upper Gastro-Intestinal Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Extraction
- -
- Demographic factors—age in years and sex.
- -
- Comorbidities—including hypertension (HTN), diabetes mellitus (DM), cardio-vascular disease (i.e., ischemic heart disease (IHD), arrythmias, valvular disorder, stroke), pulmonary disease (i.e., asthma, chronic obstructive pulmonary disease (COPD)), deep vein thrombosis (DVT), pulmonary embolism (PE) and cirrhosis.
- -
- Chronic treatment of anticoagulants and anti-platelets—including aspirin, P2Y12 inhibitors (clopidogrel, ticagrelor, prasugrel), warfarin and direct oral anticoagulants (DOACs, i.e., dabigatran, rivaroxaban, apixaban).
- -
- Hemodynamic, laboratory variables—all necessary variables at presentation for GBS calculation were collected. Other laboratory results including C-reactive protein (CRP), white blood count (WBC), platelets count, international normalization ratio (INR) and albumin were collected as well.
- -
- Medications—date regarding blood transfusion during hospital stay and treatment with tranexamic acid (TXA, i.e., Hexakapron), proton pump inhibitors (PPI), erythromycin and intravenous (IV) fluids prior to endoscopy were collected.
- -
- Endoscopic data—data regarding endoscopic diagnosis and endoscopic intervention were collected. Endoscopic intervention was defined as the use of at least one or a combination of the following interventions—band ligation, adrenaline injection, hemoclip application and/or coaptive coagulation. All EGD were performed by a trained gastroenterologist or physicians in their GI training under the supervision of a senior gastroenterologist.
2.3. Study Aim
2.4. Data Analysis
2.5. Statistical Analysis
2.6. Study Ethics and Patient Consent
3. Results
3.1. Patient Characteristics
3.2. Univariate Analysis of Risk Factors Associated with Endoscopic Intervention
3.2.1. Background Diagnosis and Clinical Features and Endoscopy Timing
3.2.2. Correlation between Medical Therapies and Endoscopic Intervention
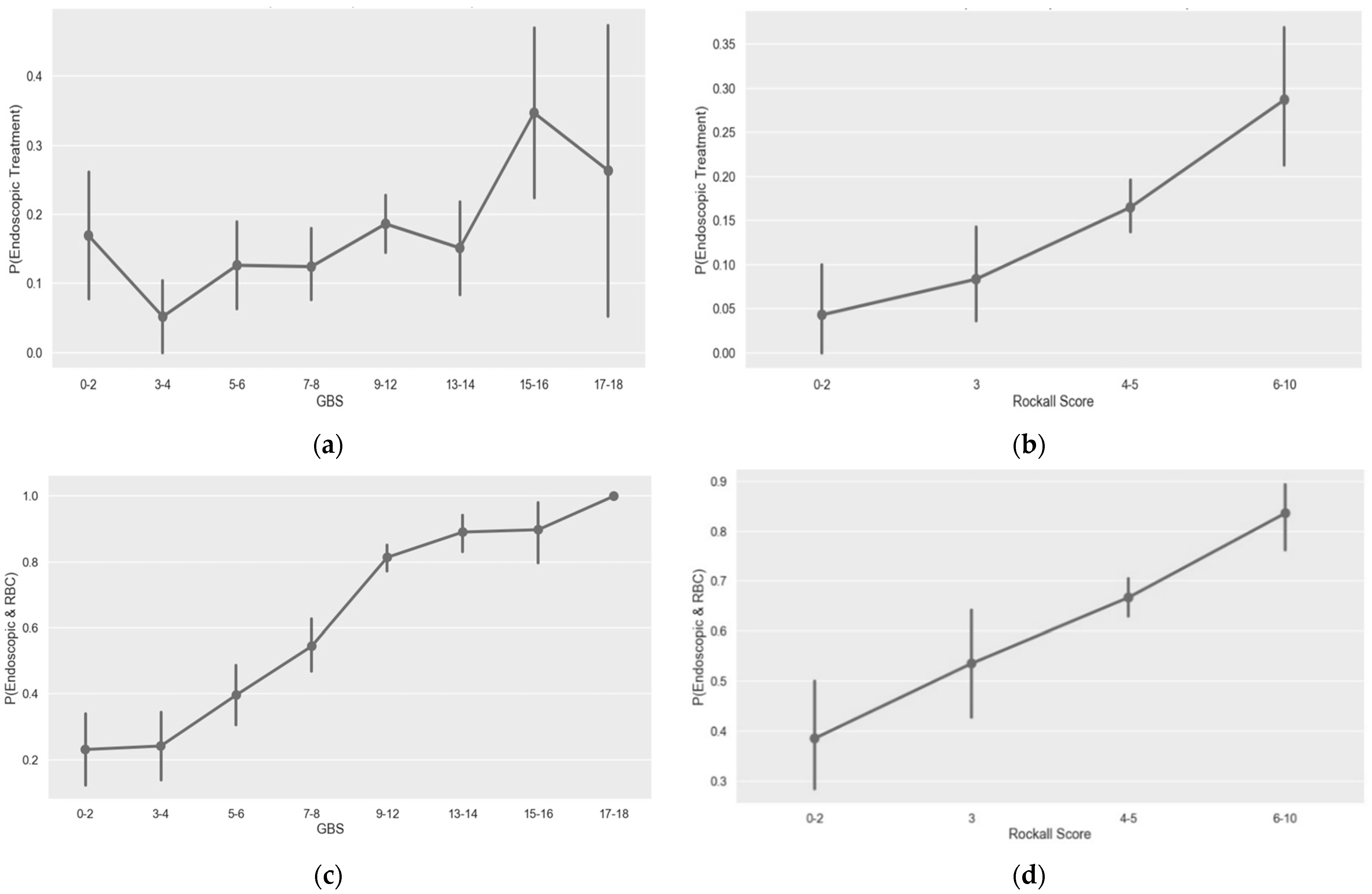
3.3. GBS and Pre-Endoscopic Rockall Score Performance for Prediction of Endoscopic Intervention
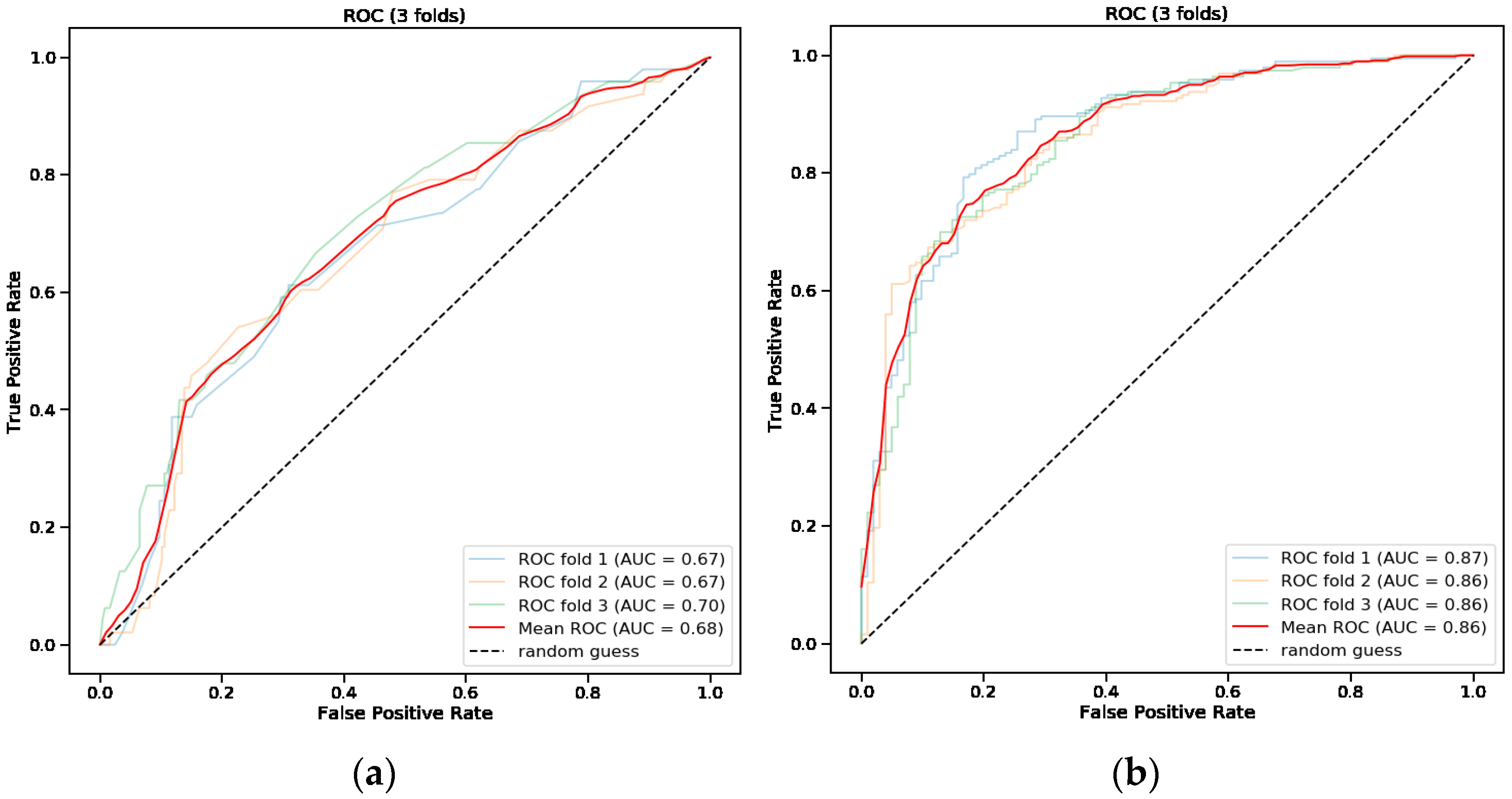
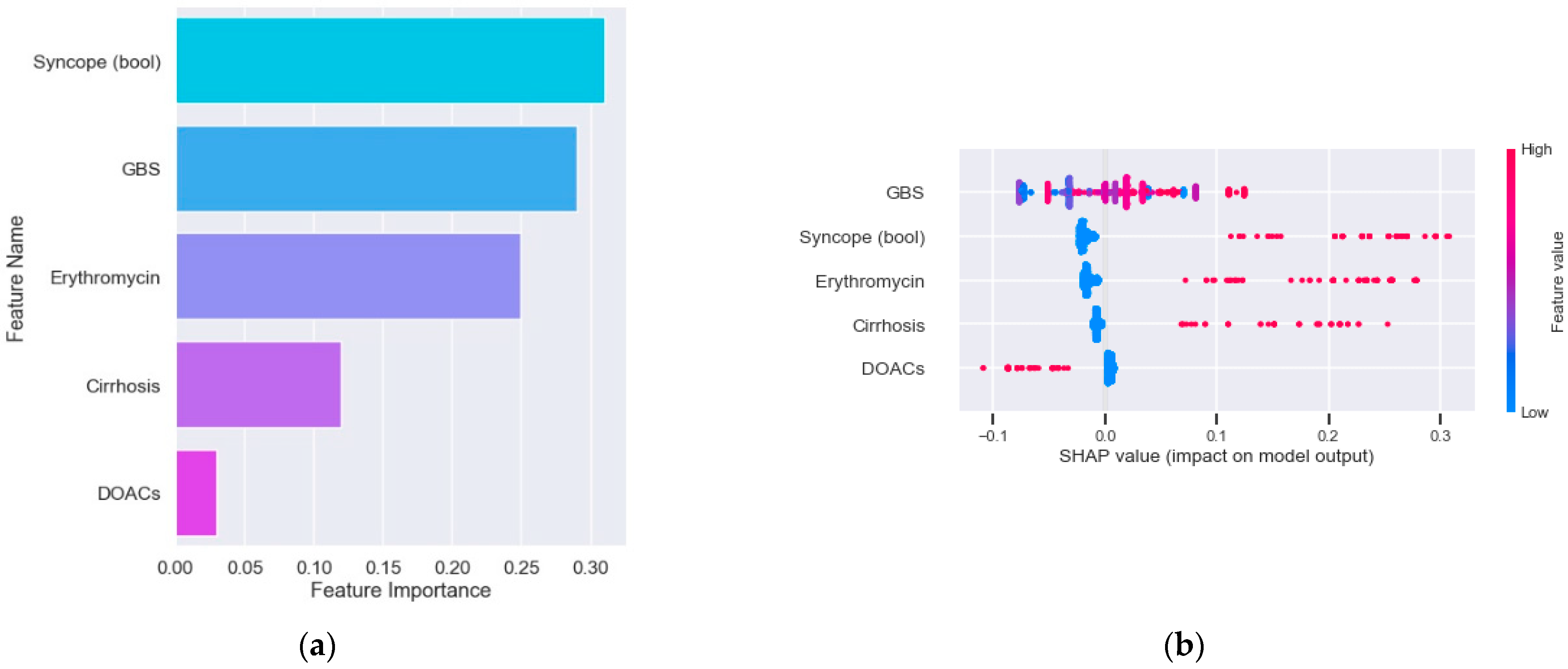
3.4. Predictive Model for Endoscopic Intervention
3.5. Predictive Model for Endoscopic Intervention and Blood Transfusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shung, D.L.; Au, B.; Taylor, R.A.; Tay, J.K.; Laursen, S.B.; Stanley, A.J.; Dalton, H.R.; Ngu, J.; Schultz, M.; Laine, L. Validation of a Machine Learning Model That Outperforms Clinical Risk Scoring Systems for Upper Gastrointestinal Bleeding. Gastroenterology 2020, 158, 160–167. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Stanley, A.J.; Morris, A.J.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.B.; Radaelli, F.; Papanikolaou, I.S.; Gonçalves, T.C.; et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 300–332. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, J.G.; Ebigbo, A.; Bhat, P.; Dinis-Ribeiro, M.; Gralnek, I.; Guy, C.; Le Moine, O.; Vilmann, P.; Antonelli, G.; Ijoma, U.; et al. Endoscopic treatment of variceal upper gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Cascade Guideline. Endosc. Int. Open 2020, 8, E990–E997. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Moon, W. Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: An evidence-based review. World J. Gastrointest. Endosc. 2019, 11, 68–83. [Google Scholar] [CrossRef]
- Hearnshaw, S.A.; Logan, R.F.; Lowe, D.; Travis, S.P.; Murphy, M.F.; Palmer, K.R. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: Results of a nationwide audit. Gut 2010, 59, 1022–1029. [Google Scholar] [CrossRef]
- Sacks, H.S.; Chalmers, T.C.; Blum, A.L.; Berrier, J.; Pagano, D. Endoscopic hemostasis. An effective therapy for bleeding peptic ulcers. JAMA 1990, 264, 494–499. [Google Scholar] [CrossRef]
- Barkun, A.N.; Bardou, M.; Kuipers, E.J.; Sung, J.J.Y.; Hunt, R.H.; Martel, M.; Sinclair, P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann. Intern. Med. 2010, 152, 101–113. [Google Scholar] [CrossRef]
- Blatchford, O.; Murray, W.R.; Blatchford, M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000, 356, 1318–1321. [Google Scholar] [CrossRef]
- Rockall, T.A.; Logan, R.F.; Devlin, H.B.; Northfield, T.C. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996, 38, 316–321. [Google Scholar] [CrossRef]
- Stanley, A.J.; Laine, L.; Dalton, H.R.; Ngu, J.H.; Schultz, M.; Abazi, R.; Zakko, L.; Thornton, S.; Wilkinson, K.; Khor, C.J.L.; et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: International multicentre prospective study. BMJ 2017, 356, i6432. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Hoversten, P.; Leggett, C.L. Upper Gastrointestinal Bleeding: Etiologies and Management. Mayo Clin. Proc. 2019, 94, 697–703. [Google Scholar] [CrossRef]
- Abougergi, M.S. Epidemiology of Upper Gastrointestinal Hemorrhage in the USA: Is the Bleeding Slowing Down? Dig. Dis. Sci. 2018, 63, 1091–1093. [Google Scholar] [CrossRef]
- Saltzman, J.R.; Tabak, Y.P.; Hyett, B.H.; Sun, X.; Travis, A.C.; Johannes, R.S. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest. Endosc. 2011, 74, 1215–1224. [Google Scholar] [CrossRef]
- Jairath, V.; Martel, M.; Logan, R.F.; Barkun, A.N. Why do mortality rates for nonvariceal upper gastrointestinal bleeding differ around the world? A systematic review of cohort studies. Can. J. Gastroenterol. 2012, 26, 537–543. [Google Scholar] [CrossRef]
- Loperfido, S.; Baldo, V.; Piovesana, E.; Bellina, L.; Rossi, K.; Groppo, M.; Caroli, A.; Bò, N.D.; Monica, F.; Fabris, L.; et al. Changing trends in acute upper-GI bleeding: A population-based study. Gastrointest. Endosc. 2009, 70, 212–224. [Google Scholar] [CrossRef]
- Oakland, K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol. 2019, 42–43, 101610. [Google Scholar] [CrossRef]
- Cook, D.J.; Guyatt, G.H.; Salena, B.J.; Laine, L.A. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology 1992, 102, 139–148. [Google Scholar] [CrossRef]
- Bryant, R.V.; Kuo, P.; Williamson, K.; Yam, C.; Schoeman, M.N.; Holloway, R.H.; Nguyen, N.Q. Performance of the Glasgow-Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest. Endosc. 2013, 78, 576–583. [Google Scholar] [CrossRef]
- Rahman, R.; Nguyen, D.L.; Sohail, U.; Almashhrawi, A.A.; Ashraf, I.; Puli, S.R.; Bechtold, M.L. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: An updated meta-analysis and systematic review. Ann. Gastroenterol. 2016, 29, 312–317. [Google Scholar] [CrossRef]
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am. J. Gastroenterol. 2021, 116, 899–917. [Google Scholar] [CrossRef]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; Al-Samkari, H.; DeLoughery, T.G.; Shatzel, J.J. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur. J. Haematol. 2020, 104, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, H.-I.T. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): An international randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 1927–1936. [Google Scholar] [CrossRef]
- Toya, Y.; Nakamura, S.; Tomita, K.; Matsuda, N.; Abe, K.; Abiko, Y.; Orikasa, S.; Akasaka, R.; Chiba, T.; Uesugi, N.; et al. Dabigatran-induced esophagitis: The prevalence and endoscopic characteristics. J. Gastroenterol. Hepatol. 2016, 31, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Moledina, S.M.; Komba, E. Risk factors for mortality among patients admitted with upper gastrointestinal bleeding at a tertiary hospital: A prospective cohort study. BMC Gastroenterol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, K.U.; Kim, S.J.; Seo, S.I.; Kim, H.S.; Jang, M.K.; Kim, H.Y.; Shin, W.G. Predictors for the need for endoscopic therapy in patients with presumed acute upper gastrointestinal bleeding. Korean J. Intern. Med. 2019, 34, 288–295. [Google Scholar] [CrossRef]
- Haddad, F.G.; El Imad, T.; Nassani, N.; Kwok, R.; Al Moussawi, H.; Polavarapu, A.; Ahmed, M.; El Douaihy, Y.; Deeb, L. In-hospital acute upper gastrointestinal bleeding: What is the scope of the problem? World J. Gastrointest. Endosc. 2019, 11, 561–572. [Google Scholar] [CrossRef]
- Hearnshaw, S.A.; Logan, R.F.; Lowe, D.; Travis, S.P.; Murphy, M.F.; Palmer, K.R. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011, 60, 1327–1335. [Google Scholar] [CrossRef]
| Parameters | Total N (%)/Median (IQR) 883 Patients | Endoscopic Intervention N (%)/Median (IQR) 145 Patients | No Endoscopic Intervention N (%)/Median (IQR) 738 Patients | p-Value | |
|---|---|---|---|---|---|
| Number of patients (%) | 883 (100%) | 145 (16.4%) | 738 (83.6%) | ||
| Age (median, IQR) | 69.0 (58.0–79.0) | 68.0 (57.0–75.0) | 69.0 (59.0–80.0) | 0.14 | |
| Male gender (N, %) | 552 (62.5%) | 90 (62.1%) | 462 (62.6%) | 0.97 | |
| Time to endoscopy (hours)-(median, IQR) | 16.0 (5.7–24.03) | 6.8 (3.17–16.37) | 17.0 (8.6–24.96) | <0.01 | |
| Pre endoscopy Rockall Score (mean ± SD) | 4.2 ± 1.4 | 4.7 ± 1.3 | 4.1 ± 1.4 | <0.01 | |
| GBS (median, IQR) | 9.0 (6.0–12.0) | 11.0 (8.0–13.0) | 9.0 (6.0–12.0) | <0.01 | |
| Pre- endoscopy treatment (N, %) | Erythromycin | 66 (7.5%) | 25 (17.2%) | 41 (5.6%) | <0.01 |
| TXA | 292 (33.1%) | 63 (43.4%) | 229 (31.0%) | <0.01 | |
| Fluids | 433 (49.0%) | 77 (53.1%) | 356 (48.2%) | 0.32 | |
| PPI | 781 (88.4%) | 132 (91.0%) | 649 (87.9%) | 0.35 | |
| PCC | 15 (1.7%) | 4 (2.8%) | 11 (1.5%) | 0.46 | |
| Vitamin K | 90 (10.2%) | 17 (11.7%) | 73 (9.9%) | 0.60 | |
| VKA | 68 (7.7%) | 10 (6.9%) | 58 (7.9%) | 0.82 | |
| Chronic treatment (N, %) | DOACs | 47 (5.3%) | 3 (2.1%) | 44 (6.0%) | 0.08 |
| P2Y12 inhibitors | 95 (10.8%) | 18 (12.4%) | 77 (10.4%) | 0.57 | |
| Acetylsalicylic acid | 269 (30.5%) | 47 (32.4%) | 222 (30.1%) | 0.64 | |
| Enoxaparin | 42 (4.8%) | 7 (4.8%) | 35 (4.7%) | 0.86 | |
| INR (median, IQR) | 1.09 (0.98–1.27) | 1.13 (0.99–1.32) | 1.08 (0.98–1.26) | 0.12 | |
| HGB (median, IQR) | 9.25 (7.5–11.2) | 8.89 (7.31–10.85) | 9.34 (7.5–11.27) | 0.22 | |
| Heart rate (median, IQR) | 89.0 (76.0–100.0) | 92.0 (77.0–102.0) | 88.0 (76.0–100.0) | 0.07 | |
| MAP (median, IQR) | 86.33 (75.67–95.33) | 84.67 (74.0–95.33) | 87.0 (76.33–95.58) | 0.17 | |
| Syncope (N, %) | 68 (7.7%) | 28 (19.3%) | 40 (5.4%) | <0.01 | |
| Cirrhosis (N, %) | 41 (4.6%) | 13 (9.0%) | 28 (3.8%) | 0.01 | |
| Cardiac arrhythmia (N, %) | 84 (9.5%) | 18 (12.4%) | 66 (8.9%) | 0.25 | |
| CHF (N, %) | 95 (10.8%) | 18 (12.4%) | 77 (10.4%) | 0.57 | |
| IHD (N, %) | 144 (16.3%) | 21 (14.5%) | 123 (16.7%) | 0.59 | |
| Renal failure (N, %) | 56 (6.3%) | 11 (7.6%) | 45 (6.1%) | 0.62 | |
| COPD (N, %) | 26 (2.9%) | 3 (2.1%) | 23 (3.1%) | 0.67 | |
| HTN (N, %) | 307 (34.8%) | 53 (36.6%) | 254 (34.4%) | 0.69 | |
| DM (N, %) | 202 (22.9%) | 31 (21.4%) | 171 (23.2%) | 0.71 | |
| Cardiac valvular disease (N, %) | 55 (6.2%) | 9 (6.2%) | 46 (6.2%) | 0.86 | |
| Asthma (N, %) | 26 (2.9%) | 5 (3.4%) | 21 (2.8%) | 0.90 | |
| Melena (bool) (N, %) | 556 (63.0%) | 92 (63.4%) | 464 (62.9%) | 0.97 | |
| DVT (N, %) | 15 (1.7%) | 2 (1.4%) | 13 (1.8%) | 0.97 | |
| Stroke (N, %) | 15 (1.7%) | 2 (1.4%) | 13 (1.8%) | 0.97 | |
| Glasgow-Blatchford Score | Pre-Endoscopic Rockall Score | New Modified Model * | |
|---|---|---|---|
| AUC | 0.54 | 0.56 | 0.68 |
| TPR (sensitivity) | 0.81 | 0.24 | 0.55 |
| TNR (specificity) | 0.28 | 0.88 | 0.71 |
| PPV | 0.18 | 0.29 | 0.27 |
| NPV | 0.88 | 0.86 | 0.89 |
| Glasgow-Blatchford Score | Pre-Endoscopic Rockall Score | New Modified Model * | |
|---|---|---|---|
| AUC | 0.70 | 0.56 | 0.86 |
| TPR (sensitivity) | 0.87 | 0.18 | 0.77 |
| TNR (specificity) | 0.53 | 0.93 | 0.79 |
| PPV | 0.78 | 0.84 | 0.88 |
| NPV | 0.69 | 0.37 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veisman, I.; Oppenheim, A.; Maman, R.; Kofman, N.; Edri, I.; Dar, L.; Klang, E.; Sina, S.; Gabriely, D.; Levy, I.; et al. A Novel Prediction Tool for Endoscopic Intervention in Patients with Acute Upper Gastro-Intestinal Bleeding. J. Clin. Med. 2022, 11, 5893. https://doi.org/10.3390/jcm11195893
Veisman I, Oppenheim A, Maman R, Kofman N, Edri I, Dar L, Klang E, Sina S, Gabriely D, Levy I, et al. A Novel Prediction Tool for Endoscopic Intervention in Patients with Acute Upper Gastro-Intestinal Bleeding. Journal of Clinical Medicine. 2022; 11(19):5893. https://doi.org/10.3390/jcm11195893
Chicago/Turabian StyleVeisman, Ido, Amit Oppenheim, Ronny Maman, Nadav Kofman, Ilan Edri, Lior Dar, Eyal Klang, Sigal Sina, Daniel Gabriely, Idan Levy, and et al. 2022. "A Novel Prediction Tool for Endoscopic Intervention in Patients with Acute Upper Gastro-Intestinal Bleeding" Journal of Clinical Medicine 11, no. 19: 5893. https://doi.org/10.3390/jcm11195893
APA StyleVeisman, I., Oppenheim, A., Maman, R., Kofman, N., Edri, I., Dar, L., Klang, E., Sina, S., Gabriely, D., Levy, I., Beylin, D., Beylin, O., Shekel, E., Horesh, N., & Kopylov, U. (2022). A Novel Prediction Tool for Endoscopic Intervention in Patients with Acute Upper Gastro-Intestinal Bleeding. Journal of Clinical Medicine, 11(19), 5893. https://doi.org/10.3390/jcm11195893






