Early Epilepsy Surgery in Benign Cerebral Tumors: Avoid Your ‘Low-Grade’ Becoming a ‘Long-Term’ Epilepsy-Associated Tumor
Abstract
1. Introduction
2. Patients and Methods
2.1. Neuroimaging
2.2. Statistical Analysis
3. Results
3.1. Seizure Outcome
3.2. Neuropsychological Outcome
4. Discussion
4.1. Predictors of Seizure Freedom
4.2. Cognitive Decline in Patients with LEAT and Improvement following Epilepsy Surgery
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.G.; Pfafflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Leenstra, S.; van Veelen, C.W.; van Rijen, P.C.; Hulsebos, T.J.; Tersmette, A.C.; Yankaya, B.; Troost, D. Glioneuronal tumors and medically intractable epilepsy: A clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy Res. 2001, 43, 179–191. [Google Scholar] [CrossRef]
- Lamberink, H.J.; Otte, W.M.; Blumcke, I.; Braun, K.P.J.; European Epilepsy Brain Bank writing group; Study Group; European Reference Network EpiCARE. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: A retrospective multicentre cohort study. Lancet Neurol. 2020, 19, 748–757. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Abraham, M.; Vilanilam, G.; Menon, R.; Menon, D.; Kumar, H.; Cherian, A.; Radhakrishnan, N.; Kesavadas, C.; Thomas, B.; et al. Surgery for “Long-term epilepsy associated tumors (LEATs)”: Seizure outcome and its predictors. Clin. Neurol. Neurosurg. 2016, 141, 98–105. [Google Scholar] [CrossRef]
- Luyken, C.; Blumcke, I.; Fimmers, R.; Urbach, H.; Elger, C.E.; Wiestler, O.D.; Schramm, J. The spectrum of long-term epilepsy-associated tumors: Long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia 2003, 44, 822–830. [Google Scholar] [CrossRef]
- Giulioni, M.; Marucci, G.; Pelliccia, V.; Gozzo, F.; Barba, C.; Didato, G.; Villani, F.; Di Gennaro, G.; Quarato, P.P.; Esposito, V.; et al. Epilepsy surgery of “low grade epilepsy associated neuroepithelial tumors”: A retrospective nationwide Italian study. Epilepsia 2017, 58, 1832–1841. [Google Scholar] [CrossRef]
- Thom, M.; Blumcke, I.; Aronica, E. Long-term epilepsy-associated tumors. Brain Pathol. 2012, 22, 350–379. [Google Scholar] [CrossRef]
- Englot, D.J.; Berger, M.S.; Barbaro, N.M.; Chang, E.F. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia 2012, 53, 51–57. [Google Scholar] [CrossRef]
- Blumcke, I.; Aronica, E.; Becker, A.; Capper, D.; Coras, R.; Honavar, M.; Jacques, T.S.; Kobow, K.; Miyata, H.; Muhlebner, A.; et al. Low-grade epilepsy-associated neuroepithelial tumours—The 2016 WHO classification. Nat. Rev. Neurol. 2016, 12, 732–740. [Google Scholar] [CrossRef]
- Blumcke, I.; Aronica, E.; Urbach, H.; Alexopoulos, A.; Gonzalez-Martinez, J.A. A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol. 2014, 128, 39–54. [Google Scholar] [CrossRef]
- Slegers, R.J.; Blumcke, I. Low-grade developmental and epilepsy associated brain tumors: A critical update 2020. Acta Neuropathol. Commun. 2020, 8, 27. [Google Scholar] [CrossRef]
- Sherman, E.M.; Wiebe, S.; Fay-McClymont, T.B.; Tellez-Zenteno, J.; Metcalfe, A.; Hernandez-Ronquillo, L.; Hader, W.J.; Jette, N. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia 2011, 52, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; Thompson, P. Red flags in epilepsy surgery: Identifying the patients who pay a high cognitive price for an unsuccessful surgical outcome. Epilepsy Behav. 2018, 78, 269–272. [Google Scholar] [CrossRef]
- Rosenow, F.; Bast, T.; Czech, T.; Feucht, M.; Hans, V.H.; Helmstaedter, C.; Huppertz, H.J.; Noachtar, S.; Oltmanns, F.; Polster, T.; et al. Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the Austrian, German, and Swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment. Epilepsia 2016, 57, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Conradi, N.; Hermsen, A.; Krause, K.; Gorny, I.; Strzelczyk, A.; Knake, S.; Rosenow, F. Hemispheric language lateralization in presurgical patients with temporal lobe epilepsy: Improving the retest reliability of functional transcranial Doppler sonography. Epilepsy Behav. 2019, 91, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Wieser, H.G.; Blume, W.T.; Fish, D.; Goldensohn, E.; Hufnagel, A.; King, D.; Sperling, M.R.; Luders, H.; Pedley, T.A.; Commission on Neurosurgery of the International League Against Epilepsy. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001, 42, 282–286. [Google Scholar] [CrossRef]
- Brückner, K. Standard der neuropsychologischen Testung in der prächirurgischen Epilepsiediagnostik. Zeitschrift für Epileptologie 2012, 25, 259–263. [Google Scholar] [CrossRef]
- Conradi, N.; Behrens, M.; Kannemann, T.; Merkel, N.; Strzelczyk, A.; Reif, P.S.; Rosenow, F.; Hermsen, A. Factorial validity of a neuropsychological test battery and its ability to discern temporal lobe epilepsy from frontal lobe epilepsy—A retrospective study. Seizure 2020, 74, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, S.R.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Devinsky, O.; Vickrey, B.G.; Cramer, J.; Perrine, K.; Hermann, B.; Meador, K.; Hays, R.D. Development of the quality of life in epilepsy inventory. Epilepsia 1995, 36, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.; Conradi, N.; Freiman, T.M.; Spyrantis, A.; Konczalla, J.; Hattingen, E.; Wagner, M.; Harter, P.N.; Mueller, M.; Leyer, A.C.; et al. Postoperative outcomes and surgical ratio at a newly established epilepsy center: The first 100 procedures. Epilepsy Behav. 2021, 116, 107715. [Google Scholar] [CrossRef]
- Kofler, F.; Berger, C.; Waldmannstetter, D.; Lipkova, J.; Ezhov, I.; Tetteh, G.; Kirschke, J.; Zimmer, C.; Wiestler, B.; Menze, B.H. BraTS Toolkit: Translating BraTS Brain Tumor Segmentation Algorithms Into Clinical and Scientific Practice. Front. Neurosci. 2020, 14, 125. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jiang, H.; Wu, H.; Ding, Y.; Wang, S.; Ming, W.; Zhu, J. Epilepsy surgery for low-grade epilepsy-associated neuroepithelial tumor of temporal lobe: A single-institution experience of 61 patients. Neurol. Sci. 2022, 43, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Chang, E.F. Rates and predictors of seizure freedom in resective epilepsy surgery: An update. Neurosurg. Rev. 2014, 37, 389–404; discussion 385–404. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, M.; Rubboli, G.; Marucci, G.; Martinoni, M.; Volpi, L.; Michelucci, R.; Marliani, A.F.; Bisulli, F.; Tinuper, P.; Castana, L.; et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: Lesionectomy compared with tailored resection. J. Neurosurg. 2009, 111, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Faramand, A.M.; Barnes, N.; Harrison, S.; Gunny, R.; Jacques, T.; Tahir, M.Z.; Varadkar, S.M.; Cross, H.J.; Harkness, W.; Tisdall, M.M. Seizure and cognitive outcomes after resection of glioneuronal tumors in children. Epilepsia 2018, 59, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Devaux, B.; Chassoux, F.; Landre, E.; Turak, B.; Laurent, A.; Zanello, M.; Mellerio, C.; Varlet, P. Surgery for dysembryoplastic neuroepithelial tumors and gangliogliomas in eloquent areas. Functional results and seizure control. Neurochirurgie 2017, 63, 227–234. [Google Scholar] [CrossRef]
- Roessler, K.; Heynold, E.; Buchfelder, M.; Stefan, H.; Hamer, H.M. Current value of intraoperative electrocorticography (iopECoG). Epilepsy Behav. 2019, 91, 20–24. [Google Scholar] [CrossRef]
- Robertson, F.C.; Ullrich, N.J.; Manley, P.E.; Al-Sayegh, H.; Ma, C.; Goumnerova, L.C. The Impact of Intraoperative Electrocorticography on Seizure Outcome After Resection of Pediatric Brain Tumors: A Cohort Study. Neurosurgery 2019, 85, 375–383. [Google Scholar] [CrossRef]
- Yao, P.S.; Zheng, S.F.; Wang, F.; Kang, D.Z.; Lin, Y.X. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J. Neurosurg. 2018, 128, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Ou, S.; Song, T.; Hu, J.; You, L.; Wang, Y.; Wang, Y. Intraoperative electrocorticography-guided microsurgical management for patients with onset of supratentorial neoplasms manifesting as epilepsy: A review of 65 cases. Epileptic Disord. 2014, 16, 175–184. [Google Scholar] [CrossRef]
- Thom, M.; Toma, A.; An, S.; Martinian, L.; Hadjivassiliou, G.; Ratilal, B.; Dean, A.; McEvoy, A.; Sisodiya, S.M.; Brandner, S. One hundred and one dysembryoplastic neuroepithelial tumors: An adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J. Neuropathol. Exp. Neurol. 2011, 70, 859–878. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.Y.; Kim, S.H.; Jang, J.; Kim, H.; Han, S.; Lim, J.S.; Son, G.; Choi, J.; Park, B.O.; Heo, W.D.; et al. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat. Med. 2018, 24, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Conradi, N.; Behrens, M.; Hermsen, A.M.; Kannemann, T.; Merkel, N.; Schuster, A.; Freiman, T.M.; Strzelczyk, A.; Rosenow, F. Assessing Cognitive Change and Quality of Life 12 Months After Epilepsy Surgery-Development and Application of Reliable Change Indices and Standardized Regression-Based Change Norms for a Neuropsychological Test Battery in the German Language. Front. Psychol. 2020, 11, 582836. [Google Scholar] [CrossRef]
- Vogt, V.L.; Witt, J.A.; Delev, D.; Grote, A.; von Lehe, M.; Becker, A.J.; Schramm, J.; Elger, C.E.; Helmstaedter, C. Cognitive features and surgical outcome of patients with long-term epilepsy-associated tumors (LEATs) within the temporal lobe. Epilepsy Behav. 2018, 88, 25–32. [Google Scholar] [CrossRef]
- Ko, A.; Kim, S.H.; Kim, S.H.; Park, E.K.; Shim, K.W.; Kang, H.C.; Kim, D.S.; Kim, H.D.; Lee, J.S. Epilepsy Surgery for Children With Low-Grade Epilepsy-Associated Tumors: Factors Associated With Seizure Recurrence and Cognitive Function. Pediatr. Neurol. 2019, 91, 50–56. [Google Scholar] [CrossRef]
- Ramantani, G.; Kadish, N.E.; Anastasopoulos, C.; Brandt, A.; Wagner, K.; Strobl, K.; Mayer, H.; Schubert-Bast, S.; Stathi, A.; Korinthenberg, R.; et al. Epilepsy surgery for glioneuronal tumors in childhood: Avoid loss of time. Neurosurgery 2014, 74, 648–657; discussion 657. [Google Scholar] [CrossRef]
- Van Breemen, M.S.; Wilms, E.B.; Vecht, C.J. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007, 6, 421–430. [Google Scholar] [CrossRef]
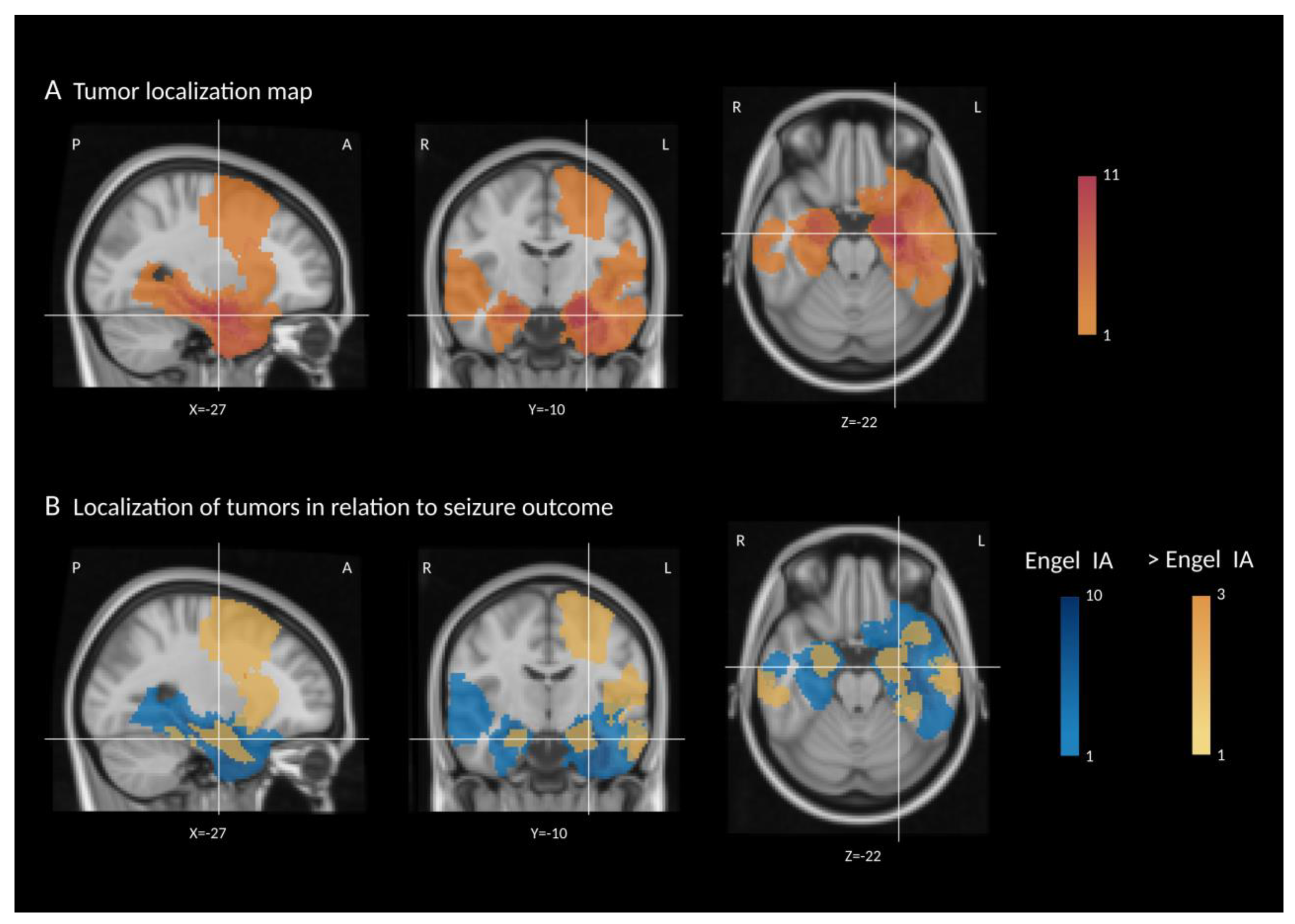
| Patient No | Sex | Age | Age at Onset | Epilepsy Duration | Side Left = 1 Right = 0 | Temporal Lobe Epilepsy 1 = Yes 0 = No | FBTCS before Surgery 1 = Yes 0 = No | Histopathology GG = 1 DNET = 2 Other = 3 | No of ASM, Total | No of ASM, at Time of Surgery | ASM after Surgery None = 1, Reduced = 2 Unchanged = 0 | Complete Resection According to Post-Op MRI 1 = Yes 0 = No | SW in 6-Months Follow Up EEG 1 = Yes 0 = No | Engel/ILAE Most Recent Visit | Follow Up (Months) | Tumor Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 4 | 3 | 1 | 0 | 1 | 0 | 2 | 4 | 2 | 2 | 0 | 1 | IA/1 | 48 | 38,934 |
| 2 | f | 6 | 2 | 4 | 1 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | IA/1 | 24 | 6532 |
| 3 | m | 6 | 5 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | IA/1 | 24 | 89,092 |
| 4 | f | 7 | 5 | 2 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 1 | 0 | IA/1 | 40 | 3842 |
| 5 | f | 8 | 8 | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 0 | IA/1 | 18 | 2852 |
| 6 | m | 9 | 8 | 2 | 1 | 1 | 0 | 2 | 4 | 2 | 2 | 1 | 0 | IA/1 | 20 | 18,484 |
| 7 | f | 12 | 11 | 2 | 1 | 0 | 1 | 2 | 3 | 2 | 1 | 0 | 0 | IA/1 | 48 | 5003 |
| 8 | m | 12 | 13 | 0 | 0 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 0 | IA/1 | 12 | 20,936 |
| 9 | f | 12 | 7 | 5 | 1 | 0 | 0 | 1 | 5 | 2 | 0 | 0 | 1 | IIIA/4 | 12 | 53,120 |
| 10 | f | 13 | 3 | 11 | 0 | 0 | 0 | 2 | 3 | 2 | 1 | 1 | 1 | IA/1 | 36 | 7909 |
| 11 | m | 14 | 8 | 6 | 1 | 1 | 0 | 1 | 3 | 2 | 2 | 1 | 0 | IA/1 | 12 | 1494 |
| 12 | m | 15 | 15 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | IA/1 | 24 | 46,627 |
| 13 | m | 16 | 14 | 3 | 1 | 1 | 1 | 1 | 6 | 2 | 1 | 1 | 0 | IA/1 | 24 | 4367 |
| 14 | m | 16 | 15 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | ID/3 | 24 | 1336 |
| 15 | f | 16 | 14 | 2 | 1 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 0 | IA/1 | 30 | 6991 |
| 16 | f | 16 | 14 | 2 | 1 | 0 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | ID/3 | 18 | 49,102 |
| 17 | m | 17 | 14 | 3 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | IA/1 | 60 | 8196 |
| 18 | m | 17 | 1 | 16 | 1 | 1 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | IB/2 | 6 | 9135 |
| 19 | m | 18 | 15 | 3 | 1 | 1 | 1 | 1 | 6 | 2 | 0 | 1 | 1 | IIA/3 | 48 | 4259 |
| 20 | m | 18 | 14 | 3 | 1 | 1 | 0 | 1 | 3 | 1 | 0 | 1 | 0 | IB/2 | 48 | 159 |
| 21 | m | 19 | 11 | 8 | 1 | 1 | 1 | 1 | 5 | 2 | 1 | 0 | 0 | IA/1 | 60 | 6836 |
| 22 | f | 19 | 7 | 12 | 0 | 0 | 1 | 2 | 5 | 2 | 2 | 0 | 0 | IA/1 | 36 | 8875 |
| 23 | m | 20 | 14 | 7 | 1 | 1 | 0 | 1 | 4 | 2 | 1 | 1 | 1 | IA/1 | 48 | 21,044 |
| 24 | f | 20 | 13 | 7 | 0 | 1 | 1 | 1 | 5 | 3 | 2 | 1 | 0 | IA/1 | 30 | 4061 |
| 25 | f | 22 | 17 | 6 | 1 | 1 | 0 | 1 | 4 | 2 | 1 | 0 | 0 | IA/1 | 42 | 3404 |
| 26 | f | 22 | 17 | 5 | 1 | 1 | 1 | 2 | 7 | 2 | 1 | 1 | 0 | IA/1 | 60 | 10,893 |
| 27 | m | 23 | 19 | 4 | 0 | 1 | 0 | 1 | 5 | 2 | 2 | 1 | 0 | IA/1 | 36 | 923 |
| 28 | f | 24 | 17 | 7 | 0 | 1 | 0 | 1 | 4 | 1 | 0 | 1 | 0 | IIA/3 | 36 | 3099 |
| 29 | f | 25 | 2 | 2 | 0 | 1 | 1 | 3 | 8 | 3 | 2 | 1 | 0 | IA/1 | 60 | 1228 |
| 30 | m | 26 | 9 | 17 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | IB/2 | 24 | 3859 |
| 31 | m | 28 | 16 | 12 | 1 | 1 | 0 | 1 | 3 | 2 | 0 | 1 | 0 | IA/1 | 24 | 29,233 |
| 32 | f | 30 | 22 | 8 | 1 | 1 | 1 | 1 | 4 | 2 | 2 | 1 | 0 | IA/1 | 24 | 1054 |
| 33 | m | 31 | 15 | 10 | 1 | 1 | 1 | 1 | 4 | 2 | 2 | 1 | 0 | IA/1 | 36 | 11,003 |
| 34 | f | 33 | 21 | 13 | 0 | 1 | 0 | 2 | 3 | 2 | 2 | 1 | 0 | IA/1 | 36 | 2753 |
| 35 | m | 40 | 15 | 25 | 1 | 1 | 1 | 2 | 8 | 2 | 0 | 1 | 0 | IA/1 | 12 | 4635 |
| Mdn = 17 | Md = 14 | Mdn = 4 | left: 23 | total n = 27 | total n = 19 | Mdn = 4 | Mdn = 2 | unchanged = 8 | total n = 27 | total n = 6 | n = 27 IA/1 | Mdn = 30 | Mdn = 6532 |
| Cognitive Domains Assessed | Before Surgery | After Surgery | p-Value |
|---|---|---|---|
| Attentional functions (n = 21) | 22.9% | 14.3% | 0.102 |
| Verbal memory (n = 23) | 14.3% | 17.1% | 0.414 |
| Nonverbal memory (n = 23) | 45.7% | 17.1% | 0.011 * |
| Executive functions (n = 23) | 25.7% | 20.0% | 0.999 |
| OVERALL relevant deficits in one or more domains (n = 28) | 65.7% | 51.4% | 0.564 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, C.; Conradi, N.; Neuhaus, E.; Konczalla, J.; Freiman, T.M.; Spyrantis, A.; Weber, K.; Harter, P.; Rosenow, F.; Strzelczyk, A.; et al. Early Epilepsy Surgery in Benign Cerebral Tumors: Avoid Your ‘Low-Grade’ Becoming a ‘Long-Term’ Epilepsy-Associated Tumor. J. Clin. Med. 2022, 11, 5892. https://doi.org/10.3390/jcm11195892
Mann C, Conradi N, Neuhaus E, Konczalla J, Freiman TM, Spyrantis A, Weber K, Harter P, Rosenow F, Strzelczyk A, et al. Early Epilepsy Surgery in Benign Cerebral Tumors: Avoid Your ‘Low-Grade’ Becoming a ‘Long-Term’ Epilepsy-Associated Tumor. Journal of Clinical Medicine. 2022; 11(19):5892. https://doi.org/10.3390/jcm11195892
Chicago/Turabian StyleMann, Catrin, Nadine Conradi, Elisabeth Neuhaus, Jürgen Konczalla, Thomas M. Freiman, Andrea Spyrantis, Katharina Weber, Patrick Harter, Felix Rosenow, Adam Strzelczyk, and et al. 2022. "Early Epilepsy Surgery in Benign Cerebral Tumors: Avoid Your ‘Low-Grade’ Becoming a ‘Long-Term’ Epilepsy-Associated Tumor" Journal of Clinical Medicine 11, no. 19: 5892. https://doi.org/10.3390/jcm11195892
APA StyleMann, C., Conradi, N., Neuhaus, E., Konczalla, J., Freiman, T. M., Spyrantis, A., Weber, K., Harter, P., Rosenow, F., Strzelczyk, A., & Schubert-Bast, S. (2022). Early Epilepsy Surgery in Benign Cerebral Tumors: Avoid Your ‘Low-Grade’ Becoming a ‘Long-Term’ Epilepsy-Associated Tumor. Journal of Clinical Medicine, 11(19), 5892. https://doi.org/10.3390/jcm11195892








