Clinical Validation of the Shock Index, Modified Shock Index, Delta Shock Index, and Shock Index-C for Emergency Department ST-Segment Elevation Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables and Outcome Measures
2.3. Data Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. Association between the SI, MSI, Delta-SI, SIC, TIMI Risk Scales and Patients’ Outcome
3.3. Odds Ratio Using Cutoff Values of the SI, MSI, SIC, and TIMI Risk Scales
3.4. Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive for the SI, MSI, SIC, and TIMI Risk Scales
4. Discussion
5. Conclusions
6. Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Executive summary: Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef]
- Krishnan, U.; Brejt, J.A.; Schulman-Marcus, J.; Swaminathan, R.V.; Feldman, D.N.; Goyal, P.; Wong, S.C.; Minutello, R.M.; Bergman, G.; Singh, H.; et al. Temporal Trends in the Clinical Acuity of Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Med. 2018, 131, 100.e9–100.e20. [Google Scholar] [CrossRef]
- Iannaccone, M.; Venuti, G.; di Simone, E.; De Filippo, O.; Bertaina, M.; Colangelo, S.; Boccuzzi, G.; de Piero, M.E.; Attisani, M.; Barbero, U.; et al. Comparison of ECMO vs. ECpella in Patients With Non-Post-Pericardiotomy Cardiogenic Shock: An Updated Meta-Analysis. Cardiovasc. Revasc. Med. 2022, 40, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Cenko, E.; Yoon, J.; Kedev, S.; Stankovic, G.; Vasiljevic, Z.; Krljanac, G.; Kalpak, O.; Ricci, B.; Milicic, D.; Manfrini, O.; et al. Sex differences in outcomes after STEMI: Effect modification by treatment strategy and age. JAMA Intern. Med. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Vogel, B.; Claessen, B.E.; Arnold, S.V.; Chan, D.; Cohen, D.J.; Giannitsis, E.; Gibson, C.M.; Goto, S.; Katus, H.A.; Kerneis, M.; et al. ST-segment elevation myocardial infarction. Nat. Rev. Dis. Primers 2019, 5, 39. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Hemradj, V.V.; Ottervanger, J.P.; de Boer, M.J.; Suryapranata, H.; For the Zwolle Myocardial Infarction Study Group. Shock Index More Sensitive Than Cardiogenic Shock in ST-Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Circ. J. 2017, 81, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Chiu, I.-M.; Tsai, M.-T.; Lin, C.-F.; Lin, C.-F. Delta Shock Index During Emergency Department Stay Is Associated with in Hospital Mortality in Critically Ill Patients. Front. Med. 2021, 8, 648375. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, A.; Blackburn, T.; Mitra, B.; Fitzgerald, M. Review article: Shock Index for prediction of critical bleeding post-trauma: A systematic review. Emerg. Med. Australas. 2014, 26, 223–228. [Google Scholar] [CrossRef]
- Yussof, S.J.M.; Zakaria, M.I.; Mohamed, F.L.; Bujang, M.A.; Lakshmanan, S.; Asaari, A.H. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med. J. Malays. 2012, 67, 406–411. [Google Scholar]
- Prasad, K.D.; Abhinov, T.; Himabindu, K.; Rajesh, K.; Moorthy, D.K. Modified Shock Index as an Indicator for Prognosis Among Sepsis Patients with and Without Comorbidities Presenting to the Emergency Department. Cureus 2021, 13, e20283. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Fuernau, G.; Eitel, C.; de Waha, S.; Desch, S.; Metzler, B.; Schuler, G.; Thiele, H.; Eitel, I. Shock Index as a Predictor of Myocardial Damage and Clinical Outcome in ST-Elevation Myocardial Infarction. Circ. J. 2016, 80, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Misumida, N.; Luger, D.; Kanei, Y. Shock Index as a predictor for In-hospital mortality in patients with non-ST-segment elevation myocardial infarction. Cardiovasc. Revasc. Med. 2016, 17, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Q.; Xu, J.-S.; Su, H.; Li, J.-X.; Wang, W.-Y.; Hong, K.; Cheng, X.-S. Modified Shock Index is a Predictor for 7-Day Outcomes in Patients With STEMI. Am. J. Emerg. Med. 2015, 33, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Ran, P.; Wei, X.-B.; Lin, Y.-W.; Li, G.; Huang, J.-L.; He, X.-Y.; Yang, J.-Q.; Yu, D.-Q.; Chen, J.-Y. Shock Index-C: An Updated and Simple Risk-Stratifying Tool in ST-Segment Elevation Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 657817. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Booysen, H.L.; Woodiwiss, A.J.; Raymond, A.; Sareli, P.; Hsu, H.-C.; Dessein, P.H.; Norton, G.R. Chronic kidney disease epidemiology collaboration-derived glomerular filtration rate performs better at detecting preclinical end-organ changes than alternative equations in black Africans. J. Hypertens. 2016, 34, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Killip, T., 3rd; Kimball, J.T. Treatment of myocardial infarction in a coronary care unit: A two year experience with 250 patients. Am. J. Cardiol. 1967, 20, 457–464. [Google Scholar] [CrossRef]
- Abe, N.; Miyashita, Y.; Hashizume, N.; Motoki, H.; Tosaka, A.; Miura, T.; Tsujimura, T.; Ishihara, T.; Uematsu, M.; Ishihara, R.; et al. Long-Term Prognostic Implications of the Admission Shock Index in Patients With Acute Myocardial Infarction Who Received Percutaneous Coronary Intervention. Angiology 2016, 68, 339–345. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, Z.; Fang, M.; Shu, Z. The prognostic value of shock index for the outcomes of acute myocardial infarction patients: A systematic review and meta-analysis. Medicine 2017, 96, e8014. [Google Scholar] [CrossRef]
- Abreu, G.; Azevedo, P.; Braga, C.G.; Vieira, C.; Pereira, M.A.; Martins, J.; Arantes, C.; Rodrigues, C.; Salgado, A.; Marques, J. Modified shock index: A bedside clinical index for risk assessment of ST-segment elevation myocardial infarction at presentation. Rev. Port. Cardiol. (Engl. Ed.) 2018, 37, 481–488. [Google Scholar] [CrossRef]
- Schmitz, T.; Harmel, E.; Linseisen, J.; Kirchberger, I.; Heier, M.; Peters, A.; Meisinger, C. Shock index and modified shock index are predictors of long-term mortality not only in STEMI but also in NSTEMI patients. Ann. Med. 2022, 54, 900–908. [Google Scholar] [CrossRef]
- Bruijns, S.; Guly, H.R.; Bouamra, O.; Lecky, F.; Wallis, L. The value of the difference between ED and prehospital vital signs in predicting outcome in trauma. Emerg. Med. J. 2013, 31, 579–582. [Google Scholar] [CrossRef]
- Joseph, B.; Haider, A.; Ibraheem, K.; Kulvatunyou, N.; Tang, A.; Azim, A.; O’Keeffe, T.; Gries, L.; Vercruysse, G.; Rhee, P. Revitalizing vital signs: The role of delta shock index. Shock 2016, 46 (Suppl. S1), 50–54. [Google Scholar] [CrossRef]
- Asmar, S.; Zeeshan, M.; Khurrum, M.; Con, J.; Chehab, M.; Bible, L.; Latifi, R.; Joseph, B. Delta Shock Index Predicts Outcomes in Pediatric Trauma Patients Regardless of Age. J. Surg. Res. 2020, 259, 182–191. [Google Scholar] [CrossRef]
- Kohn, J.R.; Dildy, G.A.; Eppes, C.S. Shock index and delta-shock index are superior to existing maternal early warning criteria to identify postpartum hemorrhage and need for intervention. J. Matern. Neonatal Med. 2018, 32, 1238–1244. [Google Scholar] [CrossRef]
- Vandromme, M.J.; Griffin, R.L.; Kerby, J.D.; McGwin, G., Jr.; Rue, L.W., 3rd; Weinberg, J.A. Identifying risk for massive transfusion in the relatively normotensive patient: Utility of the prehospital shock index. J. Trauma 2011, 70, 384–388; discussion 388–390. [Google Scholar] [CrossRef]
- Bilkova, D.; Motovska, Z.; Widimsky, P.; Dvorak, J.; Lisa, L.; Budesinsky, T. Shock Index: A Simple Clinical Parameter for Quick Mortality Risk Assessment in Acute Myocardial Infarction. Can. J. Cardiol. 2011, 27, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Jang, W.J.; Bin Song, Y.; Lima, J.A.; Guallar, E.; Choe, Y.H.; Choi, S.; Kim, E.K.; Hahn, J.-Y.; Choi, S.-H.; et al. Shock Index as a Predictor of Myocardial Injury in ST-segment Elevation Myocardial Infarction. Am. J. Med. Sci. 2016, 352, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, F.; Farbiarz, J.; Alvarez, D.; Martínez, C. Comparison between logistic regression and neural networks to predict death in patients with suspected sepsis in the emergency room. Crit. Care 2005, 9, R150–R156. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Green, J.; Horeczko, T.; Hagar, Y.; Garg, N.; Suarez, A.; Panacek, E.; Shapiro, N. Shock Index and Early Recognition of Sepsis in the Emergency Department: Pilot Study. West. J. Emerg. Med. 2013, 14, 168–174. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Liu, J.-H.; Fang, Z.A.; Shan, G.-L.; Xu, J.; Qi, Z.-W.; Zhu, H.-D.; Wang, Z.; Yu, X.-Z. Modified shock index and mortality rate of emergency patients. World J. Emerg. Med. 2012, 3, 114–117. [Google Scholar] [CrossRef]
- Althunayyan, S.M.; Alsofayan, Y.M.; Khan, A.A. Shock index and modified shock index as triage screening tools for sepsis. J. Infect. Public Health 2019, 12, 822–826. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, e344–e426. [Google Scholar]
- Lakhani, M.S.; Qadir, F.; Hanif, B.; Farooq, S.; Khan, M. Correlation of thrombolysis in myocardial infarction (TIMI) risk score with extent of coronary artery disease in patients with acute coronary syndrome. J. Pak. Med. Assoc. 2010, 60, 197–200. [Google Scholar]
- Cakar, M.A.; Sahinkus, S.; Aydin, E.; Vatan, M.B.; Keser, N.; Akdemir, R.; Gunduz, H. Relation between the GRACE score and severity of atherosclerosis in acute coronary syndrome. J. Cardiol. 2014, 63, 24–28. [Google Scholar] [CrossRef][Green Version]
- Investigators, G. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) project: A multinational registry of patients hospitalized with acute coronary syndromes. Am. Heart J. 2001, 141, 190–199. [Google Scholar]
- Chotechuang, Y.; Phrommintikul, A.; Kuanprasert, S.; Muenpa, R.; Ruengorn, C.; Patumanond, J.; Chaichuen, T.; Thanachaikun, N.; Benjanuwatra, T.; Sukonthasarn, A. GRACE score and cardiovascular outcomes prediction among the delayed coronary intervention after post-fibrinolytic STEMI patients in a limited PCI-capable hospital. Open Heart 2020, 7, e001133. [Google Scholar] [CrossRef] [PubMed]
- Correia, L.C.L.; Garcia, G.; Kalil, F.; Ferreira, F.; Carvalhal, M.; Oliveira, R.; Silva, A.; Vasconcelos, I.; Henri, C.; Noya-Rabelo, M. Prognostic Value of TIMI Score versus GRACE Score in ST-segment Elevation Myocardial Infarction. Arq. Bras. Cardiol. 2014, 103, 98–106. [Google Scholar] [CrossRef] [PubMed]
| Survival to Hospital Discharge | Mortality | p | |
|---|---|---|---|
| Characteristics of ST-elevation myocardial infarction | n = 1436 | n = 116 | |
| Age | 60.3 ± 12.6 | 69.3 ± 12.8 | <0.001 |
| Male sex | 1217 | 80 | <0.001 |
| Body mass index | 25.6 ± 3.9 | 25.3 ± 4.6 | 0.466 |
| Hypertension | 938 | 76 | 0.966 |
| Diabetes | 550 | 62 | 0.001 |
| Current smoker | 812 | 43 | <0.001 |
| Dyslipidemia | 1103 | 75 | 0.003 |
| History of myocardial infarction | 121 | 17 | 0.023 |
| History of PCI | 122 | 16 | 0.054 |
| Killip II to IV | 506 | 103 | <0.001 |
| Anterior wall STEMI | 752 | 60 | 0.894 |
| Mechanical circulatory support | 194 | 77 | <0.001 |
| ECMO intervention | 15 | 34 | <0.001 |
| Troponin I | 3.8 ± 12.6 | 13.7 ± 24.7 | <0.001 |
| Fatal arrhythmia | 104 | 37 | <0.001 |
| Survival to Hospital Discharge (n = 1436) | Mortality (n = 116) | p | |
|---|---|---|---|
| ED shock index | 0.57 (0.46, 0.71) | 0.74 (0.57, 0.93) | <0.001 |
| ED modified shock index | 0.76 (0.63, 0.93) | 0.97 (0.76, 1.25) | <0.001 |
| ED mean arterial pressure | 102 (87, 119) | 91.5 (73.8, 109.3) | <0.001 |
| ED shock index-C | −16.2 (−39.3, 8.5) | 35.2 (5.5, 59.0) | <0.001 |
| TIMI risk scores | 4 (2, 6) | 7 (6, 9) | <0.001 |
| Admission shock index | 0.65 ± 0.21 | 0.81 ± 0.37 | <0.001 |
| Admission modified shock index | 0.85 (0.72, 1.01) | 1.04 (0.83, 1.28) | <0.001 |
| Admission mean arterial pressure | 98 (86, 110) | 88 (70, 107) | <0.001 |
| Admission shock index-C | −10.3 (−33.9, 13.3) | 32.7 (5.4, 55.3) | <0.001 |
| Positive delta shock index | 578 | 39 | 0.333 |
| Positive delta modified shock index | 573 | 42 | 0.759 |
| Overall | |||||
|---|---|---|---|---|---|
| Survival to Hospital Discharge | |||||
| Threshold | AUC | Lower | Upper | p | |
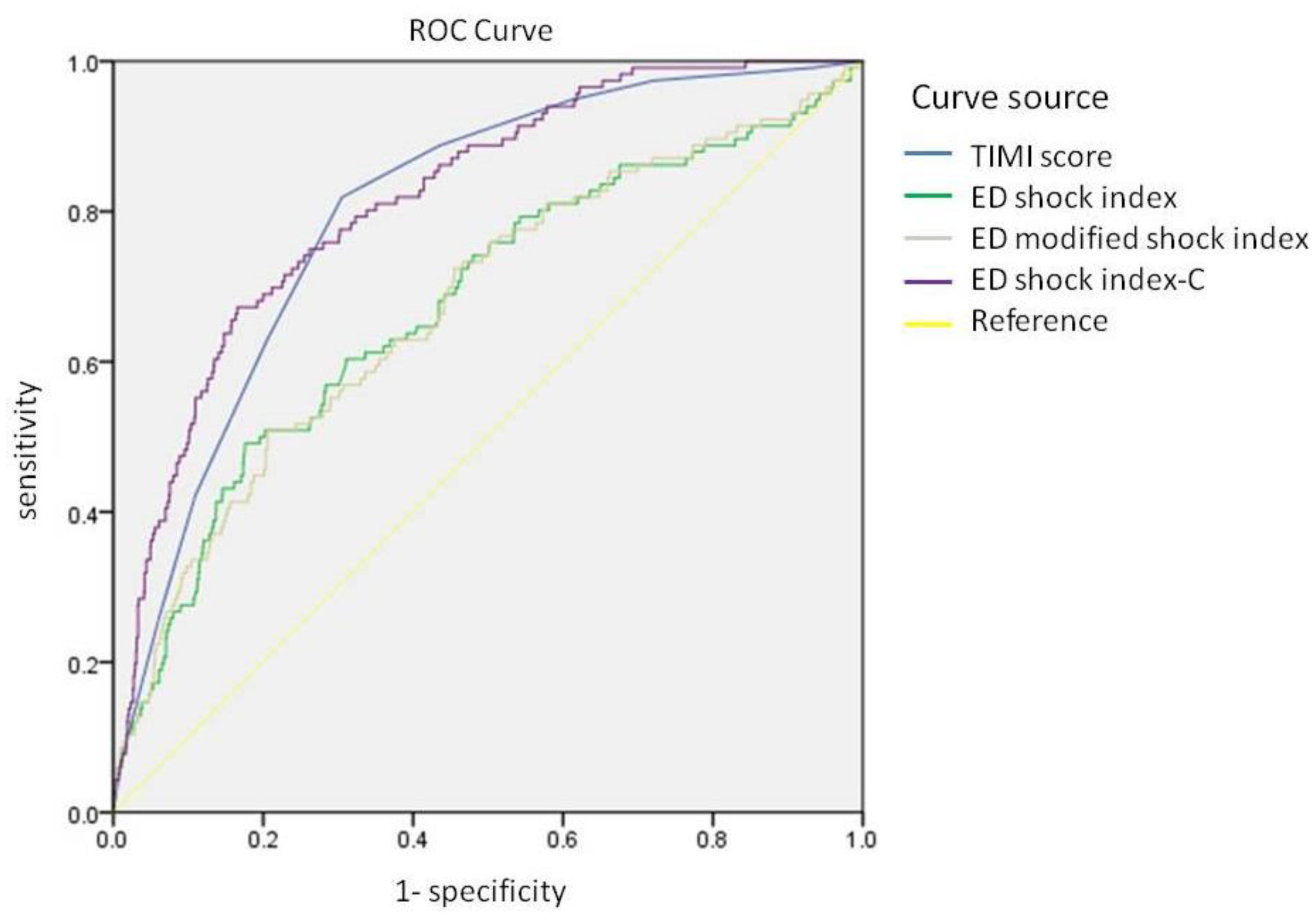
| ED Shock index | 0.75 | 0.676 | 0.620 | 0.731 | <0.001 |
| ED Modified shock index | 0.97 | 0.674 | 0.620 | 0.729 | <0.001 |
| ED shock index-C | 21.00 | 0.818 | 0.780 | 0.856 | <0.001 |
| Admission shock index | 0.75 | 0.662 | 0.605 | 0.719 | <0.001 |
| Admission modified shock index | 1.03 | 0.670 | 0.611 | 0.730 | <0.001 |
| Admission shock index-C | 28.00 | 0.792 | 0.748 | 0.836 | <0.001 |
| TIMI risk scores | 5.50 | 0.801 | 0.764 | 0.839 | <0.001 |
| Adjusted Odds Ratios for In-Hospital Mortality | ||||
|---|---|---|---|---|
| OR | 95% CI | p | ||
| ED shock index > 0.75 | 2.609 | 1.649 | 4.129 | <0.001 |
| ED modified shock index > 0.97 | 1.689 | 1.057 | 2.697 | 0.028 |
| ED shock index-C > 21.0 | 4.058 | 2.515 | 6.547 | <0.001 |
| TIMI score > 5.5 | 3.614 | 2.016 | 6.480 | <0.001 |
| Admission shock index > 0.75 | 2.759 | 1.727 | 4.407 | <0.001 |
| Admission modified shock index > 1.03 | 2.234 | 1.455 | 30712 | <0.001 |
| Admission shock index-C > 28 | 3.099 | 1.908 | 5.034 | <0.001 |
| Positive delta shock index | 0.757 | 0.481 | 1.191 | 0.229 |
| Sensitivity, Specificity and Negative Predictive Value of STEMI in Hospital Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment Using ED Shock Index-C > 21 | Assessment Using ED Shock Index > 0.75 | Assessment Using ED Modified Shock Index > 1 | Assessment Using TIMI Score > 5.5 | ||||||||
| Assessment Using Shock Index-C > 21 | Survival to Hospital Discharge | Mortality | Assessment Using ED Shock Index > 0.75 | Survival to Hospital Discharge | Mortality | Assessment Using ED Modified Shock Index > 1 | Survival to Hospital Discharge | Mortality | Assessment Using TIMI Score > 5.5 | Survival to Hospital Discharge | Mortality |
| No. of positive results | 237 | 78 | No. of positive results | 264 | 57 | No. of positive results | 257 | 48 | No. of positive results | 437 | 95 |
| No. of negative results | 1198 | 38 | No. of negative results | 1171 | 59 | No. of negative results | 1178 | 68 | No. of negative results | 999 | 21 |
| Sensitivity, % (95% CI) | 67.2 (58.6–75.9) | Sensitivity, % (95% CI) | 49.1 (39.9–58.4) | Sensitivity, % (95% CI) | 41.4 (32.3–50.5) | Sensitivity, % (95% CI) | 81.9 (74.8–89.0) | ||||
| Specificity, % (95% CI) | 83.5 (81.6–85.4) | Specificity, % (95% CI) | 81.6 (79.6–83.6) | Specificity, % (95% CI) | 82.1 (84.1–80.1) | Specificity, % (95% CI) | 69.6 (67.2–72.0) | ||||
| Positive Predictive Value, % | 24.8 (20.2–29.6) | Positive Predictive Value, % | 17.8 (13.6–22.0) | Positive Predictive Value, % | 15.7 (11.6–19.8) | Positive Predictive Value, % | 17.9 (14.6–21.1) | ||||
| Negative predictive value, % | 96.9 (96.0–97.9) | Negative predictive value, % | 95.2 (94.0–96.4) | Negative predictive value, % | 94.5 (93.3–95.8) | Negative predictive value, % | 97.9 (97.1–98.8) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-Y.; Lin, C.-F.; Liu, P.-H.; Chen, F.-C.; Chiu, I.-M.; Cheng, F.-J. Clinical Validation of the Shock Index, Modified Shock Index, Delta Shock Index, and Shock Index-C for Emergency Department ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2022, 11, 5839. https://doi.org/10.3390/jcm11195839
Chiang C-Y, Lin C-F, Liu P-H, Chen F-C, Chiu I-M, Cheng F-J. Clinical Validation of the Shock Index, Modified Shock Index, Delta Shock Index, and Shock Index-C for Emergency Department ST-Segment Elevation Myocardial Infarction. Journal of Clinical Medicine. 2022; 11(19):5839. https://doi.org/10.3390/jcm11195839
Chicago/Turabian StyleChiang, Charng-Yen, Chien-Fu Lin, Peng-Huei Liu, Fu-Cheng Chen, I-Min Chiu, and Fu-Jen Cheng. 2022. "Clinical Validation of the Shock Index, Modified Shock Index, Delta Shock Index, and Shock Index-C for Emergency Department ST-Segment Elevation Myocardial Infarction" Journal of Clinical Medicine 11, no. 19: 5839. https://doi.org/10.3390/jcm11195839
APA StyleChiang, C.-Y., Lin, C.-F., Liu, P.-H., Chen, F.-C., Chiu, I.-M., & Cheng, F.-J. (2022). Clinical Validation of the Shock Index, Modified Shock Index, Delta Shock Index, and Shock Index-C for Emergency Department ST-Segment Elevation Myocardial Infarction. Journal of Clinical Medicine, 11(19), 5839. https://doi.org/10.3390/jcm11195839





