Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Definition and Management of Abnormal Uterine Bleeding
4. Definition and Management of Dysmenorrhea
5. The Progesterone-Releasing Intrauterine System
6. Levonorgestrel-Releasing Intrauterine Systems
Type of Devices Available
- (1)
- LNG-IUS-20: Total content of 52.5 mg; LNG is initially released at a rate of 20 μg/day, and—as already mentioned—in 2021, the US FDA approved its use for contraception for up to 8 years [47]. It is licensed for HMB for up to 5 years.
- (2)
- LNG-IUS 12: Total content of 19.5 mg; LNG is initially released at a rate of 13 μg/day and has a duration of action of 5 years [62]. The device is not licensed for HMB.
- (3)
- LNG-IUS 8: Total content of 13.5 mg; LNG is initially released at a rate of 8 μg/day and has a duration of action of 3 years [63]. The device is not licensed for HMB.
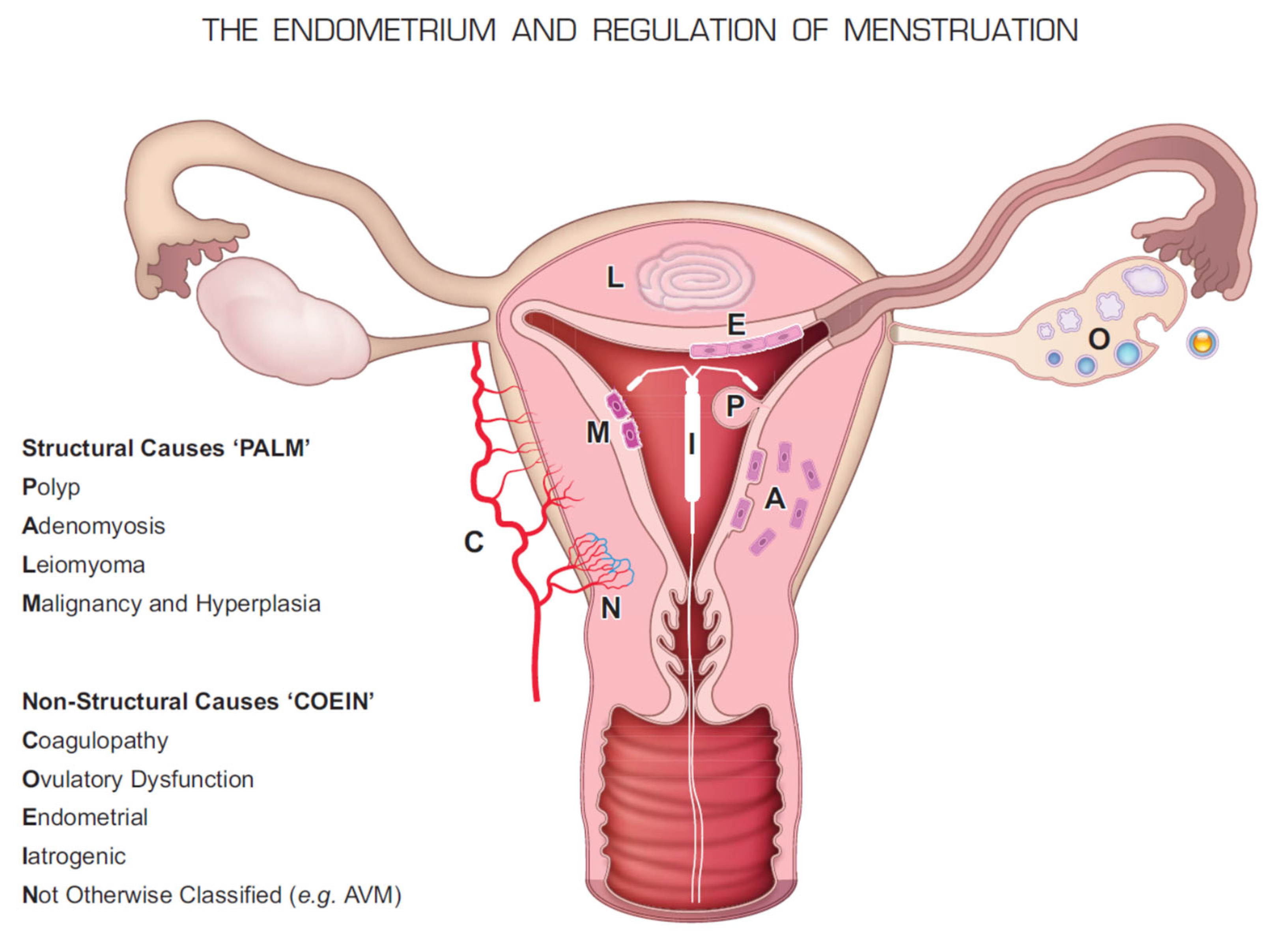
7. Causes of HMB and Dysmenorrhea and the Molecular Basis for the Use of LNG-IUS
8. Use in Abnormal Uterine Bleeding
8.1. Use in Women with Bleeding Disorders
8.2. The Effect of the LNG-IUS on the Endometrium
8.3. Comparison of Blood Loss with Cu-IUD
9. Use in Dysmenorrhea
10. Specific Issues
10.1. Barriers to Use
10.2. Use in Young Women
10.3. Safety, Side Effects, and Reasons for Failure
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Sparzak, P.B. Abnormal Uterine Bleeding; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ju, H.; Jones, M.; Mishra, G. The Prevalence and Risk Factors of Dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tadese, M.; Kassa, A.; Muluneh, A.A.; Altaye, G. Prevalence of dysmenorrhoea, associated risk factors and its relationship with academic performance among graduating female university students in Ethiopia: A cross-sectional study. BMJ Open 2021, 11, e043814. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; The FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408, Erratum in Int. J. Gynecol. Obstet. 2019, 144, 237. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Balen, A.H.; Cho, S.; Critchley, H.; Díaz, I.; Ferriani, R.; Henry, L.; Mocanu, E.; van der Spuy, Z.M. FIGO Committee on Menstrual Disorders and Related Health Impacts; FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO Ovulatory Disorders Classification System. Hum. Reprod. 2022, deac180, Advance online publication. [Google Scholar] [CrossRef]
- Bradley, L.D.; Gueye, N.-A. The medical management of abnormal uterine bleeding in reproductive-aged women. Am. J. Obstet. Gynecol. 2016, 214, 31–44. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.A.; Fogleman, C.D. Dysmenorrhea. Am. Fam. Physician 2021, 104, 164–170. [Google Scholar]
- Smith, R.P. Dysmenorrhea and Menorrhagia: A Clinician’s Guide; Springer Nature: London, UK; Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- ACOG Practice Bulletin No. 110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet. Gynecol. 2010, 115, 206–218. [CrossRef]
- Armstrong, C. ACOG Recommendations on Emergency Contraception. Am. Fam. Physician 2010, 82, 1278. [Google Scholar]
- Warner, P.E.; Critchley, H.O.; Lumsden, M.A.; Campbell-Brown, M.; Douglas, A.; Murray, G. Menorrhagia II: Is the 80-mL blood loss criterion useful in management of complaint of menorrhagia? Am. J. Obstet. Gynecol. 2004, 190, 1224–1229. [Google Scholar] [CrossRef]
- Warner, P.E.; Critchley, H.O.; Lumsden, M.A.; Campbell-Brown, M.; Douglas, A.; Murray, G.D. Menorrhagia I: Measured blood loss, clinical features, and outcome in women with heavy periods: A survey with follow-up data. Am. J. Obstet. Gynecol. 2004, 190, 1216–1223. [Google Scholar] [CrossRef]
- Hallberg, L.; Högdahl, A.-M.; Nilsson, L.; Rybo, G. Menstrual Blood Loss–A Population Study: Variation at different ages and attempts to define normality. Acta Obstet. Gynecol. Scand. 1966, 45, 320–351. [Google Scholar] [CrossRef] [PubMed]
- NICE. NICE Guideline [NG88]: Heavy Menstrual Bleeding: Assessment and Management; National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- American College of Obstetricians and Gynecologists. Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet. Gynecol. 2013, 122, 176–185. [Google Scholar] [CrossRef]
- Matteson, K.A.; Rahn, D.D.; Wheeler, T.L., 2nd; Casiano, E.A.; Siddiqui, N.Y.; Harvie, H.S.; Mamik, M.M.; Balk, E.M.; Sung, V.W.; Society of Gynecologic Surgeons Systematic Review Group. Nonsurgical Management of Heavy Menstrual Bleeding: A Systematic Review. Obstet. Gynecol. 2013, 121, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.; Critchley, H.; Fraser, I. Research and clinical management for women with abnormal uterine bleeding in the reproductive years: More than PALM-COEIN. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Heikinheimo, O.; Fraser, I. The current status of hormonal therapies for heavy menstrual bleeding. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 40, 111–120. [Google Scholar] [CrossRef]
- Davies, J.; Kadir, R.A. Heavy menstrual bleeding: An update on management. Thromb. Res. 2017, 151 (Suppl. 1), S70–S77. [Google Scholar] [CrossRef]
- Rodriguez, M.B.; Lethaby, A.; Farquhar, C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2019, 9, CD000400. [Google Scholar] [CrossRef]
- Lethaby, A.; Augood, C.; Duckitt, K.; Farquhar, C. Nonsteroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2007, CD000400. [Google Scholar] [CrossRef][Green Version]
- Rodriguez, M.B.; Lethaby, A.; Jordan, V. Progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2020, 6, CD002126. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef]
- NICE, National Institute for Health Care Excellence. Dysmenorrhoea; NICE: London, UK, 2020. [Google Scholar]
- ACOG Committee Opinion No. 760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet. Gynecol. 2018, 132, e249–e258. [CrossRef]
- Margatho, D.; Carvalho, N.M.; Eloy, L.; Bahamondes, L. Assessment of biomarkers in women with endometriosis-associated pain using the ENG contraceptive implant or the 52 mg LNG-IUS: A non-inferiority randomised clinical trial. Eur. J. Contracept. Reprod. Health Care 2018, 23, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Margatho, D.; Cursino, K.; Benetti-Pinto, C.L.; Bahamondes, L. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: Randomized clinical trial. Fertil. Steril. 2018, 110, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Margatho, D.; Carvalho, N.M.; Bahamondes, L. Endometriosis-associated pain scores and biomarkers in users of the etonogestrel-releasing subdermal implant or the 52-mg levonorgestrel-releasing intrauterine system for up to 24 months. Eur. J. Contracept. Reprod. Health Care 2020, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.L.; Clewe, T.H. Preliminary studies on the effect of hormone-releasing intrauterine devices. Am. J. Obstet. Gynecol. 1968, 101, 564–568. [Google Scholar] [CrossRef]
- Strycker, J.C.; Doyle, L.L.; Clewe, T.H.; Lippes, J. Silastic Lippes loop with crystalline Provera. Excerpta Med. Int. Congr. Ser. 1971, 246, 100. [Google Scholar]
- Scommegna, A.; Pandya, G.N.; Christ, M.; Lee, A.W.; Cohen, M.R. Intrauterine Administration of Progesterone by a Slow Releasing Device. Fertil. Steril. 1970, 21, 201–210. [Google Scholar] [CrossRef]
- Pandya, G.N.; Scommegna, A. Intrauterine progesterone releasing device—A clinical trial. Excerpta Med. Int. Congr. Ser. 1972. [Google Scholar]
- Pharriss, B.B.; Erickson, R.; Bashaw, J.; Hoff, S.; Place, V.A.; Zaffaroni, A. Progestasert: A Uterine Therapeutic System for Long-term Contraception: I. Philosophy and Clinical Efficacy. Fertil. Steril. 1974, 25, 915–921. [Google Scholar] [CrossRef]
- Burnhill, M.S. The rise and fall and rise of the IUD. Am. J. Gynecol. Health 1989, 3, 6–10. [Google Scholar]
- Snowden, R. The Progestasert and ectopic pregnancy. BMJ 1977, 2, 1600–1601. [Google Scholar] [CrossRef][Green Version]
- Hagenfeldt, K.; Landgren, B.-M. Contraception by intrauterine release of progesterone—Effects on endometrial trace elements, enzymes and steroids. J. Steroid Biochem. 1975, 6, 895–898. [Google Scholar] [CrossRef]
- Hagenfeldt, K.; Landgren, B.-M.; Edström, K.; Johannisson, E. Biochemical and morphological changes in the human endometrium induced by the progestasert device. Contraception 1977, 16, 183–197. [Google Scholar] [CrossRef]
- Shaw, S., Jr.; Macaulay, L.; Aznar, R.; Gonzalez-Angulo, A.; Roy, S. Effects of a progesterone-releasing intrauterine contraceptive device on endometrial blood vessels: A morphometric study. Am. J. Obstet. Gynecol. 1981, 141, 821–827. [Google Scholar] [CrossRef]
- Ermin, M.; Carpino, F.; Petrozza, V.; Benagiano, G. Distribution and effect on the endometrium of progesterone released from a progestasert device. Hum. Reprod. 1989, 4, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Johansson, E.D.; Jackanicz, T.M.; Luukkainen, T. Biodegradable polylactate as a steroid-releasing polymer: Intrauterine administration of d-norgestrel. Am. J. Obstet. Gynecol. 1975, 122, 90–95. [Google Scholar] [CrossRef]
- Nilsson, C.G.; Johansson, E.D.B.; Luukkainen, T. A d-norgestrel-releasing IUD. Contraception 1976, 13, 503–514. [Google Scholar] [CrossRef]
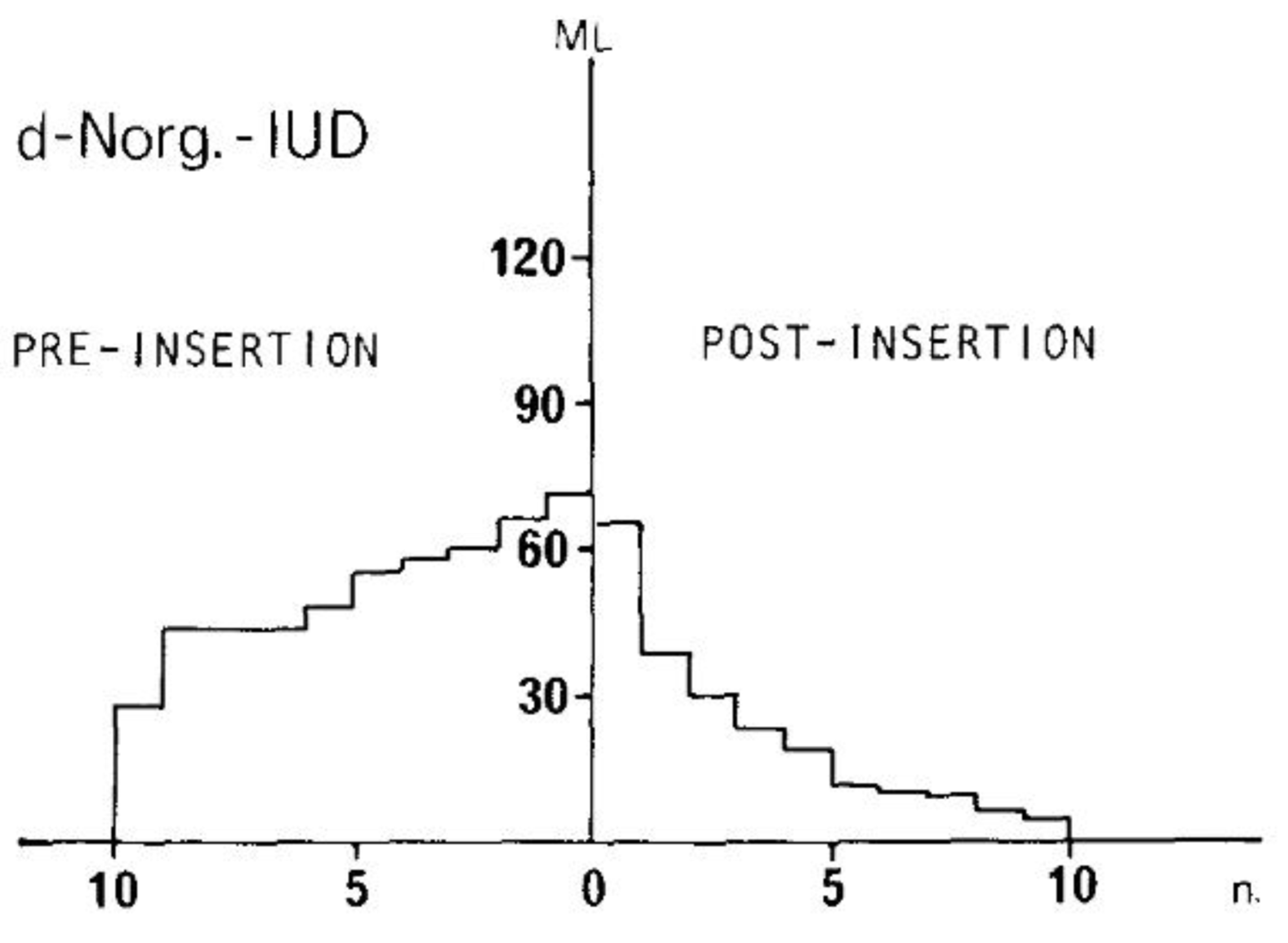
- Nilsson, C.G. Comparative quantitation of menstrual blood loss with a d-norgestrel-releasing IUD and a Nova-T-copper device. Contraception 1977, 15, 379–387. [Google Scholar] [CrossRef]
- Nilsson, C.G.; Luukkainen, T.; Arko, H. Endometrial Morphology of Women Using A d-Norgestrel-Releasing Intrauterine Device. Fertil. Steril. 1978, 29, 397–401. [Google Scholar] [CrossRef]
- Luukkainen, T.; Allonen, H.; Haukkamaa, M.; Lähteenmäki, P.; Nilsson, C.G.; Toivonen, J. Five years’ experience with levonorgestrel-releasing IUDs. Contraception 1986, 33, 139–148. [Google Scholar] [CrossRef]
- FDA. Mirena (Levonorgestrel-Releasing Intrauterine System). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf (accessed on 9 August 2022).
- Mirena. Package Insert. Bayer. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/021225s043lbl.pdf (accessed on 9 August 2022).
- Jensen, J.T.; Reinecke, I.; Lukkari-Lax, E.; Hofmann, B.M. Estimating In Vivo Levonorgestrel Release Rate and Exposure Over Eight Years with Levonorgestrel Releasing Intrauterine System 52 mg Use With Population Pharmacokinetic Approach [A03]. Obstet. Gynecol. (Ann Clin Scient Meeting Abst Suppl.) 2022, 139, 1S. [Google Scholar] [CrossRef]
- Faundes, A.; Alvarez, F.; Brache, V.; Tejada, A. The role of the levonorgestrel intrauterine device in the prevention and treatment of iron deficiency anemia during fertility regulation. Int. J. Gynecol. Obstet. 1988, 26, 429–433. [Google Scholar] [CrossRef]
- Andersson, J.K.; Rybo, G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Milsom, I.; Andersson, K.; Andersch, B.; Rybo, G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am. J. Obstet. Gynecol. 1991, 164, 879–883. [Google Scholar] [CrossRef]
- Luukkainen, T. The levonorgestrel intrauterine system: Therapeutic aspects. Steroids 2000, 65, 699–702. [Google Scholar] [CrossRef]
- Pakarinen, P.; Toivonen, J.; Luukkainen, T. Therapeutic Use of the LNG IUS, and Counseling. Semin. Reprod. Med. 2001, 19, 365–372. [Google Scholar] [CrossRef]
- Varma, R.; Sinha, D.; Gupta, J.K. Non-contraceptive uses of levonorgestrel-releasing hormone system (LNG-IUS)—A systematic enquiry and overview. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 9–28. [Google Scholar] [CrossRef]
- Bednarek, P.H.; Jensen, J. Safety, efficacy and patient acceptability of the contraceptive and non-contraceptive uses of the LNG-IUS. Int. J. Women’s Health 2009, 1, 45–58. [Google Scholar] [CrossRef]
- Rose, S.; Chaudhari, A.; Peterson, C.M. Mirena (Levonorgestrel intrauterine system): A successful novel drug delivery option in contraception. Adv. Drug Deliv. Rev. 2009, 61, 808–812. [Google Scholar] [CrossRef]
- Fraser, I.S. Non-contraceptive health benefits of intrauterine hormonal systems. Contraception 2010, 82, 396–403. [Google Scholar] [CrossRef]
- Heikinheimo, O.; Gemzell-Danielsson, K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS). Acta Obstet. Gynecol. Scand. 2012, 91, 3–9. [Google Scholar] [CrossRef]
- Sabbioni, L.; Petraglia, F.; Luisi, S. Non-contraceptive benefits of intrauterine levonorgestrel administration: Why not? Gynecol. Endocrinol. 2017, 33, 822–829. [Google Scholar] [CrossRef]
- Cristobal, I.; Lete, L.I.; de la Viuda, E.; Perulero, N.; Arbat, A.; Canals, I. One year quality of life measured with SEC-QoL in levonorgestrel 52 mg IUS users. Contraception 2016, 93, 367–371. [Google Scholar] [CrossRef]
- Gemzell-Danielsson, K.; Apter, D.; Dermout, S.; Faustmann, T.; Rosen, K.; Schmelter, T.; Merz, M.; Nelson, A. Evaluation of a new, low-dose levonorgestrel intrauterine contraceptive system over 5 years of use. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.L. Levonorgestrel-Releasing Intrauterine System (LNG-IUS 12) for Prevention of Pregnancy for Up to Five Years. Expert Rev. Clin. Pharmacol. 2017, 10, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Gemzell-Danielsson, K.; Buhling, K.J.; Dermout, S.M.; Lukkari-Lax, E.; Montegriffo, E.; Apter, D. A Phase III, single-arm study of LNG-IUS 8, a low-dose levonorgestrel intrauterine contraceptive system (total content 13.5 mg) in postmenarcheal adolescents. Contraception 2016, 93, 507–512. [Google Scholar] [CrossRef]
- Wildemeersch, D.; Jandi, S.; Pett, A.; Nolte, K.; Hasskamp, T.; Vrijens, M. Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: Results of a multicenter study. Int. J. Women’s Health 2014, 6, 727–734. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Wang, S.; Fu, X.; Lan, R.; Gong, H. Effect of modified levonorgestrel-releasing intrauterine system in human adenomyosis with heavy menstrual bleeding. J. Obstet. Gynaecol. Res. 2022, 48, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wildemeersch, D.; Andrade, A.; Goldstuck, N.D.; Hasskamp, T.; Jackers, G. Intrauterine levonorgestrel delivery with frameless fibrous delivery system: Review of clinical experience. Int. J. Women’s Health 2017, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, A.B.; Khasanov, A.A.; Suturina, L.V.; Borisova, N.I.; Reshetov, Z.S.; Foidart, J.-M.; Déri, J.A.; Tóth, V. Comparison of two levonorgestrel-releasing intrauterine systems for the treatment of heavy menstrual bleeding: A randomised, controlled, phase 3 trial. Eur. J. Contracept. Reprod. Health Care 2021, 26, 491–498. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/publications/i/item/9789240021730 (accessed on 9 August 2022).
- Creinin, M.; Kohn, J.E.; Tang, J.H.; Serna, T.B.; Society of Family Planning Clinical Affairs Committee. Society of Family Planning Committee statement on IUD nomenclature. Contraception 2022, 106, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S.; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Jones, R.L.; Lea, R.G.; Drudy, T.A.; Kelly, R.W.; Williams, A.R.W.; Baird, D.T. Role of Inflammatory Mediators in Human Endometrium during Progesterone Withdrawal and Early Pregnancy. J. Clin. Endocrinol. Metab. 1999, 84, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N.; Karube-Harada, A.; Taketani, T.; Sakata, A.; Nakamura, Y. Withdrawal of Ovarian Steroids Stimulates Prostaglandin F2α. Production Through Nuclear Factor-κB Activation via Oxygen Radicals in Human Endometrial Stromal Cells: Potential Relevance to Menstruation. J. Reprod. Dev. 2004, 50, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Osei, J.; Henderson, T.A.; Boswell, L.; Sales, K.; Jabbour, H.N.; Hirani, N. Hypoxia-Inducible Factor-1α Expression in Human Endometrium and Its Regulation by Prostaglandin E-Series Prostanoid Receptor 2 (EP2). Endocrinology 2006, 147, 744–753. [Google Scholar] [CrossRef]
- Maybin, J.A.; Hirani, N.; Jabbour, H.N.; Critchley, H.O. Novel Roles for Hypoxia and Prostaglandin E2 in the Regulation of IL-8 During Endometrial Repair. Am. J. Pathol. 2011, 178, 1245–1256. [Google Scholar] [CrossRef]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018, 9, 295. [Google Scholar] [CrossRef]
- Munro, M.G. Classification of menstrual bleeding disorders. Rev. Endocr. Metab. Disord. 2012, 13, 225–234. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Critchley, H.; Fu, Z.; Guo, S. How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod. Med. Biol. 2022, 21, e12442. [Google Scholar] [CrossRef]
- Huang, X.; Yang, N.; Fiore, V.F.; Barker, T.H.; Sun, Y.; Morris, S.W.; Ding, Q.; Thannickal, V.J.; Zhou, Y. Matrix Stiffness–Induced Myofibroblast Differentiation Is Mediated by Intrinsic Mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 340–348. [Google Scholar] [CrossRef]
- Marinković, A.; Mih, J.D.; Park, J.-A.; Liu, F.; Tschumperlin, D.J. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L169–L180. [Google Scholar] [CrossRef] [PubMed]
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431. [Google Scholar] [CrossRef] [PubMed]
- Berhan, A.; Harris, T.; Jaffar, J.; Jativa, F.; Langenbach, S.; Lönnstedt, I.; Alhamdoosh, M.; Ng, M.; Lee, P.; Westall, G.; et al. Cellular Microenvironment Stiffness Regulates Eicosanoid Production and Signaling Pathways. Am. J. Respir. Cell Mol. Biol. 2020, 63, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Marinković, A.; Liu, F.; Tschumperlin, D.J. Matrices of Physiologic Stiffness Potently Inactivate Idiopathic Pulmonary Fibrosis Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2013, 48, 422–430. [Google Scholar] [CrossRef]
- Bärnthaler, T.; Theiler, A.; Zabini, D.; Trautmann, S.; Stacher-Priehse, E.; Lanz, I.; Klepetko, W.; Sinn, K.; Flick, H.; Scheidl, S.; et al. Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J. Allergy Clin. Immunol. 2020, 145, 818–833.e11. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Guo, S. Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling. Reprod. Med. Biol. 2022, 21, e12423. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Ishikawa, H. Effects of Estradiol-17β and Progesterone on Cell Proliferation and Differentiation of the Human Endometrial Carcinoma Cell Line (HHUA) in Vitro. Asia-Oceania J. Obstet. Gynaecol. 1984, 10, 531–538. [Google Scholar] [CrossRef]
- White, J.; Sullivan, M.; Patel, L.; Croxtall, J.; D’Arcangues, C.; Belsey, E.; Elder, M. Prostaglandin production in human endometrium following continuous exposure to low-dose levonorgestrel released from a vaginal ring. Contraception 1991, 43, 401–412. [Google Scholar] [CrossRef]
- Mueller, A.; Siemer, J.; Schreiner, S.; Koesztner, H.; Hoffmann, I.; Binder, H.; Beckmann, M.; Dittrich, R. Role of estrogen and progesterone in the regulation of uterine peristalsis: Results from perfused non-pregnant swine uteri. Hum. Reprod. 2006, 21, 1863–1868. [Google Scholar] [CrossRef]
- Chen, C.-C.; Montalbano, A.P.; Hussain, I.; Lee, W.-R.; Mendelson, C.R. The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J. Biol. Chem. 2017, 292, 12560–12576. [Google Scholar] [CrossRef] [PubMed]
- Peavey, M.C.; Wu, S.-P.; Li, R.; Liu, J.; Emery, O.M.; Wang, T.; Zhou, L.; Wetendorf, M.; Yallampalli, C.; Gibbons, W.E.; et al. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc. Natl. Acad. Sci. USA 2021, 118, e2011643118. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.B.; Janowski, B.A.; Corey, D.R.; Mendelson, C. Progesterone Receptor Plays a Major Antiinflammatory Role in Human Myometrial Cells by Antagonism of Nuclear Factor-κB Activation of Cyclooxygenase 2 Expression. Mol. Endocrinol. 2006, 20, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Ascher-Landsberg, J.; Saunders, T.; Phillippe, M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am. J. Obstet. Gynecol. 2000, 183, 674–681. [Google Scholar] [CrossRef]
- Elovitz, M.; Saunders, T.; Ascher-Landsberg, J.; Phillippe, M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am. J. Obstet. Gynecol. 2000, 183, 799–804. [Google Scholar] [CrossRef]
- Nie, J.; Lu, Y.; Liu, X.; Guo, S.-W. Immunoreactivity of progesterone receptor isoform B, nuclear factor κB, and IκBα in adenomyosis. Fertil. Steril. 2009, 92, 886–889. [Google Scholar] [CrossRef]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone Receptor Isoform A But Not B Is Expressed in Endometriosis1. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef]
- Montgomery, B.E.; Daum, G.S.; Dunton, C.J. Endometrial Hyperplasia: A Review. Obstet. Gynecol. Surv. 2004, 59, 368–378. [Google Scholar] [CrossRef]
- Kim, J.; Chapman-Davis, E. Role of Progesterone in Endometrial Cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef]
- Mittal, K.; Schwartz, L.; Goswami, S.; Demopoulos, R. Estrogen and Progesterone Receptor Expression in Endometrial Polyps. Int. J. Gynecol. Pathol. 1996, 15, 345–348. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, T.; Ma, H. Progesterone ameliorates the endometrial polyp by modulating the signaling pathway of Wnt and β-catenin via regulating the expression of H19 and miR-152. J. Cell. Biochem. 2019, 120, 10164–10174. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.-W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liu, X.; Guo, S.-W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Adenomyosis and Its Rectification by a Histone Deacetylase Inhibitor and a Demethylation Agent. Reprod. Sci. 2010, 17, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone Is Essential for Maintenance and Growth of Uterine Leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Updat. 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, S.; Bagratee, J.; Moodley, J. Medical management of uterine fibroids with medroxyprogesterone acetate (Depo Provera): A pilot study. J. Obstet. Gynaecol. 2004, 24, 798–800. [Google Scholar] [CrossRef]
- Johnson, N.; Fletcher, H.; Reid, M. Depo medroxyprogesterone acetate (DMPA) therapy for uterine myomata prior to surgery. Int. J. Gynecol. Obstet. 2004, 85, 174–176. [Google Scholar] [CrossRef]
- Harmon, Q.; Baird, D. Use of depot medroxyprogesterone acetate and prevalent leiomyoma in young African American women. Hum. Reprod. 2015, 30, 1499–1504. [Google Scholar] [CrossRef]
- Benagiano, G.; Morini, A.; Aleandri, V.; Piccinno, F.; Primiero, F.M.; Abbondante, G.; Elkind-Hirsch, K. Sequential Gn-RH superagonist and medroxyprogesterone acetate treatment of uterine leiomyomata. Int. J. Gynecol. Obstet. 1990, 33, 333–343. [Google Scholar] [CrossRef]
- Palomba, S.; Sena, T.; Morelli, M.; Noia, R.; Zullo, F.; Mastrantonio, P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 102, 199–201. [Google Scholar] [CrossRef]
- Carr, B.R.; Marshburn, P.B.; Weatherall, P.T.; Bradshaw, K.D.; Breslau, N.A.; Byrd, W.; Roark, M.; Steinkampf, M.P. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: A prospective, randomized, double blind, placebo-controlled, crossover trial. J. Clin. Endocrinol. Metab. 1993, 76, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Daly, M.; Juneau-Norcross, M.; Rein, M.S.; Fine, C.; Gleason, R.; LeBoff, M. A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add-back” regimens for women with leiomyomata uteri. J. Clin. Endocrinol. Metab. 1993, 76, 1439–1445. [Google Scholar] [CrossRef]
- Teal, S.B.; Turok, D.; Chen, B.A.; Kimble, T.; Olariu, A.I.; Creinin, M.D. Five-Year Contraceptive Efficacy and Safety of a Levonorgestrel 52-mg Intrauterine System. Obstet. Gynecol. 2019, 133, 63–70. [Google Scholar] [CrossRef]
- Magalhaes, J.; Ferreira-Filho, E.S.; Soares-Junior, J.M.; Baracat, E.C. Uterine volume, menstrual patterns, and contraceptive outcomes in users of the levonorgestrel-releasing intrauterine system: A cohort study with a five-year follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 56–62. [Google Scholar] [CrossRef]
- Brosens, I.A. Endometriosis—A disease because it is characterized by bleeding. Am. J. Obstet. Gynecol. 1997, 176, 263–267. [Google Scholar] [CrossRef]
- Fedele, L.; Bianchi, S.; Raffaelli, R.; Portuese, A.; Dorta, M. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil. Steril. 1997, 68, 426–429. [Google Scholar] [CrossRef]
- Barrington, J.W.; Bowen-Simpkins, P. The levonorgestrel intrauterine system in the management of menorrhagia. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 614–616. [Google Scholar] [CrossRef]
- Peng, F.-S.; Wu, M.-Y.; Yang, J.-H.; Chen, S.-U.; Ho, H.-N.; Yang, Y.-S. Insertion of the Mirena Intrauterine System for Treatment of Adenomyosis-Associated Menorrhagia: A Novel Method. Taiwan. J. Obstet. Gynecol. 2010, 49, 160–164. [Google Scholar] [CrossRef]
- Uysal, A.; Taner, C.E.; Mun, S.; Uysal, F.; Celimli, F.H. Use of a levonorgestrel-releasing intrauterine device in the treatment of adenomyosis associated heavy menstrual bleeding. J. Pak. Med. Assoc. 2013, 63, 1349–1352. [Google Scholar]
- Grigorieva, V.; Chen-Mok, M.; Tarasova, M.; Mikhailov, A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil. Steril. 2003, 79, 1194–1198. [Google Scholar] [CrossRef]
- Mercorio, F.; De Simone, R.; Di Spiezio Sardo, A.; Cerrota, G.; Bifulco, G.; Vanacore, F.; Nappi, C. The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception 2003, 67, 277–280. [Google Scholar] [CrossRef]
- Magalhães, J.; Aldrighi, J.M.; de Lima, G.R. Uterine volume and menstrual patterns in users of the levonorgestrel-releasing intrauterine system with idiopathic menorrhagia or menorrhagia due to leiomyomas. Contraception 2007, 75, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Naki, M.M.; Tekcan, C.; Ozcan, N.; Cebi, M. Levonorgestrel-releasing intrauterine device insertion ameliorates leiomyoma-dependent menorrhagia among women of reproductive age without a significant regression in the uterine and leiomyoma volumes. Fertil. Steril. 2010, 94, 371–374. [Google Scholar] [CrossRef]
- Socolov, D.; Blidaru, I.; Tamba, B.; Miron, N.; Boiculese, V.L.; Socolov, R. Levonorgestrel releasing-intrauterine system for the treatment of menorrhagia and/or frequent irregular uterine bleeding associated with uterine leiomyoma. Eur. J. Contracept. Reprod. Health Care 2011, 16, 480–487. [Google Scholar] [CrossRef]
- Senol, T.; Kahramanoglu, I.; Dogan, Y.; Baktiroglu, M.; Karateke, A.; Suer, N. Levonorgestrel-releasing intrauterine device use as an alternative to surgical therapy for uterine leiomyoma. Clin. Exp. Obstet. Gynecol. 2015, 42, 224–227. [Google Scholar] [CrossRef]
- Kaunitz, A.M.; Bissonnette, F.; Monteiro, I.; Lukkari-Lax, E.; Muysers, C.; Jensen, J. Levonorgestrel-Releasing Intrauterine System or Medroxyprogesterone for Heavy Menstrual Bleeding: A Randomized Controlled Trial. Obstet. Gynecol. 2010, 116, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Wildemeersch, D.; Schacht, E. Treatment of menorrhagia with a novel ‘frameless’ intrauterine levonorgestrel-releasing drug delivery system: A pilot study. Eur. J. Contracept. Reprod. Health Care 2001, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kaislasuo, J.; Heikinheimo, O.; Lähteenmäki, P.; Suhonen, S. Menstrual characteristics and ultrasonographic uterine cavity measurements predict bleeding and pain in nulligravid women using intrauterine contraception. Hum. Reprod. 2015, 30, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Beelen, P.; Brink, M.J.V.D.; Herman, M.C.; Geomini, P.M.; Duijnhoven, R.G.; Bongers, M.Y. Predictive factors for failure of the levonorgestrel releasing intrauterine system in women with heavy menstrual bleeding. BMC Women’s Health 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Nam, A.; Kim, H.; Chay, D.; Park, K.; Cho, D.J.; Park, Y.; Lee, B. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am. J. Obstet. Gynecol. 2008, 198, 373.e1–373.e7. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario Levonorgestrel-Releasing Intrauterine System (52 mg) for Idiopathic Heavy Menstrual Bleeding: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2016, 16, 1–119.
- Claessens, E.A.; Cowell, C.A. Acute adolescent menorrhagia. Am. J. Obstet. Gynecol. 1981, 139, 277–280. [Google Scholar] [CrossRef]
- Kadir, R.A.; Economides, D.L.; Sabin, C.; Pollard, D.; Lee, C.A. Assessment of menstrual blood loss and gynaecological problems in patients with inherited bleeding disorders. Haemophilia 1999, 5, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A. Women and inherited bleeding disorders: Menstrual issues. Semin. Hematol. 1999, 36, 21–27. [Google Scholar]
- Kingman, C.; Kadir, R.; Lee, C.; Economides, D. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1425–1428. [Google Scholar] [CrossRef]
- Pisoni, C.N.; Cuadrado, M.J.; Khamashta, M.A.; Hunt, B.J. Treatment of menorrhagia associated with oral anticoagulation: Efficacy and safety of the levonorgestrel releasing intrauterine device (Mirena coil). Lupus 2006, 15, 877–880. [Google Scholar] [CrossRef]
- Kadir, R.A.; Chi, C. Levonorgestrel intrauterine system: Bleeding disorders and anticoagulant therapy. Contraception 2007, 75, S123–S129. [Google Scholar] [CrossRef]
- Lukes, A.S.; Reardon, B.; Arepally, G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil. Steril. 2008, 90, 673–677. [Google Scholar] [CrossRef]
- Chi, C.; Huq, F.Y.; Kadir, R.A. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: Long-term follow-up. Contraception 2011, 83, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, E.; Jamieson, M.A.; James, P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia 2013, 19, 933–938. [Google Scholar] [CrossRef]
- Lu, M.; Yang, X. Levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding in adolescents with Glanzmann’s Thrombasthenia: Illustrated case series. BMC Women’s Health 2018, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Shi, X.; Xia, M. Heavy menstrual bleeding due to primary myelofibrosis in a woman: A case report. Am. J. Transl. Res. 2021, 13, 12016–12020. [Google Scholar]
- Brull, E.P.; Fernandes, A.; Monteiro, I.; Bahamondes, L.; Juliato, C.R.T. Safety and bleeding patterns of the levonorgestrel 52-mg intrauterine system among women with thrombosis or coagulopathy. Int. J. Gynecol. Obstet. 2020, 151, 355–361. [Google Scholar] [CrossRef]
- Campos, R.R.; Baêta, T.; Silva-Filho, A.; Rezende, S.M.; Rocha, A.L.L. Use of a levonorgestrel 52-mg intrauterine system in the control of abnormal uterine bleeding in women with inherited bleeding disorders. Contraception 2020, 102, 254–258. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; El Alayli, A.; Husainat, N.; Kalot, M.A.; Shahid, S.; Aljabirii, Y.; Britt, A.; Alturkmani, H.J.; El-Khechen, H.; Motaghi, S.; et al. Gynecologic and obstetric management of women with von Willebrand disease: Summary of 3 systematic reviews of the literature. Blood Adv. 2022, 6, 228–237. [Google Scholar] [CrossRef]
- Luukkainen, T.; Nilsson, G.; Allonen, H.; Haukkamaa, M.; Toivenen, J. Intrauterine release of levonorgestrel. In Long-Acting Contraception; Papers Presented at the symposium on Long-Acting Contraception; Goldsmith, A., Toppozada, M., Eds.; Johnson-Lindroth, Inc.: Alexandria, Egypt; Chicago, IL, USA, 1983; pp. 167–173. [Google Scholar]
- Lowe, R.F.; Prata, N. Hemoglobin and serum ferritin levels in women using copper-releasing or levonorgestrel-releasing intrauterine devices: A systematic review. Contraception 2013, 87, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Saxena, P.; Firdous, N. Comparison of levonorgestrel and copper releasing intrauterine contraceptive device on body iron stores and menstrual bleeding patterns: Experience on Indian women. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 230–234. [Google Scholar] [PubMed]
- Bahamondes, L.; Petta, C.A.; Fernandes, A.; Monteiro, I. Use of the levonorgestrel-releasing intrauterine system in women with endometriosis, chronic pelvic pain and dysmenorrhea. Contraception 2007, 75, S134–S139. [Google Scholar] [CrossRef]
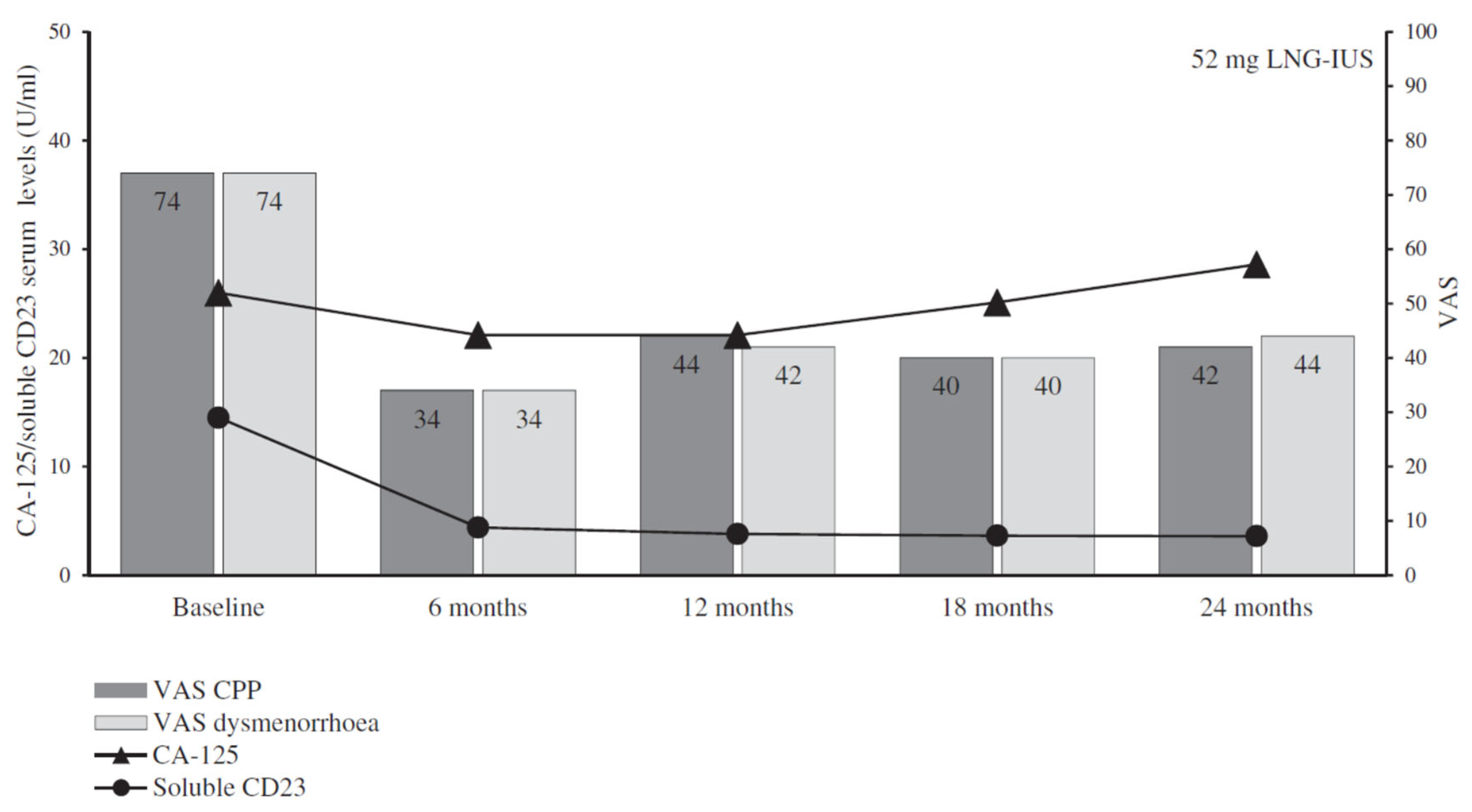
- Yucel, N.; Baskent, E.; Balci, B.K.; Goynumer, G. The levonorgestrel-releasing intrauterine system is associated with a reduction in dysmenorrhoea and dyspareunia, a decrease in CA 125 levels, and an increase in quality of life in women with suspected endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Lee, M.A.; Ko, Y.B.; Yang, J.B.; Kang, B.H.; Lee, K.H. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch. Gynecol. Obstet. 2012, 285, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lindh, I.; Milsom, I. The influence of intrauterine contraception on the prevalence and severity of dysmenorrhea: A longitudinal population study. Hum. Reprod. 2013, 28, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Kelekci, S.; Kelekci, K.H.; Yilmaz, B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception 2012, 86, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Zhang, W.Y.; Zhang, J.P.; Lu, D. The LNG-IUS study on adenomyosis: A 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009, 79, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Wildemeersch, D.; Schacht, E.; Wildemeersch, P. Treatment of primary and secondary dysmenorrhea with a novel ‘frameless’ intrauterine levonorgestrel-releasing drug delivery system: A pilot study. Eur. J. Contracept. Reprod. Health Care 2001, 6, 192–198. [Google Scholar] [CrossRef]
- Gold, M.A.; Johnson, L.M. Intrauterine devices and adolescents. Curr. Opin. Obstet. Gynecol. 2008, 20, 464–469. [Google Scholar] [CrossRef]
- Jatlaoui, T.C.; Riley, H.E.; Curtis, K.M. The safety of intrauterine devices among young women: A systematic review. Contraception 2017, 95, 17–39. [Google Scholar] [CrossRef]
- Buhling, K.J.; Hauck, B.; Dermout, S.; Ardaens, K.; Marions, L. Understanding the barriers and myths limiting the use of intrauterine contraception in nulliparous women: Results of a survey of European/Canadian healthcare providers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Worl Health Organization. Medical Eligibility Criteria for Contraceptive Use; WHO: Geneva, Switzerland, 2015.
- Hauck, B.; Costescu, D. Barriers and Misperceptions Limiting Widespread Use of Intrauterine Contraception Among Canadian Women. J. Obstet. Gynaecol. Can. 2015, 37, 606–616. [Google Scholar] [CrossRef]
- Godfrey, E.M.; Memmel, L.M.; Neustadt, A.; Shah, M.; Nicosia, A.; Moorthie, M.; Gilliam, M. Intrauterine contraception for adolescents aged 14–18 years: A multicenter randomized pilot study of Levonorgestrel-releasing intrauterine system compared to the Copper T 380A. Contraception 2010, 81, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Blunt, S.; Latthe, P. Effectiveness and tolerability of levonorgestrel intrauterine system in adolescents. J. Obstet. Gynaecol. 2010, 30, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Bayer, L.L.; Hillard, P.J.A. Use of Levonorgestrel Intrauterine System for Medical Indications in Adolescents. J. Adolesc. Health 2013, 52, S54–S58. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi-Fowode, O.A.; Bercaw-Pratt, J.L. Intrauterine Devices: Effective Contraception with Noncontraceptive Benefits for Adolescents. J. Pediatr. Adolesc. Gynecol. 2019, 32, S2–S6. [Google Scholar] [CrossRef]
- Beatty, M.N.; Blumenthal, P.D. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability. Ther. Clin. Risk Manag. 2009, 5, 561–574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bitzer, J.; Rapkin, A.; Soares, C.N. Managing the risks of mood symptoms with LNG-IUS: A clinical perspective. Eur. J. Contracept. Reprod. Health Care 2018, 23, 321–325. [Google Scholar] [CrossRef]
- Jiménez, M.F.; Vetori, D.; Fagundes, P.A.; de Freitas, F.M.; Cunha-Filho, J.S. Subendometrial microvascularization and uterine artery blood flow in IUD-induced side effects (levonorgestrel intrauterine system and copper intrauterine device). Contraception 2008, 78, 324–327. [Google Scholar] [CrossRef]

| LNG-IUS 20: Total content: 52.5 mg; daily LNG release: 20 μg; FDA-approved duration of use: 8 years for contraception, 5 years for HMB. Available as:
|
| LNG-IUS 12: Total content: 19.5 mg; daily LNG release: 13μg; duration of use: 5 years (for contraception). Available as:
|
| LNG-IUS 8: Total content: 13.5 mg; daily LNG release: 8μg; duration of use: 3 years (for contraception). Available as:
|
| Frameless 3.5 cm-long coaxial fibrous LNG-IUS: Daily LNG release: 14μg; duration of use: 3 years. Available as:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, P.; Guo, S.-W.; Habiba, M.; Benagiano, G. Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review. J. Clin. Med. 2022, 11, 5836. https://doi.org/10.3390/jcm11195836
Bianchi P, Guo S-W, Habiba M, Benagiano G. Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review. Journal of Clinical Medicine. 2022; 11(19):5836. https://doi.org/10.3390/jcm11195836
Chicago/Turabian StyleBianchi, Paola, Sun-Wei Guo, Marwan Habiba, and Giuseppe Benagiano. 2022. "Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review" Journal of Clinical Medicine 11, no. 19: 5836. https://doi.org/10.3390/jcm11195836
APA StyleBianchi, P., Guo, S.-W., Habiba, M., & Benagiano, G. (2022). Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review. Journal of Clinical Medicine, 11(19), 5836. https://doi.org/10.3390/jcm11195836








