Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
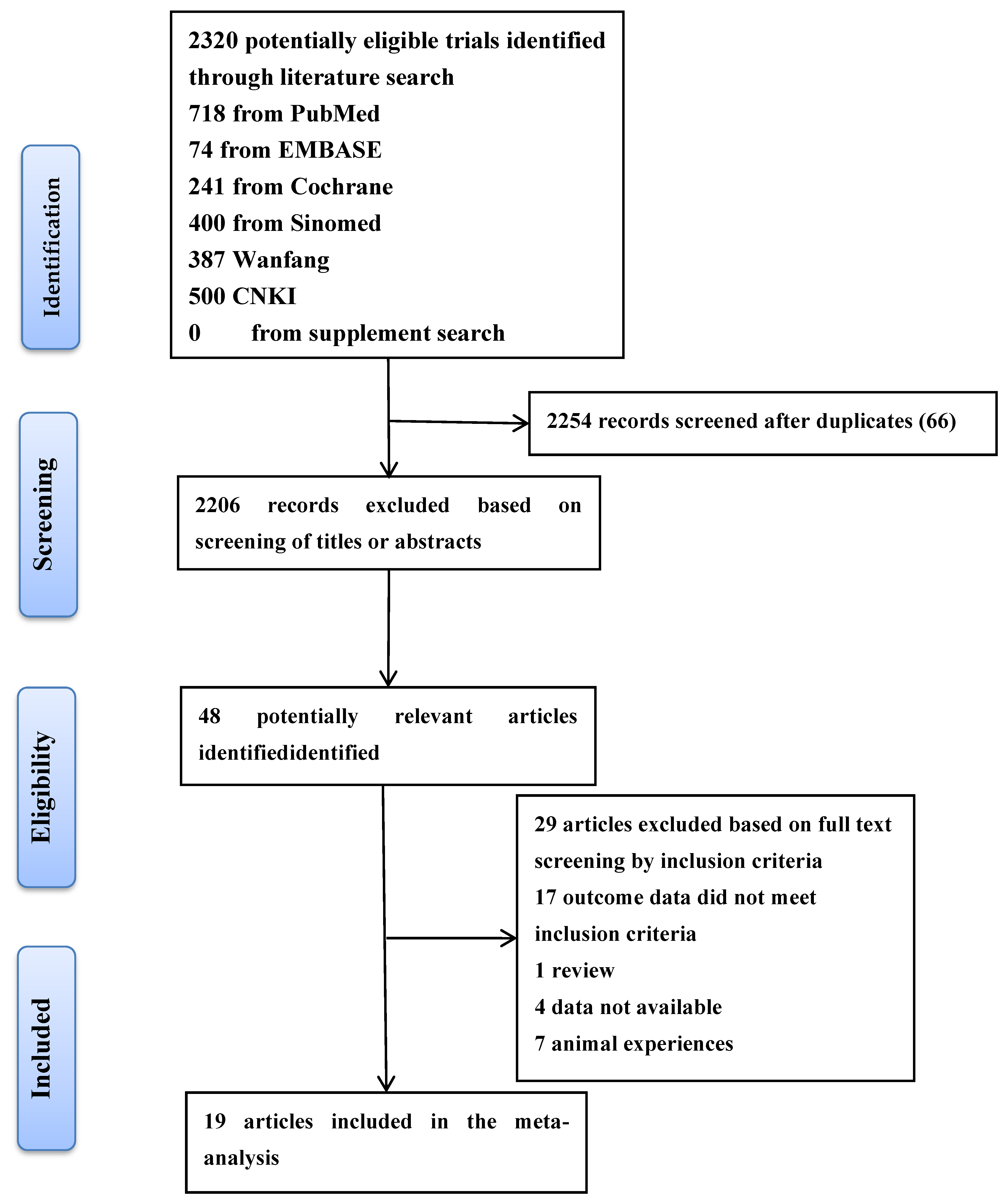
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
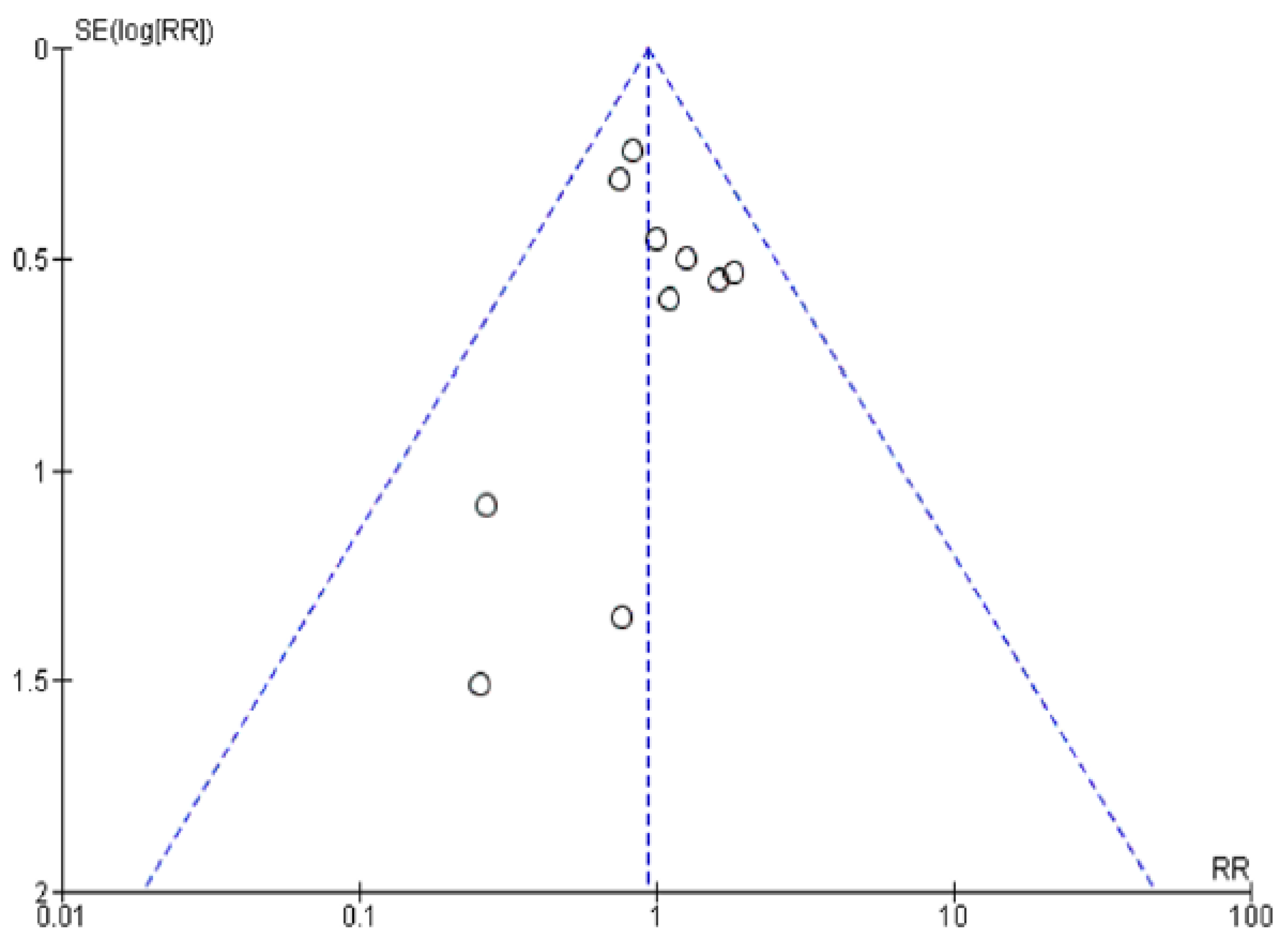
2.4. Publication Bias
2.5. Data Analysis
2.6. Study Selection
2.7. Study Characteristics
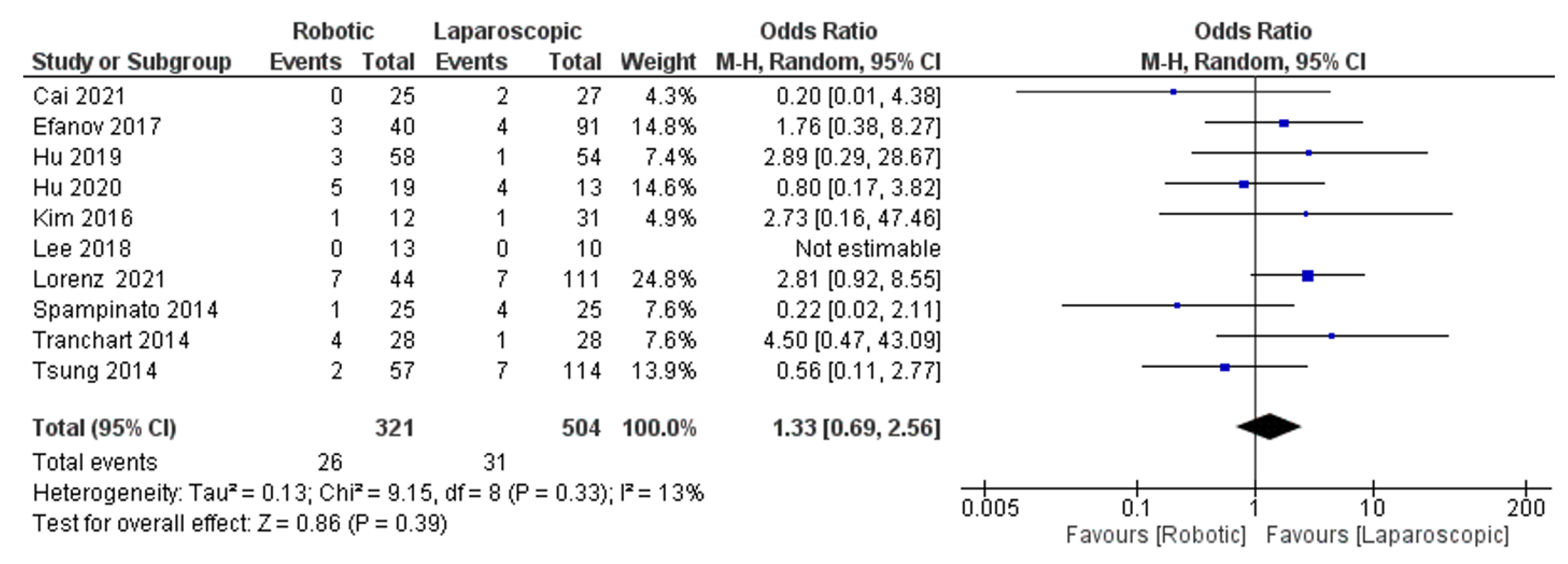
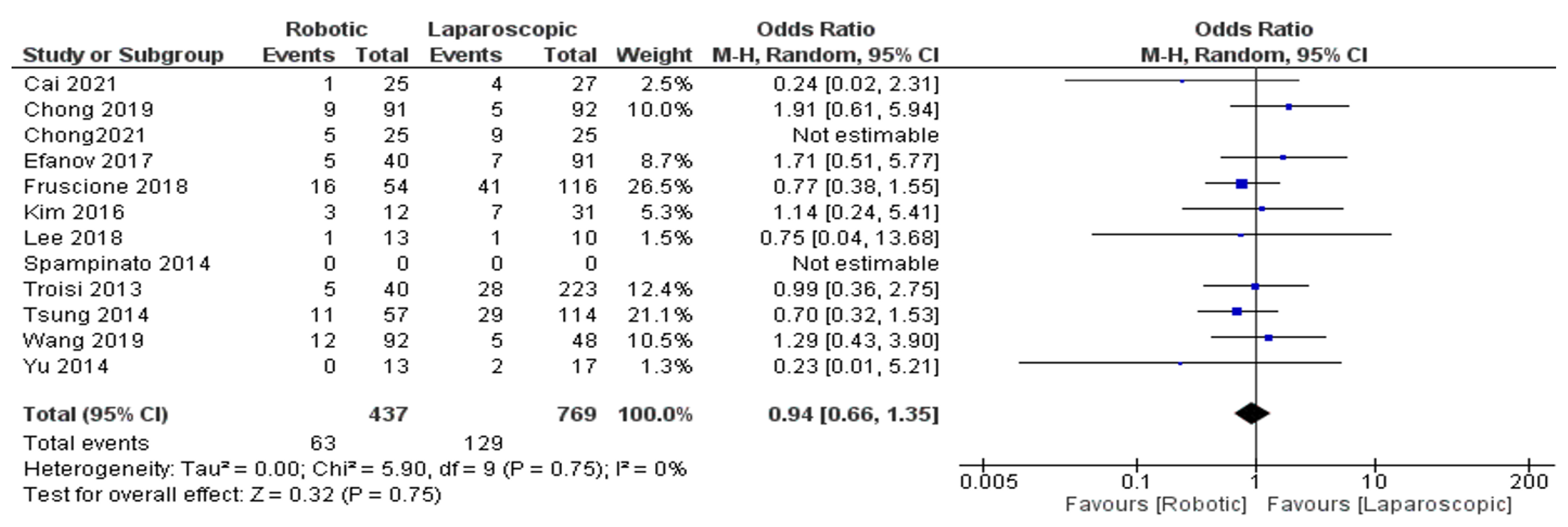
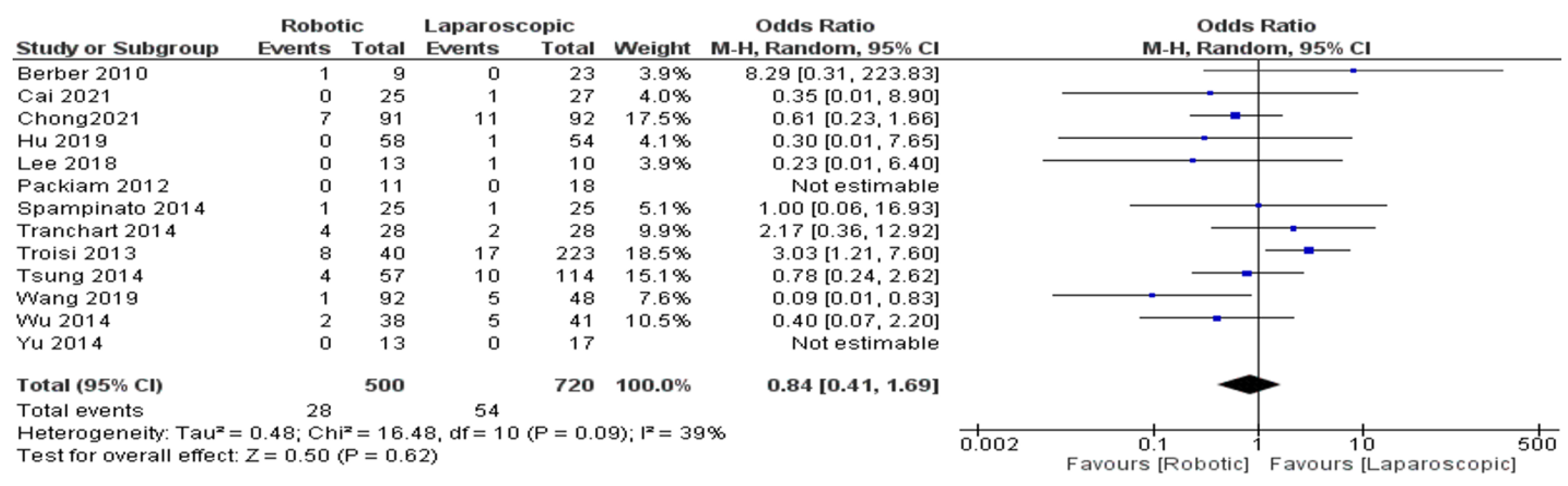
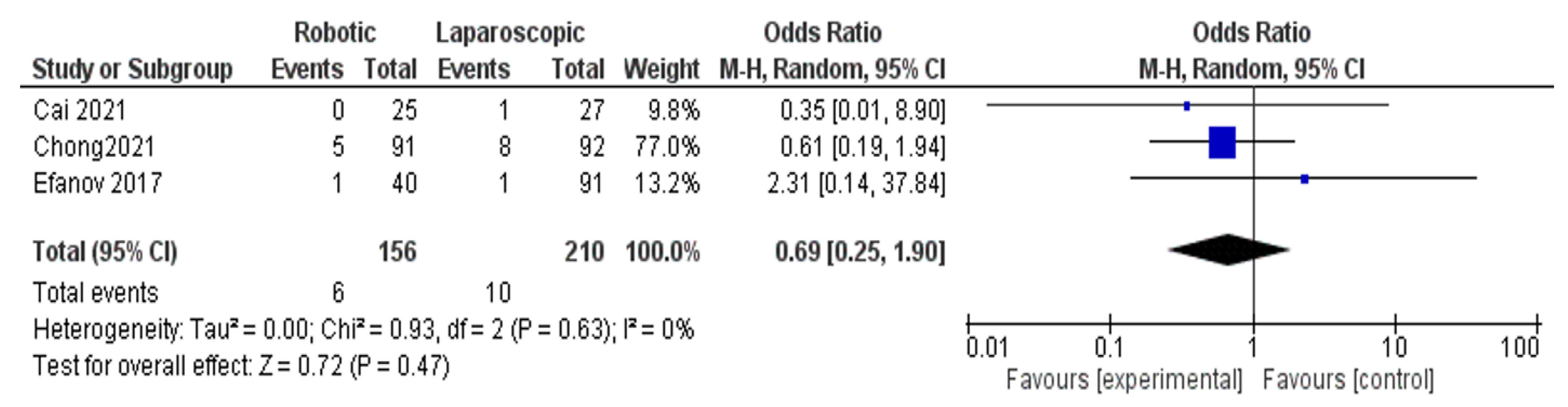
The Results of Meta-Analyses
- Blood transfusion rate
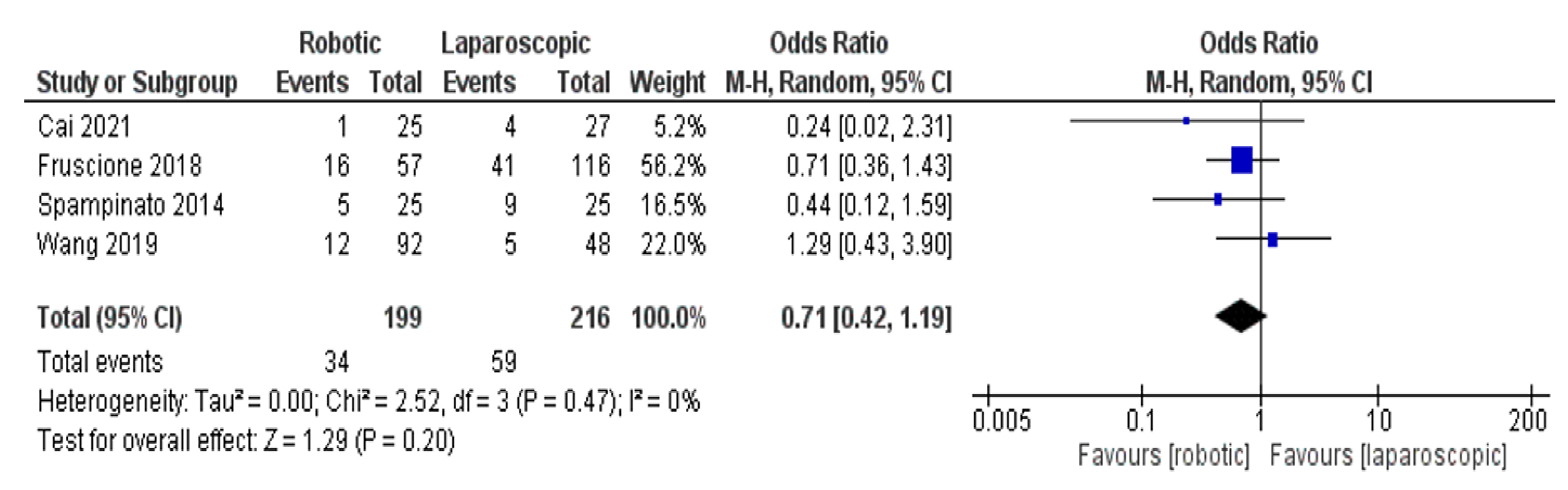
- Complications
- Conversion rate
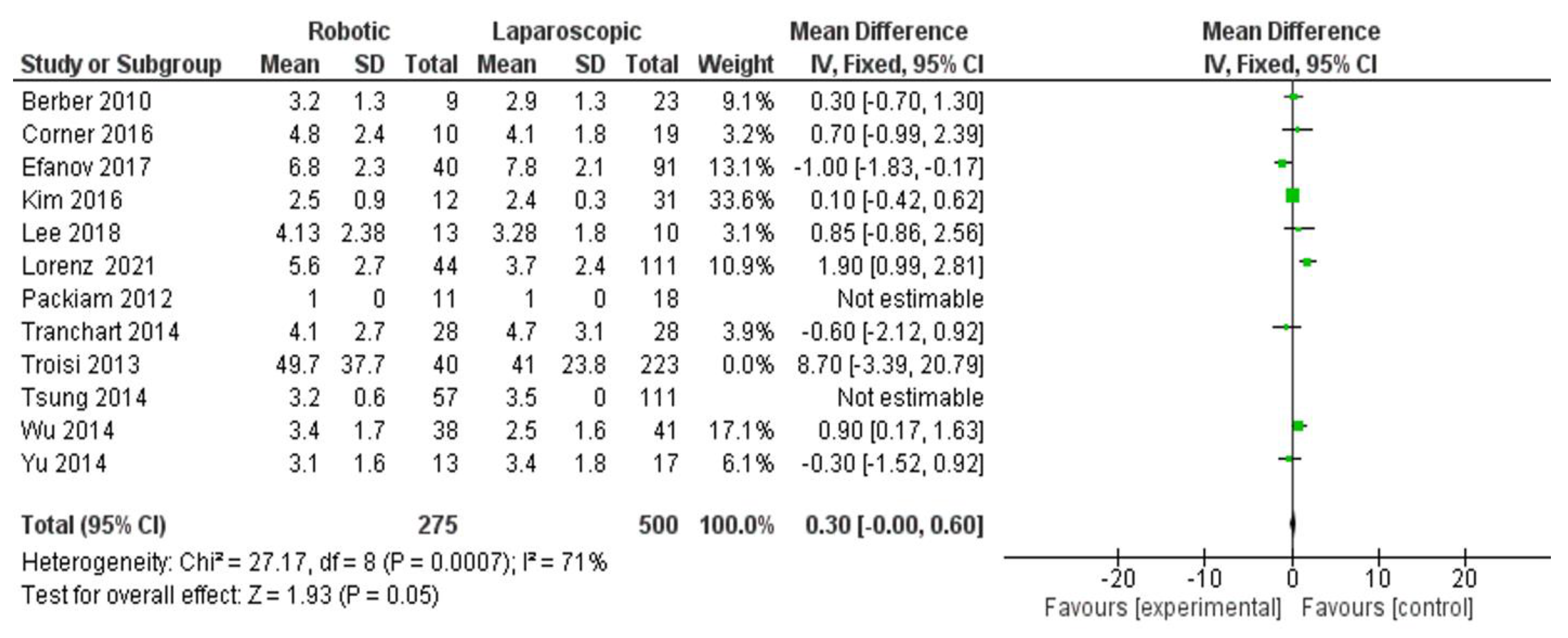
- Reoperation rate
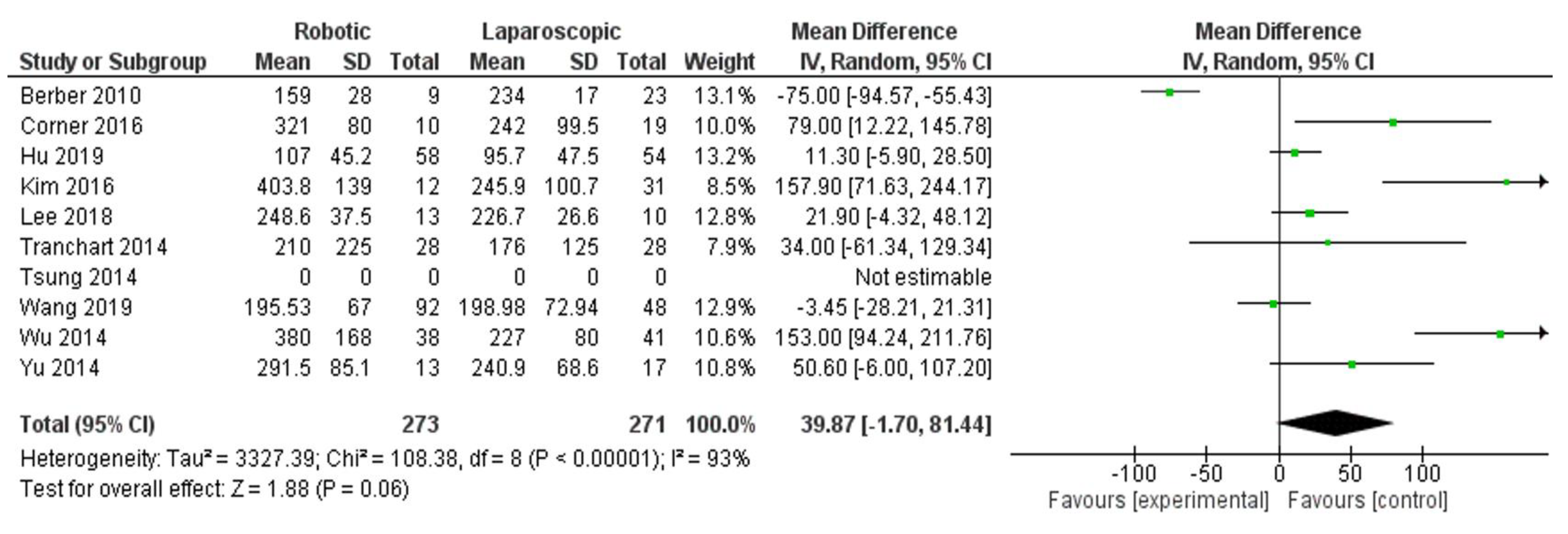
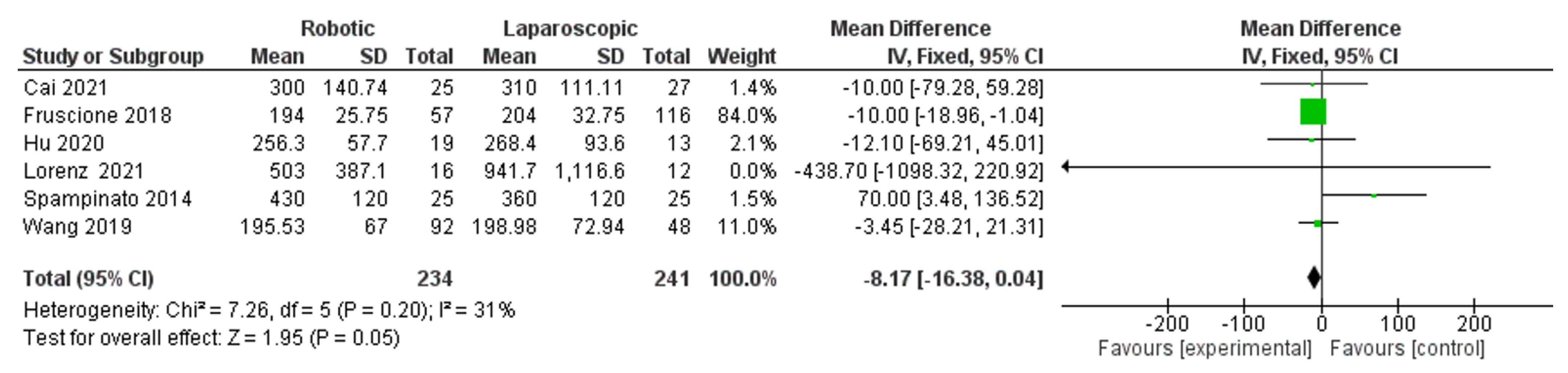
- Blood loss
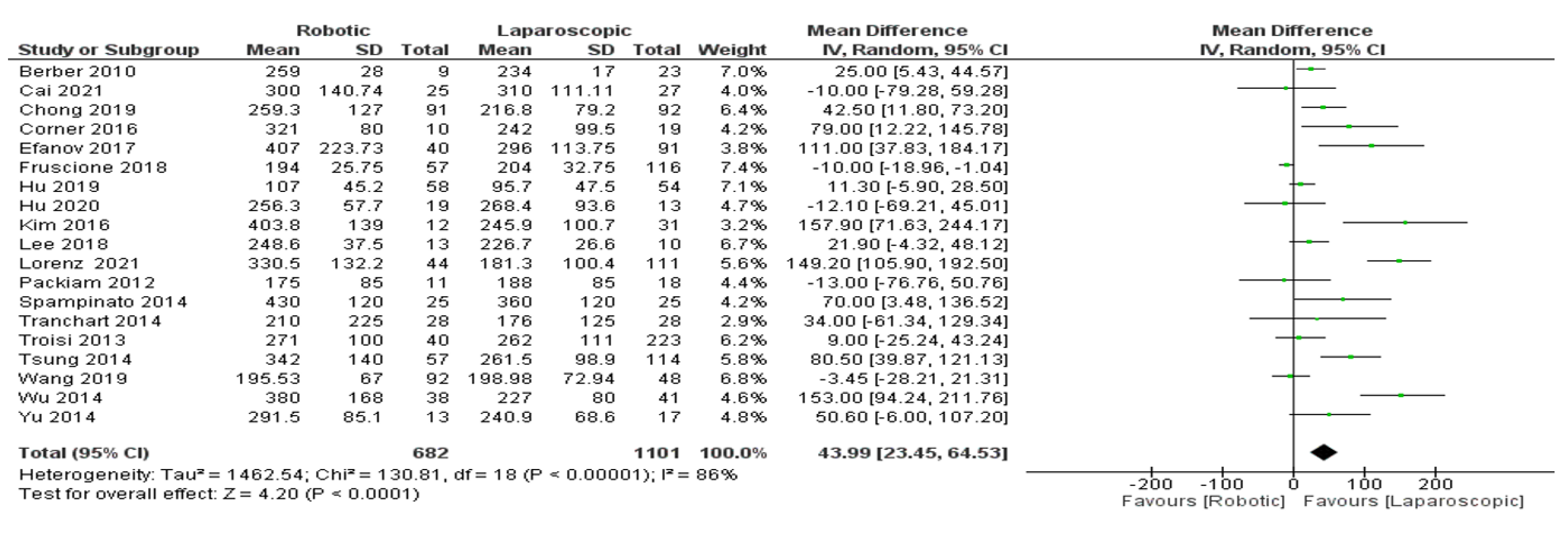
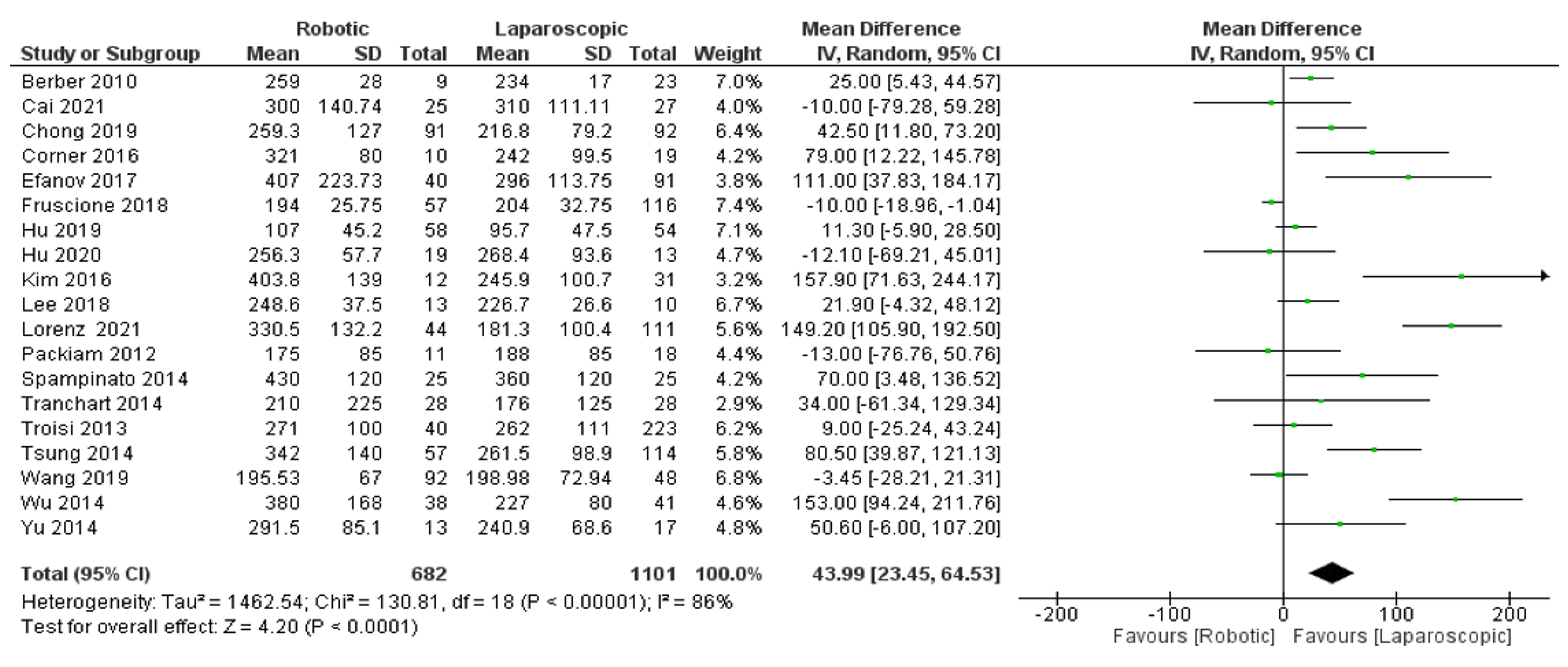
- Operation time
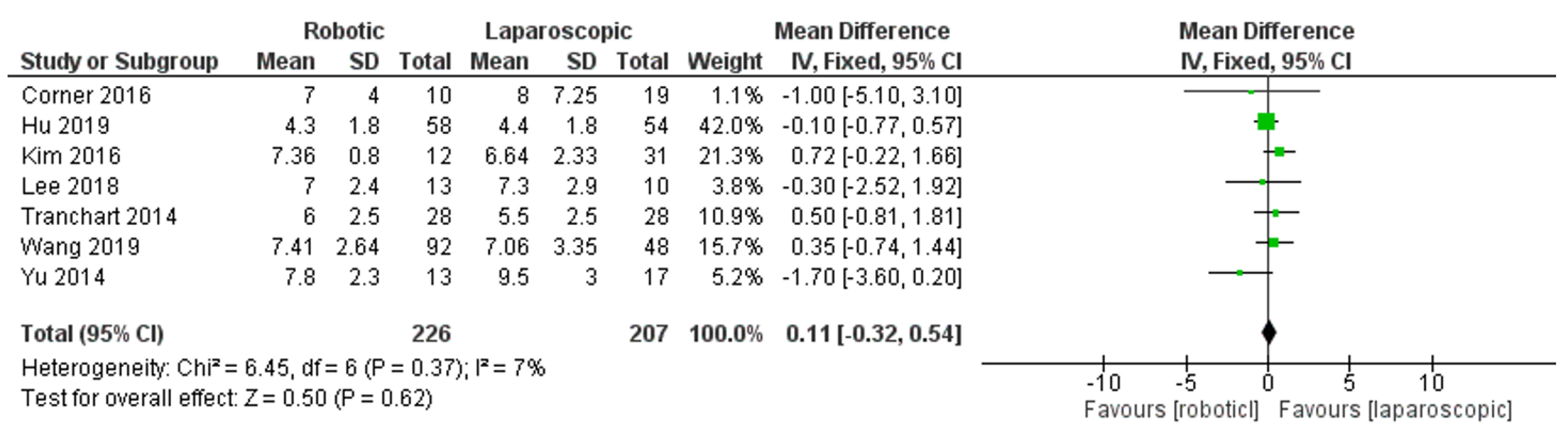
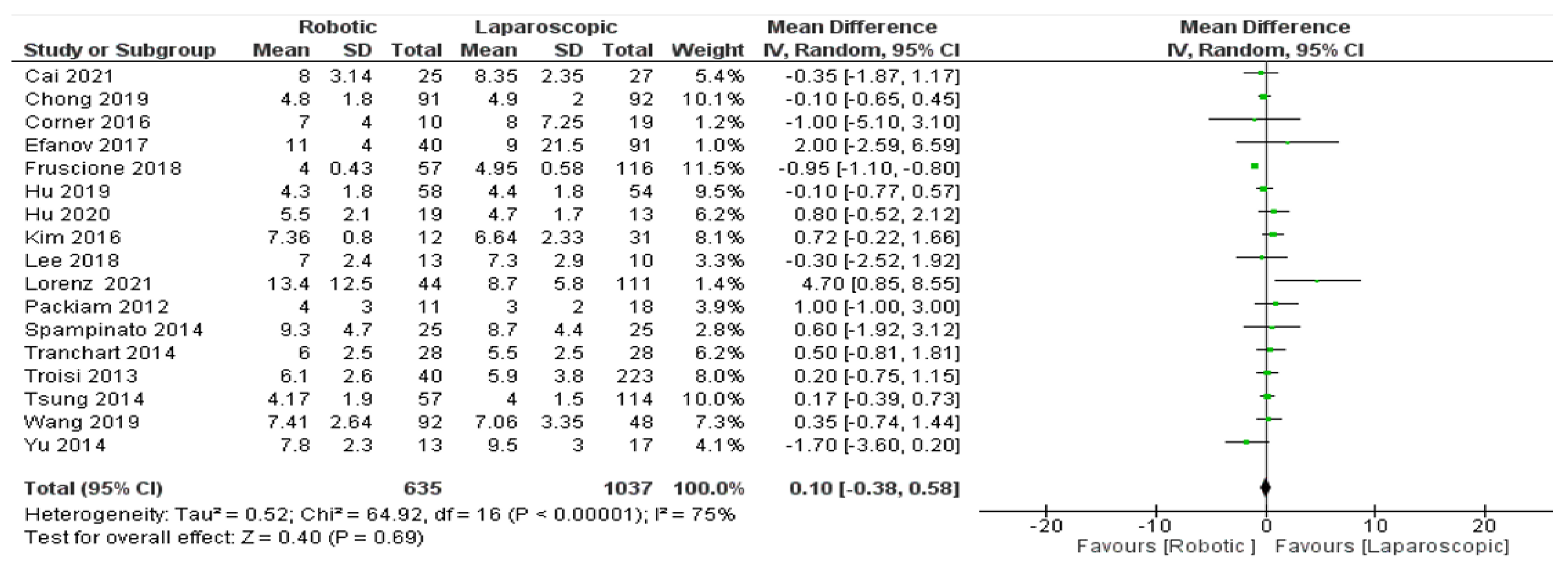
- Length of hospital stay
- Tumor size
- Subgroup analyses
- Number of patients with malignant liver tumors
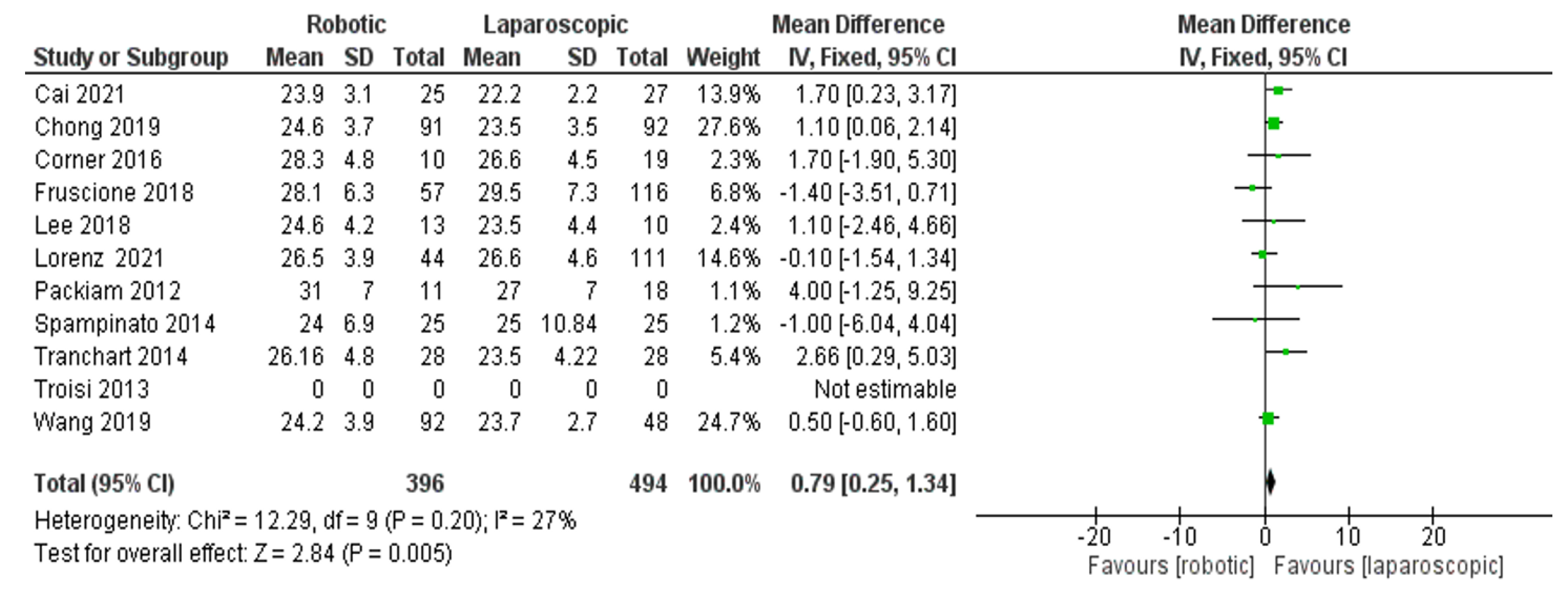
- Body mass index (BMI)
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, R.; Wakabayashi, G.; Kim, H.-J.; Choi, G.-H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; I Troisi, R.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton consensus guidelines for laparoscopic liver surgery: From indication to implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Swaid, F.; Cipriani, F.; Ratti, F.; Heres, C.; Tsung, A.; Aldrighetti, L.; Geller, D.A. Propensity score-matched analysis of pure laparoscopic versus hand-assisted/hybrid major hepatectomy at two western centers. World J. Surg. 2019, 43, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Aldrighetti, L.; Cipriani, F.; Fiorentini, G.; Catena, M.; Paganelli, M.; Ratti, F. A stepwise learning curve to define the standard for technical improvement in laparoscopic liver resections: Complexity-based analysis in 1032 procedures. Updates Surg. 2019, 71, 273–283. [Google Scholar] [CrossRef]
- Polignano, F.M.; Quyn, A.; De Figueiredo, R.S.M.; Henderson, N.A.; Kulli, C.; Tait, I.S. Laparoscopic versus open liver segmentectomy: Prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg. Endosc. 2008, 22, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Dagher, I.; O’Rourke, N.; Geller, D.A.; Cherqui, D.; Belli, G.; Gamblin, T.C.; Lainas, P.; Laurent, A.; Nguyen, K.T.; Marvin, M.R.; et al. Laparoscopic major hepatectomy: An evolution in standard of care. Ann Surg. 2009, 250, 856–860. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Marsh, J.W.; Tsung, A.; Steel, J.J.L.; Gamblin, T.C.; Geller, D.A. Comparative benefits of laparoscopic vs. open hepatic resection: A critical appraisal. Arch. Surg. 2011, 146, 348–356. [Google Scholar] [CrossRef]
- Yin, Z.; Fan, X.; Ye, H.; Yin, D.; Wang, J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: A global systemic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 1203–1215. [Google Scholar] [CrossRef]
- Usatoff, V.; Evans, P.M. Laparoscopic hepatectomy is a safe procedure for cancer patients. HPB 2009, 11, 247–251. [Google Scholar]
- Simillis, C.; Constantinides, V.A.; Tekkis, P.P.; Darzi, A.; Lovegrove, R.; Jiao, L.; Antoniou, A. Laparoscopic versus open hepatic resections for benign and malignant neoplasms—A meta-analysis. Surgery 2007, 141, 203–211. [Google Scholar] [CrossRef]
- Morino, M.; Morra, I.; Rosso, E.; Miglietta, C.; Garrone, C. Laparoscopic vs. open hepatic resection: A comparative study. Surg. Endosc. 2003, 17, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Dagher, I.; Di Giuro, G.; Dubrez, J.; Lainas, P.; Smadja, C.; Franco, D. Laparoscopic versus open right hepatectomy: A comparative study. Am. J. Surg. 2009, 198, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.J.; Altaf, K.; Javed, M.A.; Huang, W.; Mukherjee, R.; Mai, G.; Sutton, R.; Liu, X.B.; Hu, W.M. Meta-analysis of laparoscopic vs. open liver resection for hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 6657–6668. [Google Scholar] [CrossRef]
- Brytska, N.; Han, H.S.; Shehta, A.; Yoon, Y.S.; Cho, J.Y.; Choi, Y. Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with Child B or C cirrhosis. Hepatobiliary Surg. Nutr. 2015, 4, 373–378. [Google Scholar] [PubMed]
- Memeo, R.; De’Angelis, N.; De Blasi, V.; Cherkaoui, Z.; Brunetti, O.; Longo, V.; Piardi, T.; Sommacale, D.; Marescaux, J.; Mutter, D.; et al. Innovative surgical approaches for hepatocellular carcinoma. World J. Hepatol. 2016, 8, 591–596. [Google Scholar] [CrossRef]
- Memeo, R.; De’Angelis, N.; Compagnon, P.; Salloum, C.; Cherqui, D.; Laurent, A.; Azoulay, D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J. Surg. 2014, 38, 2919–2926. [Google Scholar] [CrossRef]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef]
- Sposito, C.; Battiston, C.; Facciorusso, A.; Mazzola, M.; Muscarà, C.; Scotti, M.; Romito, R.; Mariani, L.; Mazzaferro, V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 871–880. [Google Scholar] [CrossRef]
- Tsinberg, M.; Tellioglu, G.; Simpfendorfer, C.H.; Walsh, M.R.; Vogt, D.; Fung, J.; Berber, E. Comparison of laparoscopic versus open liver tumor resection: A case controlled study. Surg. Endosc. 2009, 23, 847–853. [Google Scholar] [CrossRef]
- Kitisin, K.; Packiam, V.; Bartlett, D.L.; Tsung, A. A current update on the evolution of robotic liver surgery. Minerva Chir. 2011, 66, 281. [Google Scholar]
- Ji, W.B.; Wang, H.G.; Zhao, Z.M.; Duan, W.D.; Lu, F.; Dong, J.H. Robotic-assisted laparoscopic anatomic hepatectomy in China: Initial experience. Ann. Surg. 2011, 253, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Leung, U.; Fong, Y. Robotic liver surgery. Hepatobiliary Surg. Nutr. 2014, 3, 288–294. [Google Scholar] [PubMed]
- Li, Y.; Cao, L.; Zhang, Z.; Hou, L.; Qin, Y.; Hui, X.; Li, J.; Zhao, H.; Cui, G.; Yang, K.; et al. Reporting and methodological quality of COVID-19 systematic reviews needs to be improved: An evidence mapping. J. Clin. Epidemiol. 2021, 135, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Boonpheng, B.; Thongprayoon, C.; A Mao, M.; Wijarnpreecha, K.; Bathini, T.; Kaewput, W.; Ungprasert, P.; Cheungpasitporn, W. Risk of hip fracture in patients on hemodialysis versus peritoneal dialysis: A meta-analysis of observational studies. J. Evid.-Based Med. 2019, 12, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Duffles, L.F.; Hermont, A.P.; Abreu, L.G.; Pordeus, I.A.; Silva, T.A. Association between obesity and adipokines levels in saliva and gingival crevicular fluid: A systematic review and meta-analysis. J. Evid.-Based Med. 2019, 12, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-P.; Chen, W.; Chen, L.-H.; Wan, X.-Y.; Lai, J.-M.; Yin, X.-Y. Comparison between robotic-assisted and laparoscopic left hemi-hepatectomy. Asian J. Surg. 2021, 45, 265–268. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lok, H.T.; Fung, A.K.Y.; Fong, A.K.W.; Cheung, Y.S.; Wong, J.; Lee, K.F.; Lai, P.B.S. Robotic versus laparoscopic hepatectomy: Application of the difficulty scoring system. Surg. Endosc. 2019, 34, 2000–2006. [Google Scholar] [CrossRef]
- Croner, R.S.; Perrakis, A.; Hohenberger, W.; Brunner, M. Robotic liver surgery for minor hepatic resections: A comparison with laparoscopic and open standard procedures. Langenbeck’s Arch. Surg. 2016, 401, 707–714. [Google Scholar] [CrossRef]
- Efanov, M.; Alikhanov, R.; Tsvirkun, V.; Kazakov, I.; Melekhina, O.; Kim, P.; Vankovich, A.; Grendal, K.; Berelavichus, S.; Khatkov, I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 2017, 19, 818–824. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.; Hu, M.; Zhao, Z.; Zhao, G.; Li, C.; Tan, X.; Zhang, X.; Lau, W.Y.; Liu, R. Robotic vs. laparoscopic hemihepatectomy: A comparative study from a single center. J. Surg. Oncol. 2019, 120, 646–653. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Li, C.; Wang, G.; Yin, Z.; Lau, W.Y.; Liu, R. Robotic versus laparoscopic liver resection in complex cases of left lateral sectionectomy. Int. J. Surg. 2019, 67, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tsung, A.; Geller, D.A.; Sukato, D.C.; Sabbaghian, S.; Tohme, S.; Steel, J.; Marsh, W.; Reddy, S.K.; Bartlett, D.L. Robotic versus laparoscopic hepatectomy: A matched comparison. Ann. Surg. 2014, 259, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Fruscione, M.; Pickens, R.; Baker, E.H.; Cochran, A.; Khan, A.; Ocuin, L.; Iannitti, D.A.; Vrochides, D.; Martinie, J.B. Robotic-assisted versus laparoscopic major liver resection: Analysis of outcomes from a single center. HPB 2019, 21, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, K.; Zhang, X.; Li, C.; Song, D.; Liu, R. Robotic, laparoscopic or open hemihepatectomy for giant liver haemangiomas over 10 cm in diameter. BMC Surg. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.I.; Patriti, A.; Montalti, R.; Casciola, L. Robot assistance in liver surgery: A real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 160–166. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.H.; Lee, Y.J.; Kim, S.C.; Hwang, D.W.; Song, K.B.; Shin, S.H.; Kwon, J.W.; Park, G.S.; Park, Y.J.; et al. The feasibility of robotic left-side hepatectomy with comparison of laparoscopic and open approach: Consecutive series of single surgeon. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e1982. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, J.S.; Han, D.H.; Choi, G.H.; Kim, K.S.; Choi, J.S.; Yoon, D.S. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg. Endosc. 2016, 30, 4756–4764. [Google Scholar] [CrossRef]
- Berber, E.; Akyildiz, H.Y.; Aucejo, F.; Gunasekaran, G.; Chalikonda, S.; Fung, J. Robotic versus laparoscopic resection of liver tumours. HPB 2010, 12, 583–586. [Google Scholar] [CrossRef]
- Lorenz, E.; Arend, J.; Franz, M.; Rahimli, M.; Perrakis, A.; Negrini, V.; Gumbs, A.A.; Croner, R.S. Robotic and laparoscopic liver resection—Comparative experiences at a high-volume German academic center. Langenbecks Arch. Surg. 2021, 406, 753–761. [Google Scholar] [CrossRef]
- Yu, Y.D.; Kim, K.H.; Jung, D.H.; Namkoong, J.M.; Yoon, S.Y.; Jung, S.W.; Lee, S.K.; Lee, S.G. Robotic versus laparoscopic liver resection: A comparative study from a single center. Langenbecks Arch. Surg. 2014, 399, 1039–1045. [Google Scholar] [CrossRef]
- Packiam, V.; Bartlett, D.L.; Tohme, S.; Reddy, S.; Marsh, J.W.; Geller, D.A.; Tsung, A. Minimally invasive liver resection: Robotic versus laparoscopic left lateral sectionectomy. J. Gastrointest. Surg. 2012, 16, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Tranchart, H.; Ceribelli, C.; Ferretti, S.; Dagher, I.; Patriti, A. Traditional versus robot-assisted full laparoscopic liver resection: A matched-pair comparative study. World J. Surg. 2014, 38, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.G.; Coratti, A.; Bianco, L.; Caniglia, F.; Laurenzi, A.; Puleo, F.; Ettorre, G.M.; Boggi, U. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: An Italian multi-institutional comparative study. Surg. Endosc. 2014, 28, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Hu, R.H.; Lai, H.S.; Lee, P.H. Robotic-assisted minimally invasive liver resection. Asian J. Surg. 2014, 37, 53–57. [Google Scholar] [CrossRef]
- Koffron, A.J.; Auffenberg, G.; Kung, R.; Abecassis, M. Evaluation of 300 minimally invasive liver resections at a single institution: Less is more. Ann. Surg. 2007, 246, 463–468. [Google Scholar] [CrossRef]
- Choi, S.B.; Park, J.S.; Kim, J.K.; Hyung, J.; Kim, K.S.; Yoon, D.S.; Lee, W.J.; Kim, B.R. Early experiences of robotic-assisted laparoscopic liver resection. Yonsei Med. J. 2008, 49, 632–638. [Google Scholar] [CrossRef]
- Boggi, U.; Moretto, C.; Vistoli, F.; D’Imporzano, S.; Mosca, F. Robotic suture of a large caval injury caused by endo-GIA stapler malfunction during laparoscopic wedge resection of liver segments VI and VII en-bloc with right hepatic vein. Minim. Invasive Allied Technol. 2009, 18, 306–310. [Google Scholar] [CrossRef]
- Kang, S.W.; Jeong, J.J.; Yun, J.S.; Sung, T.Y.; Lee, S.C.; Lee, Y.S.; Nam, K.H.; Chang, H.S.; Chung, W.Y.; Park, C.S. Robot-assisted endoscopic surgery for thyroid cancer: Experience with the first 100 patients. Surg. Endosc. 2009, 23, 2399–2406. [Google Scholar] [CrossRef]
- Lee, K.E.; Rao, J.; Youn, Y.K. Endoscopic thyroidectomy with the da Vinci Robot System Using the Bilateral Axillary Breast Approach Technique: Our initial experience. Surg. Laparosc. Endosc. Percutan. Tech. 2009, 19, 71–75. [Google Scholar] [CrossRef]
- Khalili, K.; Kim, T.K.; Jang, H.J.; Haider, M.A.; Khan, L.; Guindi, M.; Sherman, M. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: An analysis of diagnostic performance and resource utilization. J. Hepatol. 2011, 54, 723–728. [Google Scholar] [CrossRef]
- Lorusso, V.; Pascolo, L.; Fernetti, C.; Visigalli, M.; Anelli, P.; Tiribelli, C. In vitro and in vivo hepatic transport of the magnetic resonance imaging contrast agent B22956/1: Role of MRP proteins. Biochem. Biophys. Res. Commun. 2002, 293, 100–105. [Google Scholar] [CrossRef]
- Granito, A.; Galassi, M.; Piscaglia, F.; Romanini, L.; Lucidi, V.; Renzulli, M.; Borghi, A.; Grazioli, L.; Golfieri, R.; Bolondi, L. Impact of gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance on the non-invasive diagnosis of small hepatocellular carcinoma: A prospective study. Aliment. Pharmacol. Ther. 2012, 37, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Rao, G.; Ahmed, I. Laparoscopic or open liver resection? Let systematic review decide it. Am. J. Surg. 2012, 204, 222–231. [Google Scholar] [CrossRef]
- Mirnezami, R.; Mirnezami, A.H.; Chandrakumaran, K.; Abu Hilal, M.; Pearce, N.W.; Primrose, J.; Sutcliffe, R. Short- and long-term outcomes after laparoscopic and open hepatic resection: Systematic review and meta-analysis. HPB 2011, 13, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.; Wong, D.; Karalapillai, D.; Pearce, B.; Tan, C.O.; Tay, S.; Christophi, C.; McNicol, L.; Nikfarjam, M. The impact of fluid intervention on complications and length of hospital stay after pancreaticoduodenectomy (Whipple’s procedure). BMC Anesthesiol. 2014, 14, 35. [Google Scholar] [CrossRef]
- Guan, R.; Chen, Y.; Yang, K.; Ma, D.; Gong, X.; Shen, B.; Peng, C. Clinical efficacy of robot-assisted versus laparoscopic liver resection: A meta analysis. Asian J. Surg. 2019, 42, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Marubashi, S.; Nagano, H. Laparoscopic living-donor hepatectomy: Review of its current status. Ann. Gastroenterol. Surg. 2021, 5, 484–493. [Google Scholar] [CrossRef]
- Nouri, M.; Mohsenpour, M.A.; Katsiki, N.; Ghobadi, S.; Jafari, A.; Faghih, S.; Banach, M.; Mazidi, M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. J. Clin. Med. 2022, 11, 4503. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Sasaki, A.; Nishizuka, S.; Furukawa, T.; Kitajima, M. Our initial experience with robotic hepato-biliary-pancreatic surgery. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 481–487. [Google Scholar] [CrossRef]
- McAleese, P.; Odling-Smee, W. The effect of complications on length of stay. Ann Surg. 1994, 220, 740–744. [Google Scholar] [CrossRef]
- Huebner, M.; Hubner, M.; Cima, R.R.; Larson, D.W. Timing of complications and length of stay after rectal cancer surgery. J. Am. Coll. Surg. 2014, 218, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Sun, R.L.; Jarnagin, W.; Blumgart, L.H. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann. Surg. 1999, 229, 790. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Period | Design | Group | Total | Sex n (M%) | Mean Age | BMI (kg/m2) | Tumor Size(cm) mm | Pure Hemi Hepatectomy, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cai [26], 2021 | China | 2015–2020 | Retrospectives | RH | 25 | 12 (48.0%) | 56.4 ± 9.1 | 23.9 ± 3.1 | 5.5 ± 2.3 | YES |
| LH | 27 | 18 (66.7% | 52.7 ± 11.6 | 22.2 ± 2.2 | 4.3 ± 1.9 | |||||
| Chong [27], 2019 | China | 2003–2017 | Prospective | RH | 91 | NA | 58.7 ± 11.7 | 24.6 ± 3.7 | <3 cm, ≥3 cm | NO |
| LH | 92 | NA | 59.8 ± 11.9 | 23.5 ± 3.5 | <3 cm, ≥3 cm | NO | ||||
| Croner [28], 2016 | Germany | 2011–2015 | Retrospectives | RH | 10 | NA | 64 (45–76 | 28 (28.3) | 4.8 (2.9–10.5 | NO |
| LH | 19 | NA | 59 (32–85) | 27 (26.6) | 4.1 (1.8–8.5) | NO | ||||
| Efanov [29], 2017 | Russia | 2010–2016 | Retrospectives | RH | 40 | 9 (NA) | 45(18–76) | NA | 73 (17–142) | NO |
| LH | 91 | 36(NA) | 51(21–77) | NA | 64 (8–180) | NO | ||||
| Wang [30], 2019 | China | 2011–2017 | Retrospectives | RH | 92 | 55 (59.8) | 54.1 ± 11.2 | 24.2 ± 3.9 | <5 cm, ≥5 cm | YES |
| LH | 48 | 24 (50.0) | 49.4 ± 13.0 | 23.7 ± 2.7 | <5 cm, ≥5 cm | |||||
| Hu [31], 2019 | China | 2015–2017 | Retrospective | RL | 58 | 33 | 52.2 years | 24.7 | NA | NO |
| LH | 54 | 26 | 48.9 years | 23.8 | NA | NO | ||||
| Tsung [32], 2014 | USA | 2007–2011 | Retrospective | RH | 57 | 24 (42%) | 58.35 ± 14.6 | NA | 3.15 (2.05–5.00) | NO |
| LH | 114 | 47 (41%) | 58.72 ± 15.8 | NA | 3.50 (2.0–6.0) | NO | ||||
| Fruscione [33], 2018 | USA | 2011–2016 | Retrospective | RH | 57 | 20 (35.1) | 58.1 (15.7) | 28.1 (6.3) | NA | YES |
| LH | 116 | 52 (44.8%) | 53.2 (15.4) | 29.5 (7.3) | NA | |||||
| Hu [34], 2020 | China | 2011–2017 | Retrospectively | RH | 19 | (10.5%) | 49.2 ± 10.6 | 1.6 ± 0.2 | >10 cm | YES |
| LH | 13 | (7.7%) | 46.5 ± 8.9 | 1.6 ± 0.2 | >10 cm | |||||
| Troisi [35], 2013 | Belgium-Italy | 2004–2010 | Retrospective-comparative | RH | 40 | (67.5%) | 64.6 ± 12.1 | NA | 51.8 ± 37.6 (1–19) | NO |
| LH | 233 | 43.9% | 55.3 ± 15.7 | NA | 49.7 ± 37.7 (1–20) | NO | ||||
| Lee [36], 2018 | South Korea | 2016–2018 | Retrospective | RH | 13 | NA | 62.2 ± 9. | 24.6 ± 4.2 | 41.3 ± 23.8 | NO |
| LH | 10 | NA | 58.8 ± 11.2 | 23.5 ± 4.4 | 32.8 ± 18.0 | NO | ||||
| Kim [37], 2016 | South Korea | 2007–2013 | Retrospective | RH | 12 | 6 (50%) | 54.1 ± 12.2 | NA | 2.3 (2.0–3.6) | NO |
| LH | 31 | 18 (58%) | 56.4 ± 11.6 | NA | 2.4 (1.7–3.0) | |||||
| Berber [38], 2010 | USA | 2008–2009 | Prospective | RH | 9 | 7 (77.8%) | 66.6 ± 6.4 | NA | 3.2 ± 1.3 | NO |
| LH | 23 | 12 (52%) | 66.7 ± 9.6 | NA | 2.9 ± 1.3 | NO | ||||
| Lorenz [39], 2021 | Germany | 2010–2020 | Retrospective | RH | 44 | 24 (54.5%) | 62.6 ± 14.5 | 26.5 ± 3.9 | 5.6 ± 2.7 | |
| LH | 111 | 50 (45.0%) | 61 (55.0) | 27.0 ± 4.6 | 3.7 ± 2.4 | |||||
| Yu [40], 2014 | South Korea | 2007–2011 | Case Control | RH | 13 | 7 (53.9%) | 50.4 ± 12.2 | NA | 31.1 ± 16.0 | NO |
| LH | 17 | 9 (52.94%) | 52.5 ± 9.7 | NA | 34.8 ± 18.2 | NO | ||||
| Packiam [41], 2012 | USA | 2009–2011 | Retrospective | RH | 11 | 3 (27%) | 57 ± 16 | 31 ± 7 | 5.5(2.4–6.5) | NO |
| LH | 18 | 4 (22%) | 52 ± 17 | 29 ± 7 | 4.4 (2.6–7.1) | NO | ||||
| Tranchart [42], 2014 | France-Italy | 2008–2013 | Matched design | RH | 28 | 13 (46.4%) | 66.5 (42–84) | 26.1 (16.7–36) | 35(6–115) | NO |
| LH | 28 | 13 (46.4%) | 66(41–78) | 23.2 (16–33) | 40(6–130) | NO | ||||
| Spampinato [43], 2014 | Italy | 2009–2012 | Retrospective | RH | 25 | 13 (52%) | 63 (32–80) | 24 (16.4–21.8) | NA | NO |
| LH | 25 | 10 (40%) | 62 (33–80) | 25 (20–28.5) | NA | NO | ||||
| Wu [44], 2014 | Taiwan | 2007–2011 | Retrospective | RH | 38 | 32 (84.2%) | 60.9 ± 14.9 | NA | 3.4 ± 1.7 | NO |
| LH | 41 | 28 (68.3%) | 54.1 ± 14 | NA | 2.5 ± 1.6 |
| Author | Group | Surgical Duration (minute) | Complications | Blood Loss (mL) | Conversion to Open | Transfusion | Reoperation | Length of Hospital (day) | Hepatectomy Extend |
|---|---|---|---|---|---|---|---|---|---|
| Cai [26], 2021 | RH | 303.578 ± 149.3624 | 1/25 | 100 ± 37.037 | 0/25 | 0/25 | 0/27 | 8 ± 3.1445 | left hemihepatectomy: 25(48.07%) |
| LH | 313.57 ± 117.40 | 4/27 | 200 ± 148 | 1/27 | 6/27 | 1/27 | 8.35 ± 2.34 | left hemihepatectomy: 27(51.92%) | |
| Chong [27], 2019 | RH | 259.3 ± 127.0 | 9/91 | 274.6 ± 568.1 | 7/91 | NA | 5/91 | 4.8 ± 1.8 | LLS:39 (42.9%) Wedge resection:31 (34.1%) Left hepatectomy:39 (42.9%) Right hepatectomy:6 (6.6%) CLR: 1 (1.1% MR: 2 (2.2%) Major:19 (20.9%) Minor: 72 (79.1%) |
| LH | 216.8 ± 79.2 | 5/92 | 212.4 ± 313.4 | 11/92 | NA | 8/92 | 4.9 ± 2.0 | LLS: 40 (43.5%) Wedge resection:47 (51.1%) Left hepatectomy: 7 (51.1%) Right hepatectomy: 1 (1.1%) CLR:0 MR:0 Major: 4 (4.3%) Minor: 88 (95.7%) | |
| Croner [28], 2016 | RH | 321 ± 80 | NA | 306 mL NA | NA | NA | NA | 7 ± 4 | Minor: 10 (34.48%) |
| LH | 242 ± 99.5 | NA | 356 mL (NA) | NA | NA | NA | 8 ± 7.25 | Minor: 19 (65.515) | |
| Efanov [29], 2017 | RH | 407 ± 223.73 | 5/40 | 465 ± 500 | NA | 3/40 | 1/40 | 11 ± 4 | RH-H:0 LH-H: 2 (5%) RPS: 5 (13%) S: 1 (3%) WRPS: 3 (8%) ALS-S: 18 (45%) WRAS: 11 (28%) |
| LH | 296 ± 133.75 | 7/91 | 302 ± 550 | NA | 4/91 | 1/91 | 9 ± 21.5 | RH-H: 9 (10%) LH-H: 2 (2%) RPS: 6 (7%) S: 6 (7%) WRPS: 23 (25%) ALS-S: 24 (26%) WRS: 21 (23%) | |
| Wang [30], 2019 | RH | 195.53 ± 67.00 | 12/92 | 346.04 ± 234.17 | NA | NA | NA | 7.41 ± 2.64 | Left liver: 48 (52.2%) Right liver: 44 (47.8%) |
| LH | 198.98 ± 72.94 | 5/48 | 243.04 ± 171.87 | NA | NA | NA | 7.06 ± 3.35 | Left liver: 29 (60.4%) Right liver: 19 (21.6%) | |
| Hu [31], 2019 | RL | 107.0 ± 45.2 | NA | 80.1 ± 144.4 | 0/58 | NA | NA | 4.3 ± 1.8 | Left lateral sectionectomy:51.17 % |
| LH | 95.7 ± 47.5 | NA | 108.9 ± 180.8 | 1/54 | NA | NA | 4.4 ± 1.8 | Left lateral sectionectomy:48.21% | |
| Tsung [32],2014 | RH | 353.66 ± 143.75 | 11/57 | 195.58 ± 218.66 | 4/57 | 2\57 | NA | 4.1767± 1.90 | Major:21(NA) Minor:36(NA) |
| LH | 261.5 ± 98.9 | 29/114 | 170.34 ± 225.25 | 10/114 | 7\114 | NA | 4 ± 1.50 | Major:42(NA) Minor:72(NA) | |
| Fruscione [33], 2018 | RH | 195.537 ± 22.457 | 16/57 | 268.18 ± 103.56 | NA | NA | NA | 4 ± 0.4361 | Left: 20 (35.1) Patial: 17 (29.8) Right: 20 (35.1) |
| LH | 205.0674 ± 25.6799 | 41/116 | 405.08 ± 117.62 | NA | NA | NA | 4.9492 ± 0.5881 | Left: 22 (19.0) Patial: 48 (41.4) Right: 46 (39.7) | |
| Hu [34], 2020 | RH | 268.4 ± 93.6 | NA | 319.5 ± 206.0 | NA | 5\19 | NA | 5.5 ± 2.1 | Right:15 Left:4 |
| LH | 268.4 ± 93.6 | NA | 476.9 ± 210.8 | NA | 4\13 | NA | 4.7 ± 1.7 | Right:8 Left:5 | |
| Troisi [35], 2013 | RH | 271 ± 100 | 5/40 | NA | 8/40 | NA | NA | 6.1 ± 2.6 | Major hepatectomy:0 Left hepatectomy:0 Right hepatectomy:0 |
| LH | 262 ± 111 | 28/223 | NA | 17/223 | NA | NA | 5.9 ± 3.8 | Major hepatectomy: 37 (16.6%) Left hepatectomy:16 (7.2%) Right hepatectomy:17 (7.6%)’& other extents | |
| Lee [36], 2018 | RH | 248.6 ± 37.5 | NA | 320.3 ± 331.9 | 0/13 | 0/13 | NA | 7.0 ± 2.4 | left-sidehepatectomy Left lateral sectionectomy |
| LH | 226.7 ± 26.6 | NA | 392.8 ± 374.5 | 1/10 | 0/10 | NA | 7.3 ± 2.9 | ||
| Kim [37],2016 | RH | 403.8 ± 139.0 | NA | 206.6875 ± 125.79 | NA | 1/12 | NA | 7.36 ± 0.8386 | left lateral sectionectomy: (27.90%) |
| LH | 245.9 ± 100.7 | NA | 212.3508 ± 291.4453 | NA | 1/31 | NA | 6.6437 ± 2.3316 | left lateral sectionectomy: (72.09%) | |
| Berber [38], 2010 | RH | 258.5 ± 27.9 | NA | 136 ± 61 | 1/9 | NA | NA | NA | Segmental liver resection:6 Left lateral sectionectomy:3 |
| LH | 233.6 ± 16.4 | NA | 155 ± 54 | 0/23 | NA | NA | NA | Segmental liver resection:12 Left lateral sectionectomy:11 | |
| Lorenz [39], 2021 | RH | 330.5 ± 132.2 | 4/44 | 439.8 ± 346.3 | NA | 7/44 | NA | 13.4 ± 12.5 | Major: 16, Minor: 25 |
| LH | 181.3 ± 100.4 | 3/111 | 425.4 ± 590.1 | NA | 7/111 | NA | 8.7 ± 5.8 | Major: 12, Minor: 60 | |
| Yu [40], 2014 | RH | 291.5 ± 85.1 | 0/13 | 388.5 ± 65.0 | 0/13 | 0/13 | NA | 7.8 ± 2.3 | LLS:10, LH-H:3 |
| LH | 240.9 ± 68.6 | 2/17 | 342.6 ± 84.7 | 0/17 | 0/17 | NA | 9.5 ± 3.0 | LLS:6, LH-H:11 | |
| Packiam [41], 2012 | RH | 175 ± 85 | — | 30 ± 40 | 0/11 | 0 | NA | 4 ± 3 | LLS |
| LH | 188 ± 85 | — | 30 ± 35 | 0/18 | 0 | NA | 3 ± 2 | LLS | |
| Tranchart [42], 2014 | RH | 210 ± 125 | NA | 200 ± 150 | 4/28 | 4/28 | NA | 6 ± 2.5 | Bisegmentectomy:1,LLS:5, Segmentectomy:7, etc.. |
| LH | 176 ± 125 | NA | 150 ± 150 | 2/28 | 1/28 | NA | 5.5 ± 2.5 | Bisegmentectomy:1,LLS:5, Segmentectomy:7, etc.. | |
| Spampinato [43], 2014 | RH | 430 ± 120 | 5/25 | 250 ± 155 | 1/25 | 1/25 | NA | 9.3 ± 4.7 | Major hepatectomy |
| LH | 360 ± 120 | 9/25 | 400 ± 155 | 1/25 | 4/25 | NA | 8.7 ± 4.4 | Major hepatectomy | |
| Wu [44], 2014 | RH | 380 ± 166 | NA | 325 ± 480 | 2/38 | NA | NA | — | Major liver resection |
| LH | 227 ± 80 | NA | 173 ± 165 | 5/41 | NA | NA | — | Right &Left lobe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboudou, T.; Li, M.; Zhang, Z.; Wang, Z.; Li, Y.; Feng, L.; Chu, X.; Chen, N.; Zhou, W.; Yang, K. Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5831. https://doi.org/10.3390/jcm11195831
Aboudou T, Li M, Zhang Z, Wang Z, Li Y, Feng L, Chu X, Chen N, Zhou W, Yang K. Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(19):5831. https://doi.org/10.3390/jcm11195831
Chicago/Turabian StyleAboudou, Taslim, Meixuan Li, Zeliang Zhang, Zhengfeng Wang, Yanfei Li, Lufang Feng, Xiajing Chu, Nan Chen, Wence Zhou, and Kehu Yang. 2022. "Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 19: 5831. https://doi.org/10.3390/jcm11195831
APA StyleAboudou, T., Li, M., Zhang, Z., Wang, Z., Li, Y., Feng, L., Chu, X., Chen, N., Zhou, W., & Yang, K. (2022). Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(19), 5831. https://doi.org/10.3390/jcm11195831