A New Treatment Protocol of Combined High-Dose Levothyroxine and Repetitive Transcranial Magnetic Stimulation for the Treatment of Rapid-Cycling Bipolar Spectrum Disorders: A Cohort Evaluation of 55 Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Socio-Demographic and Clinical Characteristics
3.2. Tolerability
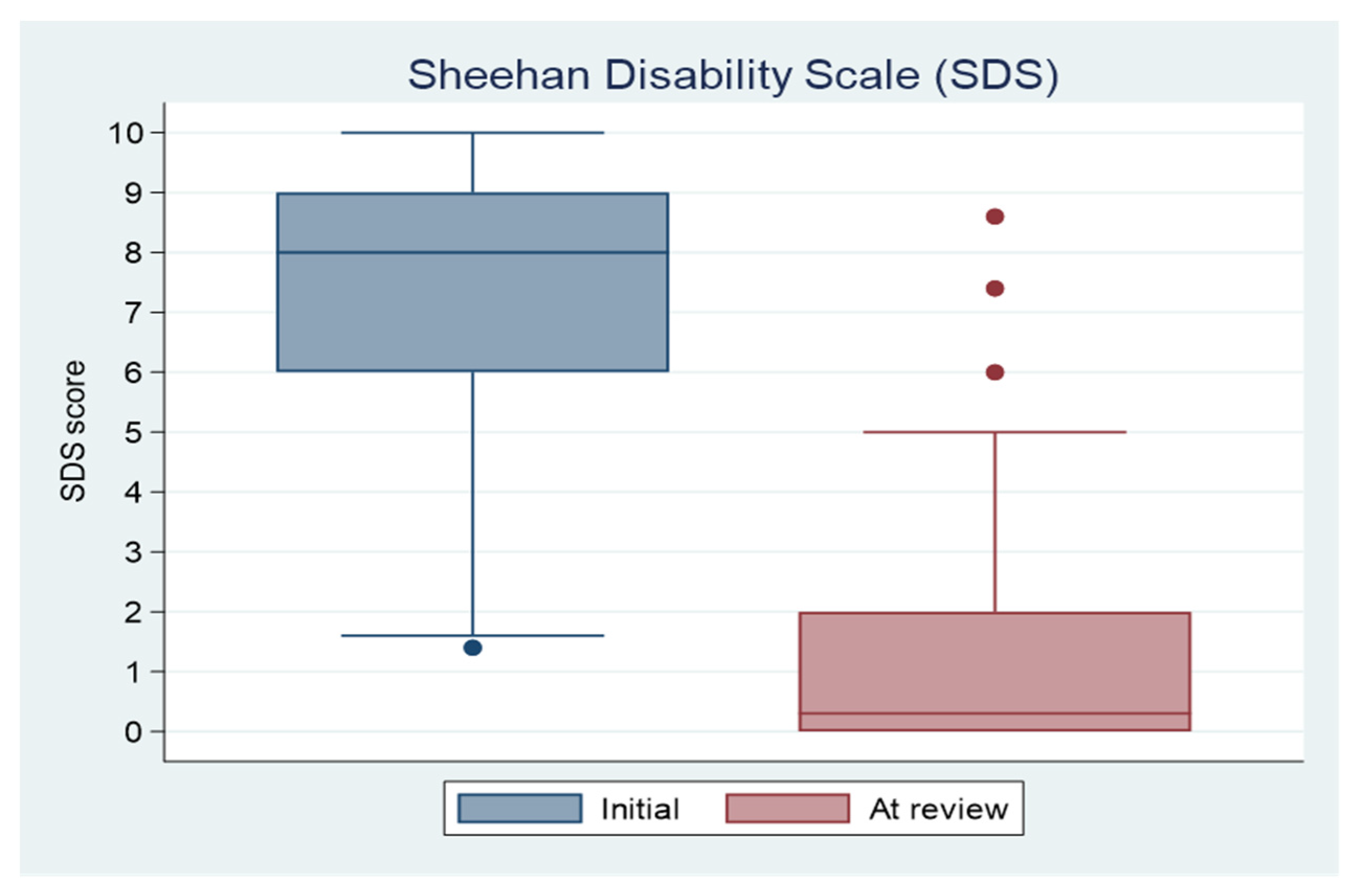
3.3. Primary Outcome
3.4. Secondary Clinical Outcomes
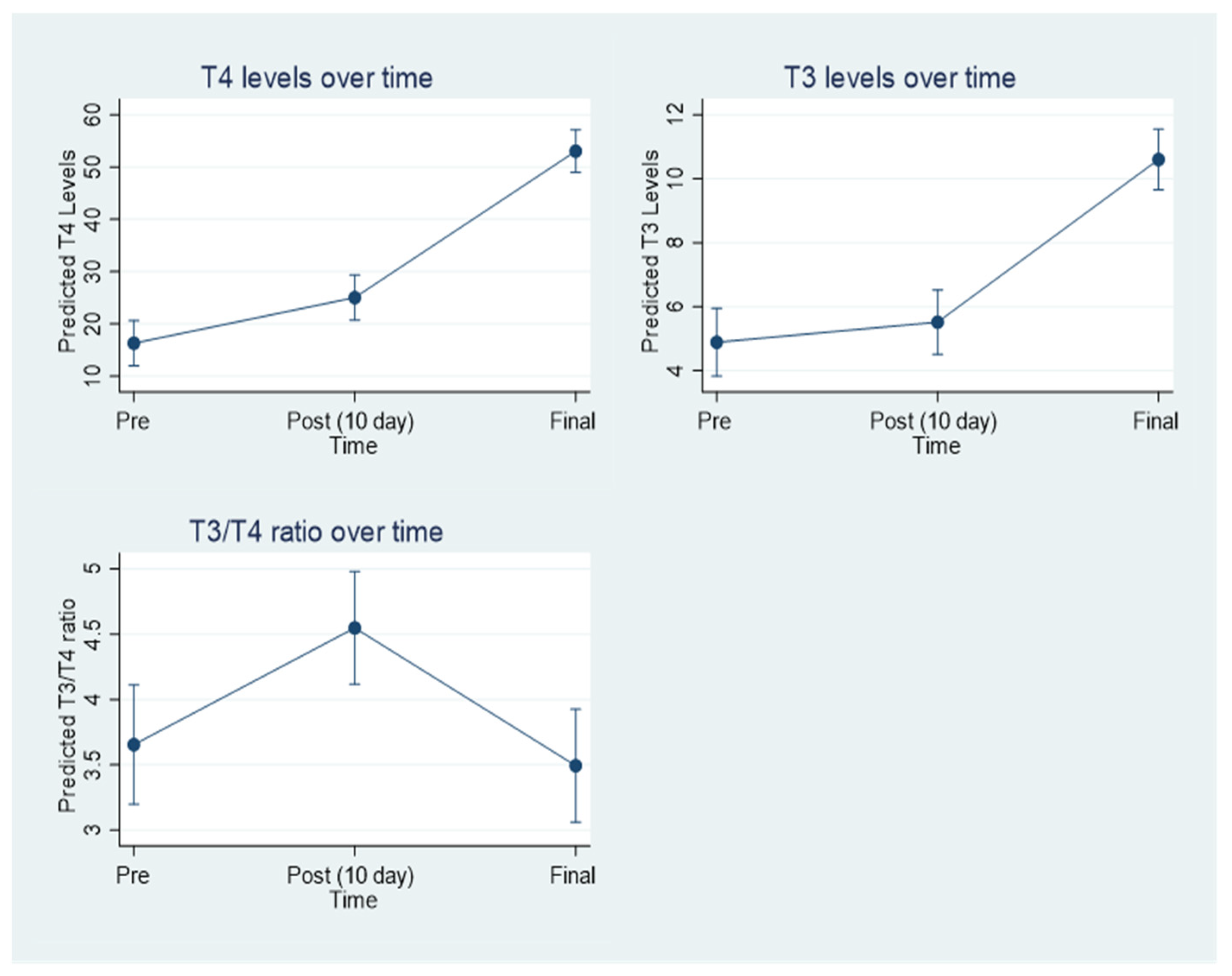
3.5. Thyroid Hormone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.; Hällgren, J.; Wahlbeck, K.; Erlinge, D.; Alfredsson, L.; Osby, U. Cardiovascular mortality in bipolar disorder: A population-based cohort study in Sweden. BMJ Open 2013, 3. Available online: https://bmjopen.bmj.com/content/3/4/e002373 (accessed on 15 July 2022). [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.; Petukhova, M.; Kessler, R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Angst, J. The Bipolar Spectrum. Br. J. Psychiatry 2007, 190, 189–191. [Google Scholar] [CrossRef]
- Kramlinger, K.G.; Post, R.M. Ultra-rapid and ultradian cycling in bipolar affective illness. Br. J. Psychiatry 1996, 168, 314–323. [Google Scholar] [CrossRef]
- Bonnín, C.M.; Sánchez-Moreno, J.; Martínez-Arán, A.; Solé, B.; Reinares, M.; Rosa, A.R.; Goikolea, J.M.; Benabarre, A.; Ayuso-Mateos, J.L.; Ferrer, M.; et al. Subthreshold symptoms in bipolar disorder: Impact on neurocognition, quality of life and disability. J. Affect. Disord. 2012, 136, 650–659. [Google Scholar] [CrossRef]
- De Dios, C.; Ezquiaga, E.; Agud, J.L.; Vieta, E.; Soler, B.; García-López, A. Subthreshold symptoms and time to relapse/recurrence in a community cohort of bipolar disorder outpatients. J. Affect. Disord. 2012, 143, 160–165. [Google Scholar] [CrossRef]
- Garriga, M.; Solé, E.; González-Pinto, A.; Selva-Vera, G.; Arranz, B.; Amann, B.L.; Saiz-Ruiz, J.; Pérez-Blanco, J.; Vieta, E. Efficacy of quetiapine XR vs. placebo as concomitant treatment to mood stabilizers in the control of subthreshold symptoms of bipolar disorder: Results from a pilot, randomized controlled trial. Eur. Neuropsychopharmacol. 2017, 27, 959–969. [Google Scholar] [CrossRef]
- Adli, M.; Whybrow, P.C.; Grof, P.; Rasgon, N.; Gyulai, L.; Baethge, C.; Glenn, T.; Bauer, M. Use of polypharmacy and self-reported mood in outpatients with bipolar disorder. Int. J. Psychiatry Clin. Pract. 2005, 9, 251–256. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hieronymus, F.; Lorentzen, R.; McGirr, A.; Ostergaad, S.D. The efficacy of repetitive transcranial magnetic stimulation (rTMS) for bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 279, 250–255. [Google Scholar] [CrossRef]
- McGirr, A.; Karmani, S.; Arsappa, R.; Berlim, M.T.; Thirthalli, J.; Muralidharan, K.; Yatham, L.N. Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry 2016, 15, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Tee, M.K.; Au, C.H. A Systematic Review and Meta-Analysis of Randomized Sham-Controlled Trials of Repetitive Transcranial Magnetic Stimulation for Bipolar Disorder. Psychiatr. Q. 2020, 91, 1225–1247. [Google Scholar] [CrossRef]
- Zamar, A.; Roberts, A.; Bedu-Addo, A.; Lulsegged, A.; Kouimtsidis, C. High-Dose Levothyroxine for bipolar disorder; the potential role of thyroid function and genetic tests. Report from twenty cases. Int. J. Psychiatr. Res. 2021, 4, 1–7. [Google Scholar]
- Pilhatsch, M.; Stamm, T.J.; Stahl, P.; Lewitzka, U.; Berghöfer, A.; Sauer, C.; Gitlin, M.; Frye, M.A.; Whybrow, P.C.; Bauer, M. Treatment of bipolar depression with supraphysiologic doses of levothyroxine: A randomized, placebo-controlled study of comorbid anxiety symptoms. Int. J. Bipolar Disord. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Walshaw, P.D.; Gyulai, L.; Bauer, M.; Bauer, M.S.; Calimlim, B.; Sugar, C.A.; Whybrow, P.C. Thyroid hormone treatment in rapid cycling bipolar disorder: A double-blind placebo-controlled trial of levothyroxine (L-T4) and triiodothyronine (T3). Bipolar Disord. 2018, 20, 594–603. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.P.W.; Fonseca, T.L.; Ueta, C.B.; McAninch, E.A.; Abdalla, S.; Wittmann, G.; Lechan, R.M.; Gereben, B.; Bianco, A.C. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J. Clin. Investig. 2015, 125, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Koopdonk-Kool, J.M.; de Vijlder, J.J.; Veenboer, G.J.; Ris-Stalpers, C.; Kok, J.H.; Vulsma, T.; Boer, K.; Visser, T.J. Type II and type III deiodinase activity in human placenta as a function of gestational age. J. Clin. Endocrinol. Metab. 1996, 81, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Barnes, T.R.E.; Young, A.H. Mixed states in bipolar disorder: Etiology, pathogenesis and treatment. In The Maudsley Prescribing Guidelines in Psychiatry; Wiley Blackwell: New York, NY, USA, 2018; p. 241. [Google Scholar]
- Ketter, T.A. Handbook of Diagnosis and Treatment of Bipolar Disorders; American Psychiatric Publishing Corporation: Washington, DC, USA, 2010; Volume 7, p. 217. [Google Scholar]
- Lovallo, W.R.; Farag, N.H.; Vincent, A.S.; Thomas, T.L.; Wilson, M.F. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharm. Biochem. Behav. 2006, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, e00045. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Pérez, M.J.; Jara, C.; Vergara, E.H.; Quintanilla, R.A. Ethanol Consumption Affects Neuronal Function: Role of the Mitocho. Mitochondrial Dis. 2017. [Google Scholar] [CrossRef]
- Sheehan, K.H.; Sheehan, D.V. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int. Clin. Psychopharmacol. 2008, 23, 70–83. [Google Scholar] [CrossRef]
- Arbuckle, R.; Frye, M.A.; Brecher, M.; Paulsson, B.; Rajagopalan, K.; Palmer, S.; Deglinnocenti, A. The psychometric validation of the Sheehan Disability Scale (SDS) in patients with bipolar disorder. Psychiatry Res. 2009, 165, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Baur, H.; Berghöfer, A.; Ströhle, A.; Hellweg, R.; Müller-Oerlinghausen, B.; Baumgartner, A. Effects of supraphysiological thyroxine administration in healthy controls and patients with depressive disorders. J. Affect. Disord. 2002, 68, 285–294. [Google Scholar] [CrossRef]
- He, B.; Li, J.; Wang, G.; Ju, W.; Lu, Y.; Shi, Y.; He, L.; Zhong, N. Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 986–990. [Google Scholar] [CrossRef] [PubMed]
- McAninch, E.A.; Jo, S.; Preite, N.Z.; Farkas, E.; Mohácsik, P.; Fekete, C.; Egri, P.; Gereben, B.; Li, Y.; Deng, Y.; et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J. Clin. Endocrinol. Metab. 2015, 100, 920–933. [Google Scholar] [CrossRef]
- Crantz, F.R.; Silva, J.E.; Larsen, P.R. An Analysis of the Sources and Quantity of 3,5,3′-Triiodothyronine Specifically Bound to Nuclear Receptors in Rat Cerebral Cortex and Cerebellum. Endocrinology 1982, 110, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and mood: Mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Palomo, A.; Dodd, S.; Anmella, G.; Carvalho, A.F.; Scaini, G.; Quevedo, J.; Pacchiarotti, I.; Vieta, E.; Berk, M. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front. Psychiatry 2021, 12, 546801. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Neurobiological basis of bipolar disorder: Mitochondrial dysfunction hypothesis and beyond. Schizophr. Res. 2017, 187, 62–66. [Google Scholar] [CrossRef]
- Mesquita, R.C.; Faseyitan, O.K.; Turkeltaub, P.E.; Buckley, E.M.; Thomas, A.; Kim, M.N.; Durduran, T.; Greenberg, J.H.; Detre, J.A.; Yodh, A.G.; et al. Blood flow and oxygenation changes due to low-frequency repetitive transcranial magnetic stimulation of the cerebral cortex. J. Biomed. Opt. 2013, 18, 067006. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamar, A.C.; Kouimtsidis, C.; Lulsegged, A.; Roberts, R.; Koutsomitros, T.; Stahl, D. A New Treatment Protocol of Combined High-Dose Levothyroxine and Repetitive Transcranial Magnetic Stimulation for the Treatment of Rapid-Cycling Bipolar Spectrum Disorders: A Cohort Evaluation of 55 Patients. J. Clin. Med. 2022, 11, 5830. https://doi.org/10.3390/jcm11195830
Zamar AC, Kouimtsidis C, Lulsegged A, Roberts R, Koutsomitros T, Stahl D. A New Treatment Protocol of Combined High-Dose Levothyroxine and Repetitive Transcranial Magnetic Stimulation for the Treatment of Rapid-Cycling Bipolar Spectrum Disorders: A Cohort Evaluation of 55 Patients. Journal of Clinical Medicine. 2022; 11(19):5830. https://doi.org/10.3390/jcm11195830
Chicago/Turabian StyleZamar, Antonis C., Christos Kouimtsidis, Abbi Lulsegged, Robin Roberts, Theodoros Koutsomitros, and Daniel Stahl. 2022. "A New Treatment Protocol of Combined High-Dose Levothyroxine and Repetitive Transcranial Magnetic Stimulation for the Treatment of Rapid-Cycling Bipolar Spectrum Disorders: A Cohort Evaluation of 55 Patients" Journal of Clinical Medicine 11, no. 19: 5830. https://doi.org/10.3390/jcm11195830
APA StyleZamar, A. C., Kouimtsidis, C., Lulsegged, A., Roberts, R., Koutsomitros, T., & Stahl, D. (2022). A New Treatment Protocol of Combined High-Dose Levothyroxine and Repetitive Transcranial Magnetic Stimulation for the Treatment of Rapid-Cycling Bipolar Spectrum Disorders: A Cohort Evaluation of 55 Patients. Journal of Clinical Medicine, 11(19), 5830. https://doi.org/10.3390/jcm11195830






