Early versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Attal, M.; Harousseau, J.L.; Stoppa, A.M.; Sotto, J.J.; Fuzibet, J.G.; Rossi, J.F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A Prospective, Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Richardson, P.G.; Laubach, J.P.; McCarthy, P.L. Management of Relapsed Multiple Myeloma after Autologous Stem Cell Transplant. Biology of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 793–798. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Hoering, A.; Cavo, M.; Miguel, J.S.; Goldschimdt, H.; Hajek, R.; Turesson, I.; Lahuerta, J.J.; Attal, M.; Barlogie, B.; et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—An IMWG Research Project. Blood Cancer J. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Jagannath, S.; Desikan, K.R.; Mattox, S.; Vesole, D.; Siegel, D.; Tricot, G.; Munshi, N.; Fassas, A.; Singhal, S.; et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 1999, 93, 55–65. [Google Scholar] [CrossRef]
- Attal, M.; Harousseau, J.L.; Facon, T.; Guilhot, F.; Doyen, C.; Fuzibet, J.G.; Monconduit, M.; Hulin, C.; Caillot, D.; Bouabdallah, R.; et al. Single versus Double Autologous Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2003, 349, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Hari, P.; Bashey, A.; Devine, S.; Efebera, Y.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer 2019, 9, 44. [Google Scholar] [CrossRef]
- Spencer, A.; Prince, H.M.; Roberts, A.W.; Prosser, I.W.; Bradstock, K.F.; Coyle, L.; Gill, D.S.; Horvath, N.; Reynolds, J.; Kennedy, N. Consolidation Therapy with Low-Dose Thalidomide and Prednisolone Prolongs the Survival of Multiple Myeloma Patients Undergoing a Single Autologous Stem-Cell Transplantation Procedure. J. Clin. Oncol. 2009, 27, 1788–1793. [Google Scholar] [CrossRef]
- Cavo, M.; Pantani, L.; Petrucci, M.T.; Patriarca, F.; Zamagni, E.; Donnarumma, D.; Crippa, C.; Boccadoro, M.; Perrone, G.; Falcone, A.; et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012, 120, 9–19. [Google Scholar] [CrossRef]
- Shustik, C.; Belch, A.; Robinson, S.; Rubin, S.H.; Dolan, S.P.; Kovacs, M.J.; Grewal, K.S.; Walde, D.; Barr, R.; Wilson, J.; et al. A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. Br. J. Haematol. 2007, 136, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shustik, C.; Belch, A.; Robinson, S.; Rubin, S.H.; Dolan, S.P.; Kovacs, M.J.; Grewal, K.S.; Walde, D.; Barr, R.; Wilson, J.; et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood 2002, 99, 3163–3168. [Google Scholar]
- Myeloma Trialists’ Collaborative Group. Interferon as therapy for multiple myeloma: An individual patient data overview of 24 randomized trials and 4012 patients. Br. J. Haematol. 2001, 113, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Fritz, E.; Ludwig, H. Interferon-α treatment in multiple myeloma: Meta-analysis of 30 randomised trials among 3948 patients. Ann. Oncol. 2000, 11, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Tricot, G.; Anaissie, E.; Shaughnessy, J.; Rasmussen, E.; Van Rhee, F.; Fassas, A.; Zangari, M.; Hollmig, K.; Pineda-Roman, M.; et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N. Engl. J. Med. 2006, 354, 1021–1030. [Google Scholar] [CrossRef]
- Attal, M.; Harousseau, J.L.; Leyvraz, S.; Doyen, C.; Hulin, C.; Benboubker, L.; Agha, I.Y.; Bourhis, J.H.; Garderet, L.; Pegourie, B.; et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood 2006, 108, 3289–3294. [Google Scholar] [CrossRef]
- Morgan, G.J.; Gregory, W.M.; Davies, F.E.; Bell, S.E.; Szubert, A.J.; Brown, J.M.; Coy, N.N.; Cook, G.; Russell, N.H.; Rudin, C.; et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood 2012, 119, 7–15. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; van der Holt, B.; Zweegman, S.; Vellenga, E.; Croockewit, S.; van Oers, M.H.; von dem Borne, P.; Wijermans, P.; Schaafsma, R.; de Weerdt, O.; et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood 2010, 115, 1113–1120. [Google Scholar] [CrossRef]
- Kagoya, Y.; Nannya, Y.; Kurokawa, M. Thalidomide maintenance therapy for patients with multiple myeloma: Meta-analysis. Leuk. Res. 2012, 36, 1016–1021. [Google Scholar] [CrossRef]
- Stewart, A.K.; Trudel, S.; Bahlis, N.J.; White, D.; Sabry, W.; Belch, A.; Reiman, T.; Roy, J.; Shustik, C.; Kovacs, M.J.; et al. A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: The National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood 2013, 121, 1517–1523. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Facon, T.; Hulin, C.; Moreau, P.; Mathiot, C.; Roussel, M.; Payen, C.; et al. Lenalidomide Maintenance After Stem-Cell Transplantation for Multiple Myeloma: Follow-Up Analysis Of The IFM 2005–02 Trial. Blood 2013, 122, 406. [Google Scholar] [CrossRef]
- Sonneveld, P.; Salwender, H.J.; Van Der Holt, B.; el Jarari, L.; Bertsch, U.; Blau, I.W.; Zweegman, S.; Weisel, K.C.; Vellenga, E.; Pfreundschuh, M.; et al. Bortezomib Induction and Maintenance in Patients with Newly Diagnosed Multiple Myeloma: Long-Term Follow-Up of the HOVON-65/GMMG-HD4 Trial; American Society of Hematology: Washington, DC, USA, 2015. [Google Scholar]
- Shah, N.; Callander, N.; Ganguly, S.; Gul, Z.; Hamadani, M.; Costa, L.; Sengsayadeth, S.; Abidi, M.; Hari, P.; Mohty, M.; et al. Hematopoietic Stem Cell Transplantation for Multiple Myeloma: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transpl. 2015, 21, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Costello, C.; Efebera, Y.; et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1685–1717. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; Lacy, M.Q.; et al. Outcomes of maintenance therapy with lenalidomide or bortezomib in multiple myeloma in the setting of early autologous stem cell transplantation. Leukemia 2018, 32, 712–718. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, H.; Masih-Khan, E.; Chen, C.I.; Prica, A.; Reece, D.; Tiedemann, R.; Trudel, S.; Kukreti, V. Tolerability and Efficacy of Post Transplant Lenalidomide Maintenance Therapy in Multiple Myeloma: A Real World Single Centre Experience. Blood 2017, 130 (Suppl. 1), 3462. [Google Scholar]
- Holstein, S.A.; Jung, S.H.; Richardson, P.G.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: A randomised, double-blind, phase 3 trial. Lancet Haematol. 2017, 4, e431–e442. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Prospective Evaluation of the Prognostic Impact of Measurable Residual Disease (MRD) within a Phase III Study Comparing a Fixed Duration Therapy versus Continuous Therapy with Daratumumab, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma Requiring a First Salvage Treatment. Available online: https://ClinicalTrials.gov/show/NCT05203003 (accessed on 5 September 2022).
- Minimal Residual Disease Guided Maintenance Therapy with Belantamab Mafodotin and Lenalidomide after Autologous Hematopoietic Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma. Available online: https://ClinicalTrials.gov/show/NCT05091372 (accessed on 5 September 2022).
- A Study of Daratumumab Plus Lenalidomide versus Lenalidomide Alone as Maintenance Treatment in Participants with Newly Diagnosed Multiple Myeloma Who Are Minimal Residual Disease Positive after Frontline Autologous Stem Cell Transplant. Available online: https://ClinicalTrials.gov/show/NCT03901963 (accessed on 5 September 2022).
| Maintenance < 3 Years (n = 102), 30% | Maintenance ≥ 3 Years (n = 238), 70% | p-Value | |||
|---|---|---|---|---|---|
| Age at Transplant (Yrs, median range) | 58 | 37–73 | 60 | 37–75 | 0.33 |
| Age ≤65 | 83 | 81.4 | 188 | 79.0 | 0.62 |
| Age >65 | 19 | 18.6 | 50 | 21.0 | |
| Sex | 0.56 | ||||
| Male | 57 | 55.9 | 141 | 59.2 | |
| Female | 45 | 44.1 | 97 | 40.8 | |
| Race | 0.02 | ||||
| Black | 6 | 5.9 | 37 | 15.5 | |
| White | 96 | 94.1 | 197 | 82.8 | |
| Others | 0 | 0.0 | 4 | 1.7 | |
| Melphalan use | 0.91 | ||||
| 140 | 12 | 11.8 | 27 | 11.3 | |
| 200 | 90 | 88.2 | 211 | 88.7 | |
| Pre-Transplant remission status | 0.15 | ||||
| CR/VGPR | 55 | 53.9 | 153 | 64.3 | |
| PR | 38 | 37.3 | 64 | 26.9 | |
| SD/PD | 9 | 8.8 | 21 | 8.8 | |
| Post-Transplant remission status | 0.01 | ||||
| CR/VGPR | 80 | 78.4 | 206 | 86.6 | |
| PR | 14 | 13.7 | 29 | 12.2 | |
| SD/PD | 8 | 7.8 | 3 | 1.3 | |
| Cytogenetic risk | 0.52 | ||||
| Standard risk | 55 | 63.2 | 137 | 67.2 | |
| High/Intermediate risk | 32 | 36.8 | 67 | 32.8 | |
| ISS staging | 0.07 | ||||
| 1 | 44 | 53.7 | 78 | 38.6 | |
| 2 | 23 | 28.0 | 76 | 37.6 | |
| 3 | 15 | 18.3 | 48 | 23.8 | |
| Maintenance < 3 Years (n = 102) | Maintenance ≥ 3 Years (n = 238) | |||
|---|---|---|---|---|
| Maintenance Drugs | n | % | n | % |
| Lenalidomide | 90 | 88.2 | 214 | 89.9 |
| Bortezomib | 15 | 14.7 | 21 | 8.8 |
| Ixazomib | 0 | 0 | 12 | 5.0 |
| Lenalidomide + Bortezomib | 0 | 0 | 2 | 0.8 |
| Lenalidomide + Cyclophosphamide | 0 | 0 | 2 | 0.8 |
| Pomalidomide | 0 | 0 | 3 | 1.3 |
| Pomalidomide + Cyclophosphamide | 0 | 0 | 1 | 0.4 |
| Thalidomide | 3 | 2.9 | 1 | 0.4 |
| Prednisone | 3 | 2.9 | 1 | 0.4 |
| Reasons for stopping * | ||||
| Patient Preference | 23 | 22.5 | 7 | 2.9 |
| Physician Decision | 1 | 1 | 0 | 0 |
| Progression | 0 | 0 | 57 | 23.9 |
| Recurrent Infection | 1 | 1 | 0 | 0 |
| SPM | 4 | 3.9 | 5 | 2.1 |
| Adverse events | 51 | 50 | 30 | 12.6 |
| Other | 22 | 21.6 | 8 | 3.4 |
| Adverse Events | Maintenance < 3 Years | Maintenance ≥ 3 Years |
|---|---|---|
| Pancytopenia | 8 | 2 |
| Neutropenia | 7 | 5 |
| Infection | 9 | 2 |
| Thrombocytopenia | 1 | 0 |
| GI | 8 | 4 |
| Neurologic deficits | 1 | 0 |
| Cardiac | 1 | 4 |
| Depression | 1 | 0 |
| Fatigue | 10 | 9 |
| Neuropathy | 5 | 4 |
| Peripheral neuropathy | 2 | 0 |
| Renal | 2 | 1 |
| Skin | 3 | 0 |
| DVT or pulmonary embolism | 0 | 1 |
| Maintenance < 3 Years | Maintenance ≥ 3 Years | |||||
|---|---|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | |||
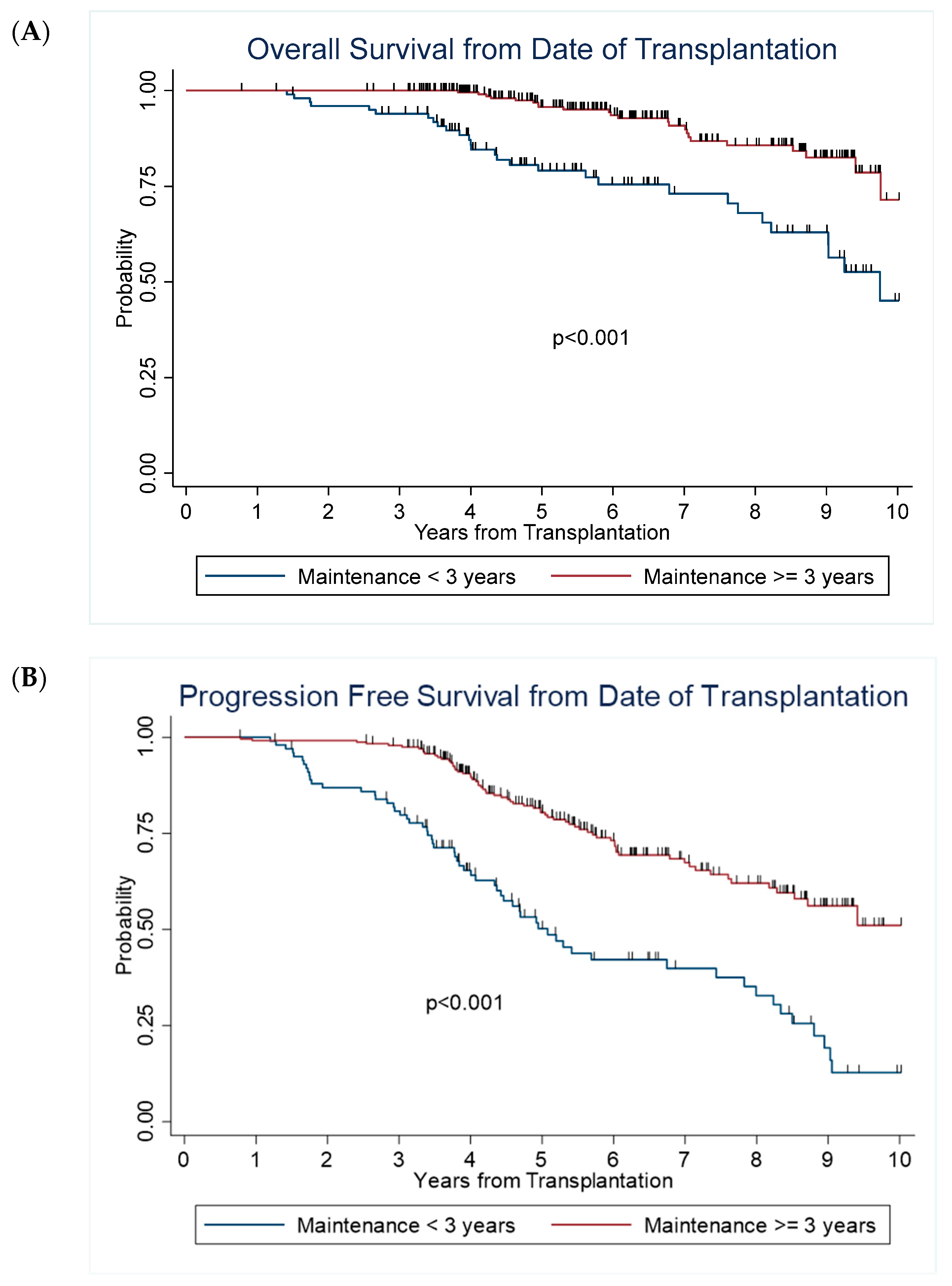
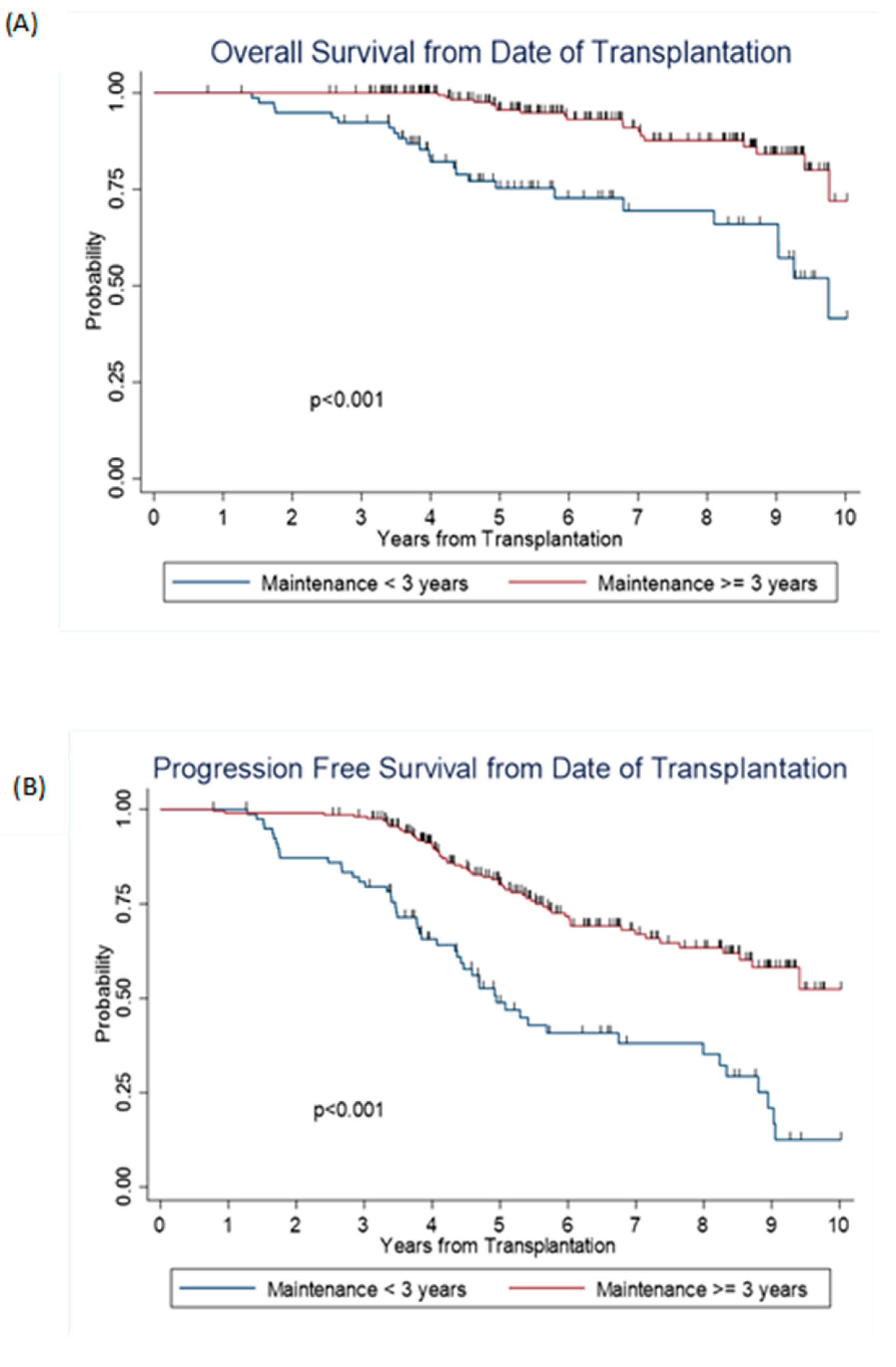
| Overall survival (OS) | ||||||
| Year 1 | 100% | 100% | ||||
| Year 3 | 94% | 87% | 97% | 100% | ||
| Year 5 | 79% | 69% | 86% | 96% | 92% | 98% |
| Year 10 | 45% | 26% | 62% | 71% | 52% | 84% |
| Median, 95% CI | 9.8 | 8.2 | NR | NR | NR | NR |
| Progression free survival (PFS) | ||||||
| Year 1 | 100% | 99% | 97% | 100% | ||
| Year 3 | 81% | 72% | 87% | 98% | 95% | 99% |
| Year 5 | 50% | 39% | 60% | 80% | 74% | 85% |
| Year 10 | 13% | 5% | 25% | 51% | 38% | 63% |
| Median, 95% CI | 5.1 | 4.4 | 7.4 | NR | 8.5 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunnelee, J.; Cottini, F.; Zhao, Q.; Faisal, M.S.; Elder, P.; Rosko, A.; Bumma, N.; Khan, A.; Umyarova, E.; Devarakonda, S.; et al. Early versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma. J. Clin. Med. 2022, 11, 5794. https://doi.org/10.3390/jcm11195794
Nunnelee J, Cottini F, Zhao Q, Faisal MS, Elder P, Rosko A, Bumma N, Khan A, Umyarova E, Devarakonda S, et al. Early versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma. Journal of Clinical Medicine. 2022; 11(19):5794. https://doi.org/10.3390/jcm11195794
Chicago/Turabian StyleNunnelee, Jordan, Francesca Cottini, Qiuhong Zhao, Muhammad Salman Faisal, Patrick Elder, Ashley Rosko, Naresh Bumma, Abdullah Khan, Elvira Umyarova, Srinivas Devarakonda, and et al. 2022. "Early versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma" Journal of Clinical Medicine 11, no. 19: 5794. https://doi.org/10.3390/jcm11195794
APA StyleNunnelee, J., Cottini, F., Zhao, Q., Faisal, M. S., Elder, P., Rosko, A., Bumma, N., Khan, A., Umyarova, E., Devarakonda, S., Benson, D. M., Efebera, Y. A., & Sharma, N. (2022). Early versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma. Journal of Clinical Medicine, 11(19), 5794. https://doi.org/10.3390/jcm11195794







