Intra Articular Injection of Autologous Microfat and Platelets-Rich Plasma in the Treatment of Wrist Osteoarthritis: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient’s Selection
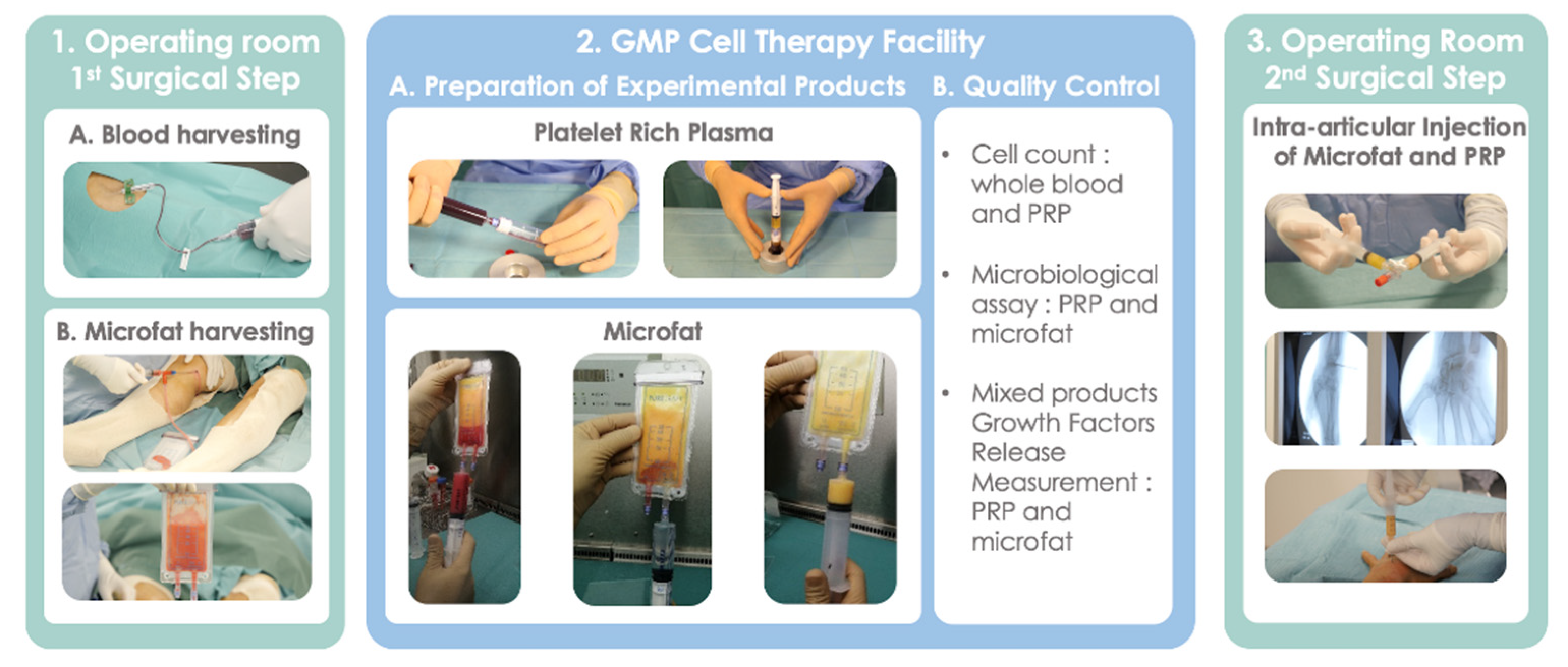
2.2. Surgical Procedure
2.3. Blood and Adipose Tissue Harvesting
2.4. Preparation of Experimental Products: MF-PRP
2.5. Biological Characterization of Injected Products
2.6. Intra-Articular Injection of MF-PRP
2.6.1. Evaluation Tools and Follow-Up
2.6.2. MRI Acquisition and Analysis
2.6.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Biological Characteristics of Mixed Products
3.3. Safety
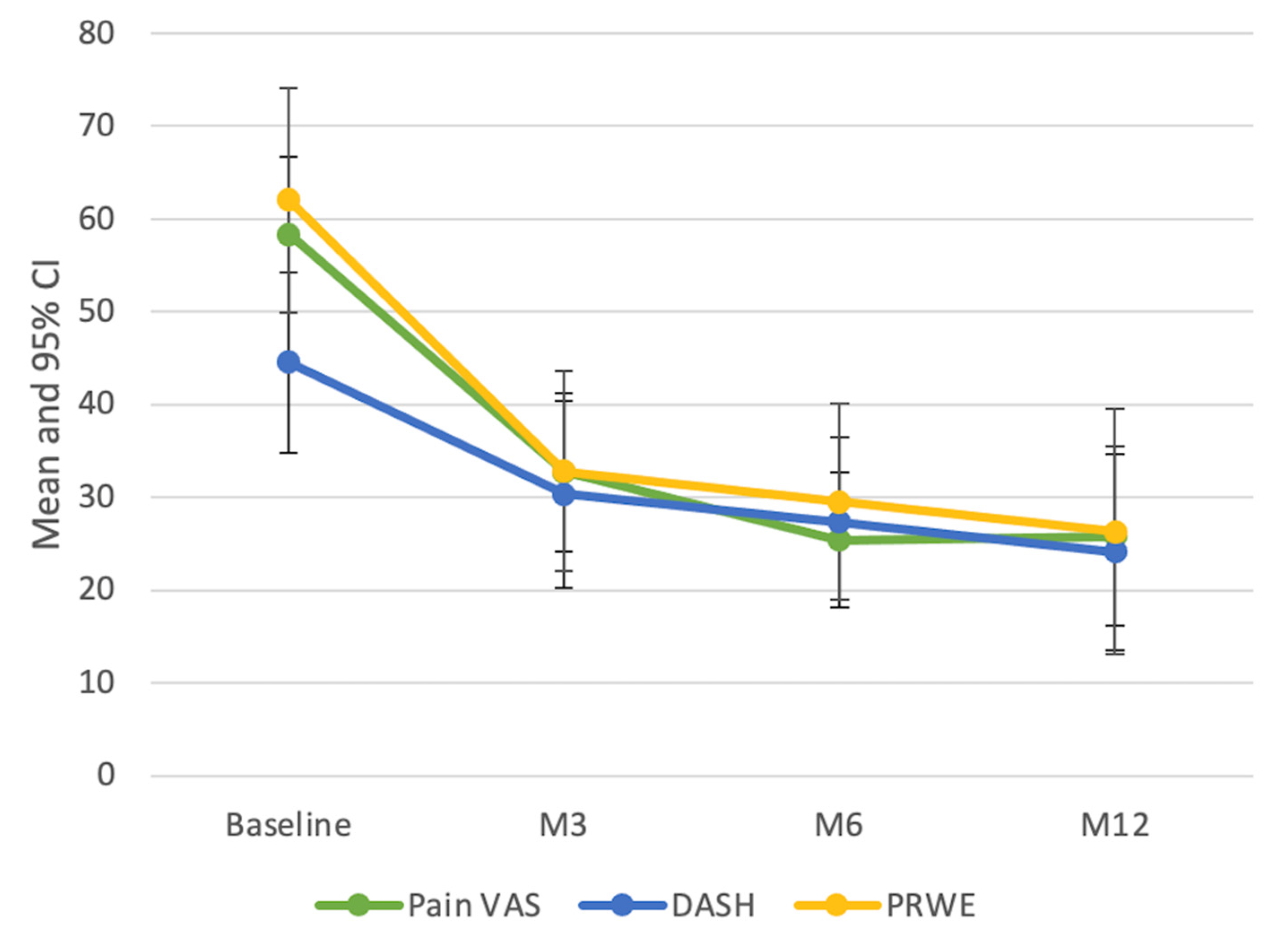
3.4. Clinical Assessment
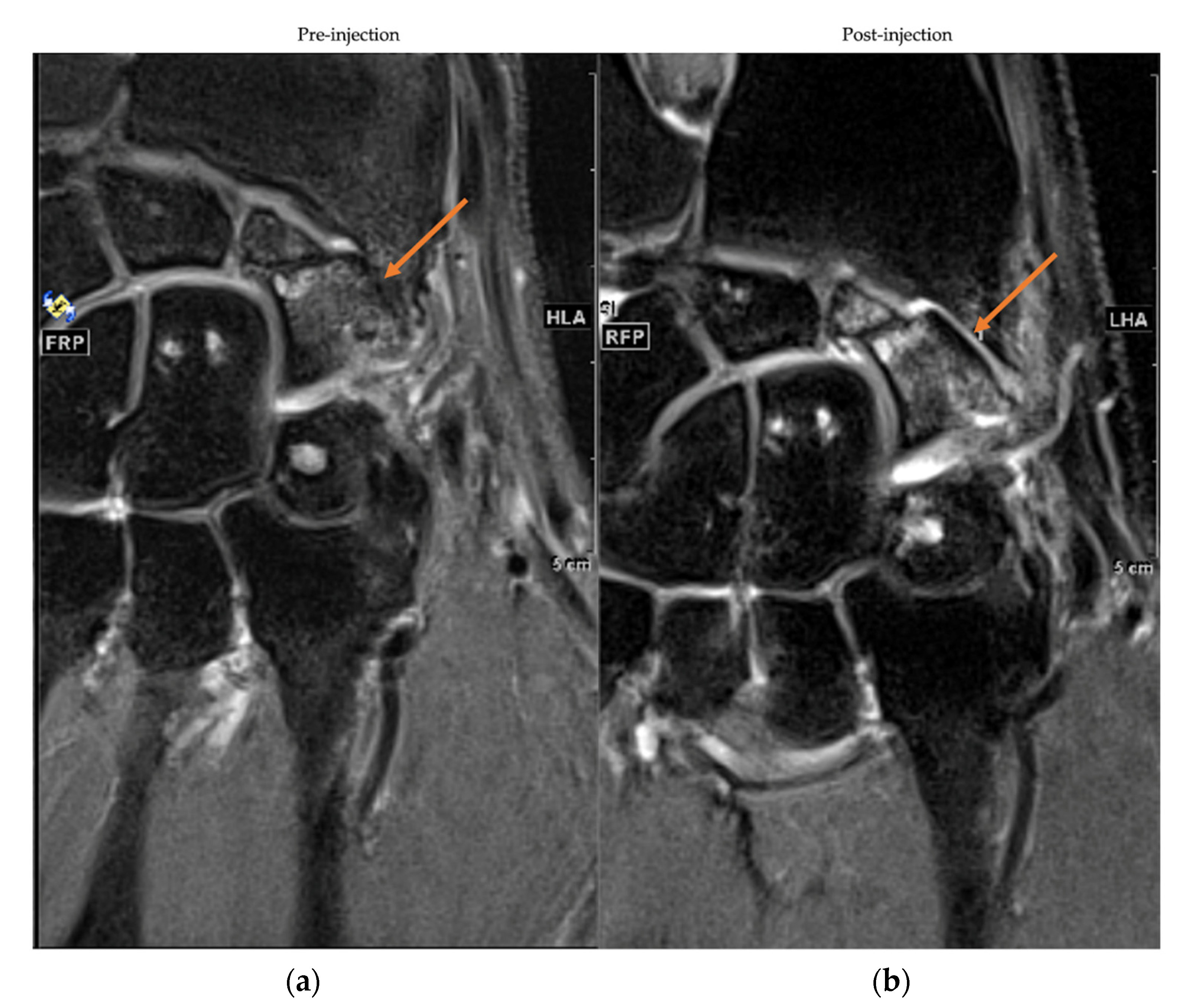
3.5. MRI Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, J.K. Degenerative arthritis of the wrist. Curr. Orthop. 1999, 13, 290–296. [Google Scholar] [CrossRef]
- Laulan, J.; Marteau, E.; Bacle, G. Wrist osteoarthritis. Orthop. Traumatol. Surg. Res. 2015, 101, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.E.; Rodner, C.M. Osteoarthritis of the wrist. J. Hand Surg. 2007, 32, 725–746. [Google Scholar] [CrossRef]
- Watson, H.K.; Ballet, F.L. The SLAC wrist: Scapholunate advanced collapse pattern of degenerative arthritis. J. Hand Surg. 1984, 9, 358–365. [Google Scholar] [CrossRef]
- Lane, N.; Shidara, K.; Wise, B. Osteoarthritis year in review 2016: Clinical. Osteoarthr. Cartil. 2017, 25, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Mahendira, D.; Towheed, T. Systematic review of non-surgical therapies for osteoarthritis of the hand: An update. Osteoarthr. Cartil. 2009, 17, 1263–1268s. [Google Scholar] [CrossRef][Green Version]
- Lue, S.; Koppikar, S.; Shaikh, K.; Mahendira, D.; Towheed, T.E. Systematic review of non-surgical therapies for osteoarthritis of the hand: An update. Osteoarthr. Cartil. 2017, 25, 1379–1389. [Google Scholar] [CrossRef]
- Fuchsberger, T.; Gonser, P.; Boesch, C.; Tonagel, F.; Fischborn, T.; Schaller, H.; Haerle, M. Corrigendum to Patient-rated long-term results after complete denervation of the wrist. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1381. [Google Scholar] [CrossRef]
- Sauerbier, M.; Kluge, S.; Bickert, B.; Germann, G. Subjective and objective outcomes after total wrist arthrodesis in patients with radiocarpal arthrosis or Kienböck’s disease. Chir. Main 2000, 19, 223–231. [Google Scholar] [CrossRef]
- Nair, R. Review Article: Total Wrist Arthroplasty. J. Orthop. Surg. 2014, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sagerfors, M.; Gupta, A.; Brus, O.; Pettersson, K. Total Wrist Arthroplasty: A Single-Center Study of 219 Cases With 5-Year Follow-up. J. Hand Surg. 2015, 40, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, G.; Boeckstyns, M.; Sorensen, A.I.; Axelsson, P.; Kroener, K.; Liverneaux, P.; Obert, L.; Merser, S. “Remotion” Total Wrist Arthroplasty: Preliminary Results of a Prospective International Multicenter Study of 215 Cases. J. Wrist Surg. 2012, 1, 17–22. [Google Scholar] [CrossRef]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-Rich Plasma Peptides: Key for Regeneration. Int. J. Pept. 2012, 2012, 532519. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204–215. [Google Scholar] [CrossRef]
- Dai, W.L.; Zhou, A.G.; Zhang, H.; Zhang, J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy 2017, 33, 659–670.e1. [Google Scholar] [CrossRef]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef]
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review With Quantitative Synthesis. Arthroscopy 2013, 29, 2037–2048. [Google Scholar] [CrossRef]
- Laudy, A.B.; Bakker, E.W.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 657–672. [Google Scholar] [CrossRef]
- English, A.; Jones, E.A.; Corscadden, D.; Henshaw, K.; Chapman, T.; Emery, P.; McGonagle, D. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology 2007, 46, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.-A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic Potential of Multipotential Cells from Human Adipose Tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.S.; Desouches, C.; Gay, A.M.; Hautier, A.; Magalon, G. Development of micro-injection as an innovative autologous fat graft technique: The use of adipose tissue as dermal filler. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1692–1699. [Google Scholar] [CrossRef]
- Alharbi, Z.; Opländer, C.; Almakadi, S.; Fritz, A.; Vogt, M.; Pallua, N. Conventional vs. micro-fat harvesting: How fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1271–1278. [Google Scholar] [CrossRef]
- Bembo, F.; Eraud, J.; Philandrianos, C.; Bertrand, B.; Silvestre, A.; Veran, J.; Sabatier, F.; Magalon, G.; Magalon, J. Combined use of platelet rich plasma and micro-fat in sport and race horses with degenerative joint disease: Preliminary clinical study in eight horses. Muscle Ligaments Tendons J. 2016, 6, 198–204. [Google Scholar] [CrossRef]
- Mayoly, A.; Iniesta, A.; Curvale, C.; Kachouh, N.; Jaloux, C.; Eraud, J.; Vogtensperger, M.; Veran, J.; Grimaud, F.; Jouve, E.; et al. Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results. Int. J. Mol. Sci. 2019, 20, 1111. [Google Scholar] [CrossRef]
- Sautereau, N.; Daumas, A.; Truillet, R.; Jouve, E.; Magalon, J.; Veran, J.; Casanova, D.; Frances, Y.; Magalon, G.; Granel, B. Efficacy of Autologous Microfat Graft on Facial Handicap in Systemic Sclerosis Patients. Plast. Reconstr. Surg. -Glob. Open 2016, 4, e660. [Google Scholar] [CrossRef]
- Graiet, H.; Lokchine, A.; Francois, P.; Velier, M.; Grimaud, F.; Loyens, M.; Berda-Haddad, Y.; Veran, J.; Dignat-George, F.; Sabatier, F.; et al. Use of platelet-rich plasma in regenerative medicine: Technical tools for correct quality control. BMJ Open Sport Exerc. Med. 2018, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Schernberg, F. Classification des fractures du scaphoïde carpien. Etude anatomo-radiologique des traits [Classification of fractures of the carpal scaphoid. An anatomo-radiologic study of characteristics]. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1988, 74, 693–695. [Google Scholar] [PubMed]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and Its Shortened Version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.M.; de Muinck Keizer, R.J.; Goslings, J.C.; Vos, L.M.; Rosenwasser, M.P.; Schep, N.W. The Minimum Clinically Important Difference of the Patient-rated Wrist Evaluation Score for Patients with Distal Radius Fractures. Clin. Orthop. Relat. Res. 2015, 473, 3235–3241. [Google Scholar] [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef]
- Barfod, K.W.; Blønd, L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan. Med. J. 2019, 66, A5565. [Google Scholar]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2018, 43, 1123–1134. [Google Scholar] [CrossRef]
- Haas, E.M.; Eisele, A.; Arnoldi, A.; Paolini, M.; Ehrl, D.; Volkmer, E.; Giunta, R.E. One-Year Outcomes of Intraarticular Fat Transplantation for Thumb Carpometacarpal Joint Osteoarthritis: Case Review of 99 Joints. Plast. Reconstr. Surg. 2020, 145, 151–159. [Google Scholar] [CrossRef]
- Malanga, G.A.; Chirichella, P.S.; Hogaboom, N.S.; Capella, T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: A prospective pilot study. Int. Orthop. 2021, 45, 473–480. [Google Scholar] [CrossRef]
- Pak, J.; Chang, J.-J.; Lee, J.H.; Lee, S.H. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef]
- Froschauer, S.M.; Holzbauer, M.; Wenny, R.; Schmidt, M.; Huemer, G.M.; Kwasny, O.; Duscher, D. Autologous Fat Transplantation for Thumb Carpometacarpal Joint Osteoarthritis (Liparthroplasty): A Case Series with Two Years of Follow-UP. J. Clin. Med. 2020, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Kemper, R.; Wirth, J.; Baur, E.M. Arthroscopic Synovectomy Combined with Autologous Fat Grafting in Early Stages of CMC Osteoarthritis of the Thumb. J. Wrist. Surg. 2018, 7, 165–171. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef]
- Adriani, E.; Moio, M.; Di Paola, B.; Salustri, W.; Alfieri, A.; Parisi, P.; Ruggiero, M.; Borab, Z.; Carlesimo, B. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints 2017, 5, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; You, D.; Zhao, S.; Zhu, Z.; Xu, M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Medicine 2020, 99, e19388. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, H.; Liang, G.; Zeng, L.F.; Yang, W.; Liu, J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2020, 21, 224. [Google Scholar] [CrossRef]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 49. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.L.; Dumonceau, R.G.; Jouve, E.; Cohen, M.; Djouri, R.; Richardet, N.; Jourdan, E.; Giraudo, L.; Dumoulin, C.; Grimaud, F.; et al. Intra-Articular Injection of Autologous Microfat and Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Comparative Study. Arthroscopy 2021, 37, 3125–3137.e3. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Maria, D.S.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Jorgensen, C.; Djouad, F.; Bouffi, C.; Mrugala, D.; Noël, D. Multipotent mesenchymal stromal cells in articular diseases. Best Pract. Res. Clin. Rheumatol. 2008, 22, 269–284. [Google Scholar] [CrossRef]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef]
- Heidari, N.; Noorani, A.; Slevin, M.; Cullen, A.; Stark, L.; Olgiati, S.; Zerbi, A.; Wilson, A. Patient-Centered Outcomes of Microfragmented Adipose Tissue Treatments of Knee Osteoarthritis: An Observational, Intention-to-Treat Study at Twelve Months. Stem Cells Int. 2020, 2020, 8881405. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Herold, C.; Rennekampff, H.O.; Groddeck, R.; Allert, S. Autologous Fat Transfer for Thumb Carpometacarpal Joint Osteoarthritis: A Prospective Study. Plast. Reconstr. Surg. 2017, 140, 327–335. [Google Scholar] [CrossRef]
- Roux, C.; Pisani, D.F.; Ben Yahia, H.; Djedaini, M.; Beranger, G.E.; Chambard, J.-C.; Ambrosetti, D.; Michiels, J.-F.; Breuil, V.; Ailhaud, G.; et al. Chondrogenic potential of stem cells derived from adipose tissue: A powerful pharmacological tool. Biochem. Biophys. Res. Commun. 2013, 440, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, P.; Bui, K.H.-T.; Ngo, D.Q.; Vu, N.B.; Truong, N.H.; Phan, N.L.-C.; Le, D.M.; Duong, T.D.; Nguyen, T.D.; Le, V.T.; et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng. Part C Methods 2009, 15, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Atashi, F.; Jaconi, M.E.; Pittet-Cuénod, B.; Modarressi, A. Autologous platelet-rich plasma: A biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng. Part C Methods 2015, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Azpeitia, E.; Andia, I. Partnership between platelet-rich plasma and mesenchymal stem cells: In vitro experience. Muscles Ligaments Tendons J. 2014, 4, 52–62. [Google Scholar] [CrossRef]
- 66. Van Buul, G.M.; Koevoet, W.L.; Kops, N.; Koen Bos, P.; Verhaar, J.A.N.; Weinans, H.; Bernsen, M.R.; Van Osch, G.J.V.M. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am. J. Sports Med. 2011, 39, 2362–2370. [Google Scholar] [CrossRef]
- Ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; de Munter, W.; Grevers, L.C.; Jeanson, J.; Noël, D.; Casteilla, L.; Jorgensen, C.; et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef]
- Nava, S.; Sordi, V.; Pascucci, L.; Tremolada, C.; Ciusani, E.; Zeira, O.; Cadei, M.; Soldati, G.; Pessina, A.; Parati, E.; et al. Long-Lasting Anti-Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int. 2019, 2019, 5901479. [Google Scholar] [CrossRef]
- Pintat, J.; Silvestre, A.; Magalon, G.; Gadeau, A.P.; Pesquer, L.; Perozziello, A.; Peuchant, A.; Mounayer, C.; Dallaudière, B. Intra-articular Injection of Mesenchymal Stem Cells and Platelet-Rich Plasma to Treat Patellofemoral Osteoarthritis: Preliminary Results of a Long-Term Pilot Study. J. Vasc. Interv. Radiol. 2017, 28, 1708–1713. [Google Scholar] [CrossRef]
- Chin, K.W.T.K.; Engelsman, A.F.; van Gulik, T.M.; Strackee, S.D. Selective denervation of the wrist for chronic pain: A systematic literature review. J. Hand Surg. Eur. Vol. 2020, 45, 265–272. [Google Scholar] [CrossRef]
- Erne, H.C.; Cerny, M.K.; Ehrl, D.; Bauer, A.T.; Schmauss, V.; Moog, P.; Broer, P.N.; Loew, S.; Schmauss, D. Autologous Fat Injection versus Lundborg Resection Arthroplasty for the Treatment of Trapeziometacarpal Joint Osteoarthritis. Plast. Reconstr. Surg. 2018, 141, 119–124. [Google Scholar] [CrossRef]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef]

| Inclusion Criteria |
|---|
|
|
|
|
|
|
|
| Exclusion Criteria |
|
| Number of Patients (n) | 12 |
| Gender (women/men) | 4 (33)/8 (67) |
| Age (years) | 53.8 ± 14.8 (24–68) |
| BMI (kg/m2) | 25.6 ± 3.8 (20–30.5) |
| Dominant hand: Right-handed | 12 (100) |
| Affected hand: Right/Left | 7 (58)/5 (42) |
| Disease duration from diagnosis (years) | 6.6 ± 9.1 (1–25) |
| Traumatic etiology: | 12 (100) |
| 4 (33.3) |
| 4 (33.3) |
| 4 (33.3) |
| Osteoarthritis grade (Kellgren and Lawrence classification), (0 to 4) | Grade 3: 3 (25) Grade 4: 9 (75) |
| Localisation of osteoarthritis (X-ray) | |
| 8 (67) |
| 4 (33) |
| Mean ± SD | Median (Min–Max) | |
|---|---|---|
| Total mean volume (mL) | 3.6 ± 0.4 | 3.8 (2.7–4) |
| Microbiological Assay, Free of germ | ||
| Microfat | 10 (83) | |
| PRP | 12 (100) | |
| Cells (millions; %) | ||
| Platelets | 741 ± 162; 95.3 ± 2.8 | 715 (480–1094) |
| Red Blood Cells | 33.3 ± 17.7; 4.5 ± 2.7 | 30 (17.5–76) |
| Leukocytes | 1.2 ± 1.3; 0.1 ± 0.1 | 0.9 (0.1–4.1) |
| Ratio platelets/cc of fat | 410 ± 88 | 407 (274–547) |
| Inflammatory cytokines (pg/mL) | ||
| IL-1b | 7.7 ± 16.6 | 1.8 (0–58) |
| TNFa | 25.3 ± 23.5 | 18.3 (0.6–84.7) |
| IL-6 | 4669 ± 3187 | 3861 (344–9960) |
| IFN γ | 4 ± 6 | 0.1 (0–18.9) |
| Anti-Inflammatory cytokines (pg/mL) | ||
| IL-10 | 12.1 ± 8.5 | 11.1 (0–25.1) |
| IL-1ra | 155 ± 149 | 125 (28–539) |
| Ratio IL1Ra/Il1b | 88.7 ± 108.4 | 39.9 (1.6–378.6) |
| Growth factors (pg/mL) | ||
| PDGF | 519 ± 340 | 490 (62–1095) |
| VEGF-a | 28.2 ± 31.3 | 24.7 (0–101.8) |
| EGF | 131.8 ± 67.3 | 135.3 (5.3–214.4) |
| FGF-2 | 6828 ± 3345 | 5904.9 (2410–12,999) |
| TGFB1 | 5019 ± 2891 | 5095.2 (1092–9271) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayoly, A.; Witters, M.; Jouve, E.; Bec, C.; Iniesta, A.; Kachouh, N.; Veran, J.; Grimaud, F.; Zavarro, A.C.; Fernandez, R.; et al. Intra Articular Injection of Autologous Microfat and Platelets-Rich Plasma in the Treatment of Wrist Osteoarthritis: A Pilot Study. J. Clin. Med. 2022, 11, 5786. https://doi.org/10.3390/jcm11195786
Mayoly A, Witters M, Jouve E, Bec C, Iniesta A, Kachouh N, Veran J, Grimaud F, Zavarro AC, Fernandez R, et al. Intra Articular Injection of Autologous Microfat and Platelets-Rich Plasma in the Treatment of Wrist Osteoarthritis: A Pilot Study. Journal of Clinical Medicine. 2022; 11(19):5786. https://doi.org/10.3390/jcm11195786
Chicago/Turabian StyleMayoly, Alice, Marie Witters, Elisabeth Jouve, Cécilia Bec, Aurélie Iniesta, Najib Kachouh, Julie Veran, Fanny Grimaud, Anouck Coulange Zavarro, Rémi Fernandez, and et al. 2022. "Intra Articular Injection of Autologous Microfat and Platelets-Rich Plasma in the Treatment of Wrist Osteoarthritis: A Pilot Study" Journal of Clinical Medicine 11, no. 19: 5786. https://doi.org/10.3390/jcm11195786
APA StyleMayoly, A., Witters, M., Jouve, E., Bec, C., Iniesta, A., Kachouh, N., Veran, J., Grimaud, F., Zavarro, A. C., Fernandez, R., Bendahan, D., Giraudo, L., Dumoulin, C., Chagnaud, C., Casanova, D., Sabatier, F., Legré, R., Jaloux, C., & Magalon, J. (2022). Intra Articular Injection of Autologous Microfat and Platelets-Rich Plasma in the Treatment of Wrist Osteoarthritis: A Pilot Study. Journal of Clinical Medicine, 11(19), 5786. https://doi.org/10.3390/jcm11195786






