Effects of DPP4 Inhibitor in Platelet Reactivity and Other Cardiac Risk Markers in Patients with Type 2 Diabetes and Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
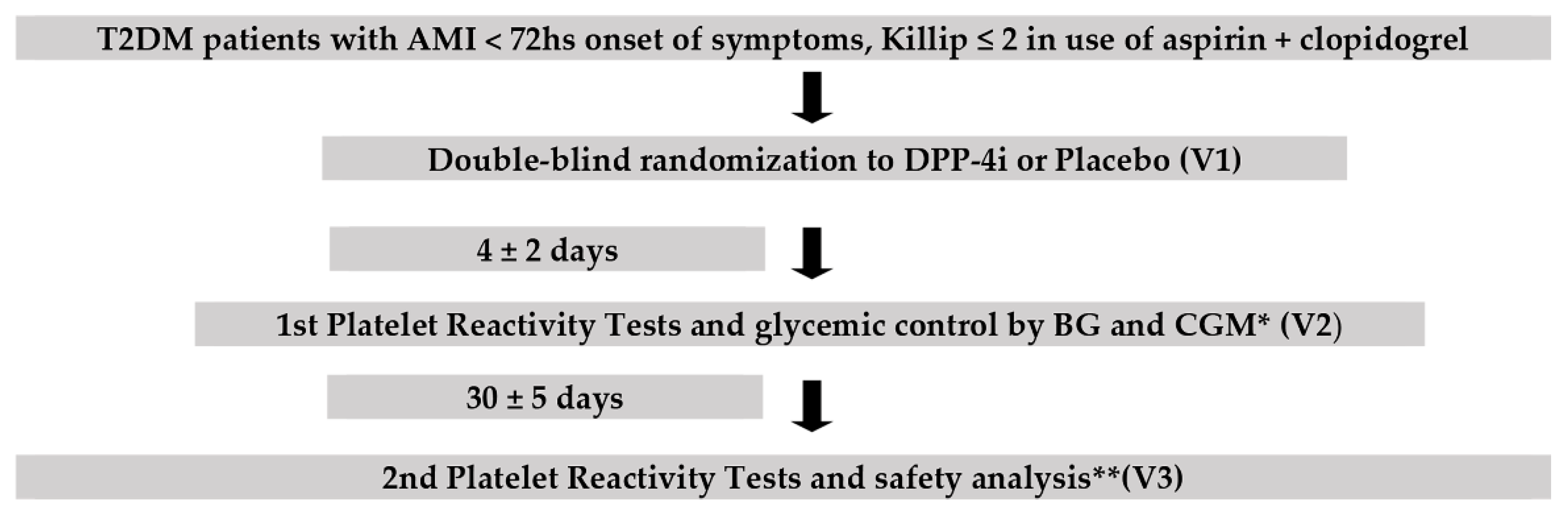
2.1. Study Design
2.2. Randomization and Masking
2.3. Participants
2.4. Inclusion and Exclusion Criteria
2.5. Study Endpoints
3. Study Procedures
3.1. Analysis of Platelet Reactivity
3.2. Other Laboratory Analysis
3.3. Glycemic Control
3.4. Ethical Aspects
3.5. Statistical Analysis
4. Results
4.1. Enrollment
4.2. Participants Baseline Characteristics
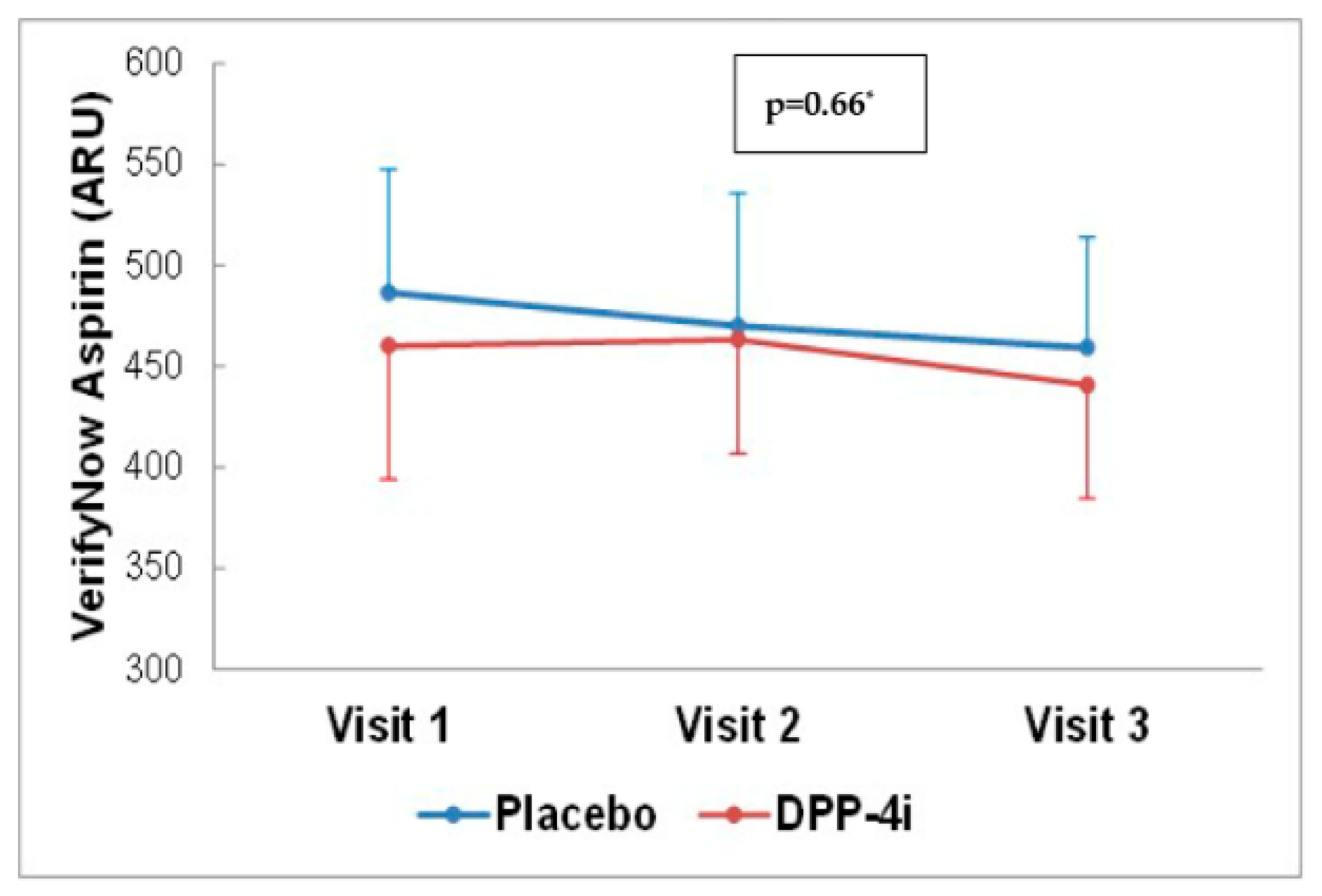
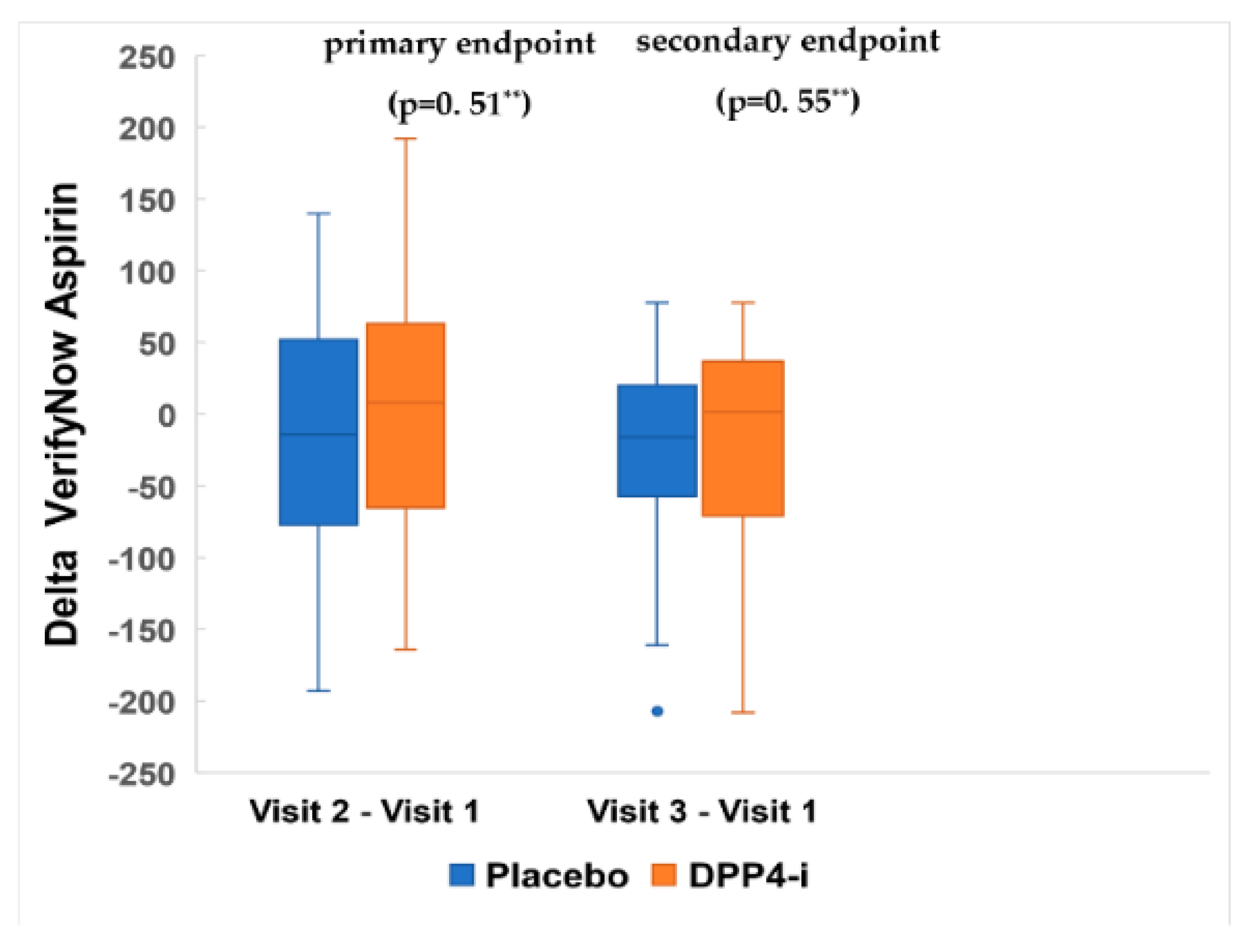
4.3. Effects of DPP-4 Inhibitor on Platelet Reactivity
4.4. Effects of DPP-4 Inhibitor on Glycemic Control
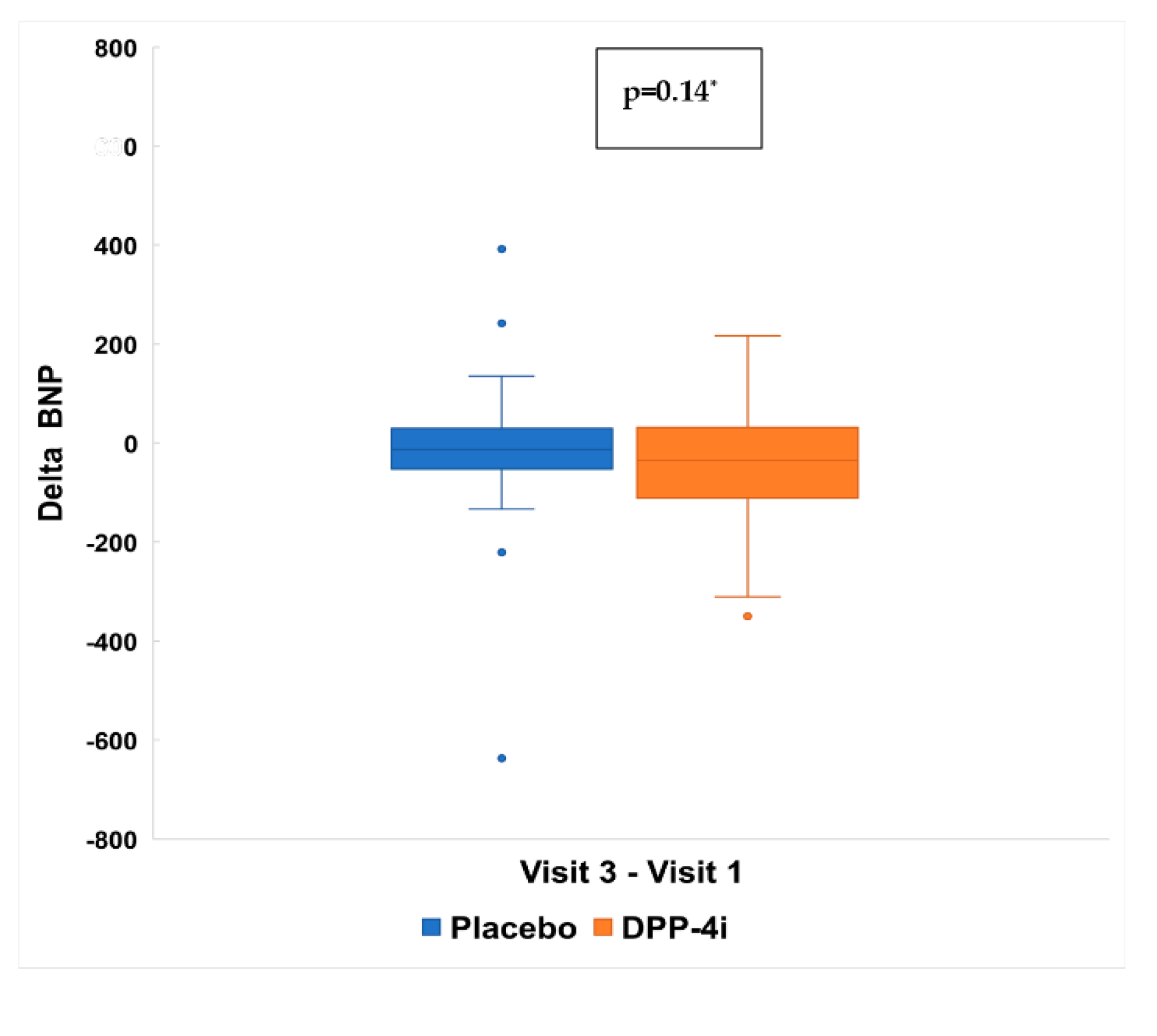
4.5. Effects of DPP4 Inhibitor on BNP
4.6. Effects of DPP4 Inhibitor on Inflammation
4.7. Safety Analyses
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- International Diabetes Federation. IDF Diabetes Atlas, 2017, 8th ed.; Suvi, K., Joao da Rocha Fernandes, B.M., Eds.; International Diabetes Fedration: Brussels, Belgium, 2017; ISBN 978-2-930229-87-4. Available online: http://www.diabetesatlas.org/ (accessed on 3 February 2020).
- International Diabetes Federation. Diabetes and Cardiovascular Disease Brussels, Belgium. 2016. Available online: http://www.idf.org/cvd (accessed on 3 February 2020).
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of Tight Glycemic Control during Acute Coronary Syndrome on CV Outcome in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, A.R.; Kietsiriroje, N.; Badimon, L.; Vilahur, G.; Gorog, A.D.; Angiolillo, D.J.; Russell, A.D.; Rocca, B.; Storey, R.F. Antithrombotic therapy in diabetes: Which, when, and for how long? Eur. Hear. J. 2021, 42, 2235–2259. [Google Scholar] [CrossRef]
- Ferreiro, J.L.; Angiolillo, D.J. Diabetes and Antiplatelet Therapy in Acute Coronary Syndrome. Circulation 2011, 123, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Paven, E.; Dillinger, J.-G.; Sollier, C.B.D.; Vidal-Trecan, T.; Berge, N.; Dautry, R.; Gautier, J.-F.; Drouet, L.; Riveline, J.-P.; Henry, P. Determinants of aspirin resistance in patients with type 2 diabetes. Diabetes Metab. 2019, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Papazafiropoulou, A.; Papanas, N.; Pappas, S.; Maltezos, E.; Mikhailidis, D.P. Effects of oral hypoglycemic agents on platelet function. J. Diabetes Its Complicat. 2015, 29, 846–851. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Pieralice, S.; Giannone, S.; Carpenito, M.; Monte, L.; Watanabe, M.; Cavallari, I.; Maddaloni, E.; Ussia, G.P.; et al. Platelet Effects of Anti-diabetic Therapies: New Perspectives in the Management of Patients with Diabetes and Cardiovascular Disease. Front. Pharmacol. 2021, 12, 670155. [Google Scholar] [CrossRef]
- Xia, C.; Goud, A.; D’Souza, J.; Dahagam, C.H.; Rao, X.; Rajagopalan, S.; Zhong, J. DPP4 inhibitors and cardiovascular outcomes: Safety on heart failure. Hear. Fail. Rev. 2017, 22, 299–304. [Google Scholar] [CrossRef]
- McGuire, D.K.; Van de Werf, F.; Armstrong, P.W.; Standl, E.; Koglin, J.; Green, J.B.; Bethel, M.A.; Cornel, J.H.; Lopes, R.D.; Halvorsen, S.; et al. Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) Study Group. Association Between Sitagliptin Use and Heart Failure Hospitalization and Related Out-comes in Type 2 Diabetes Mellitus: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 126–135. [Google Scholar] [CrossRef]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.; Mosenzon, O.; KyungAh SAVOR-TIMI 53 Steering Committee and Investigators; Umez-Eronini, A.A.; et al. Heart Failure, Saxagliptin, and Diabetes Mellitus: Observations from the SAVOR-TIMI 53 Randomized Trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2017, 40 (Suppl. 1), S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. J. Am. Coll Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Lordkipanidzé, M. Testing platelet function. Hematol Oncol Clin. N. Am. 2013, 27, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Toyoshima, M.T.K.; de Souza, A.B.C.; Admoni, S.N.; Cukier, P.; Lottenberg, S.A.; Latronico, A.C.; Nery, M. New digital tool to facilitate subcuta-neous insulin therapy orders: An inpatient insulin dose calculator. Diabetol. Metab. Syndr. 2015, 7, 114. [Google Scholar] [CrossRef]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions, a measure of diabetes instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Dorje, T.; Yan, H.; Qian, J.; Ge, J. Glycemic variability predicts cardiovascular complications in acute myocardial infarction patients with type 2 diabetes mellitus. Int. J. Cardiol. 2014, 172, 498–500. [Google Scholar] [CrossRef]
- Czerwoniuk, D.; Fendler, W.; Walenciak, L.; Mlynarski, W. GlyCulator: A glycemic variability calculation tool for continuous glucose monitoring data. J. Diabetes Sci. Technol. 2011, 5, 447–451. [Google Scholar] [CrossRef]
- Dracoulakis, M.D.A.; Gurbel, P.A.; Giugliano, R.P.; Cattaneo, M.; Martins, H.S.; Nicolau, J.C.; Filho, R.K. High Residual Platelet Reactivity during Aspirin Therapy in Patients with Non-St Segment Elevation Acute Coronary Syndrome: Comparison Between Initial and Late Phases. Arq. Bras. Cardiol. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiolo-gy/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016, 134, e123–e155. [Google Scholar]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary ar-tery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- Nicolau, J.C.; Feitosa Filho, G.S.; Petriz, J.L.; Furtado, R.H.D.M.; Precoma, D.B.; Lemke, W.; Lopes, R.D.; Timerman, A.; Marin Neto, J.A.; Bezerra Neto, L.; et al. Brazilian Society of Cardiology Guide-lines on Unstable Angina and Acute Myocardial Infarction without ST-Segment Elevation. Arq. Bras. Cardiol. 2021, 117, 181–264. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.B.; Grove, E.L.; Neergaard-Petersen, S.; Würtz, M.; Hvas, A.M.; Kristensen, S.D. Determinants of reduced antiplatelet effect of aspirin in patients with stable coronary artery disease. PLoS ONE 2015, 10, e0126767. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P.; Liani, R.; Davì, G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015, 120, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Gupta, A.K.; Verma, A.K.; Kailashiya, J.; Singh, S.K.; Kumar, N. Sitagliptin: Antiplatelet effect in diabetes and healthy volunteers. Platelets 2012, 23, 565–570. [Google Scholar] [CrossRef]
- Cameron-Vendrig, A.; Reheman, A.; Siraj, M.A.; Xu, X.R.; Wang, Y.; Lei, X.; Afroze, T.; Shikatani, E.; El-Mounayri, O.; Noyan, H.; et al. Glucagon-Like Peptide 1 Receptor Activation At-tenuates Platelet Aggregation and Thrombosis. Diabetes 2016, 65, 1714–1723. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Feng, Z.; Wan, Q.; Wu, J. Linagliptin Regulates the Mitochondrial Respiratory Reserve to Alter Platelet Activation and Arterial Thrombosis. Front. Pharmacol. 2020, 11, 585612. [Google Scholar] [CrossRef]
- Barale, C.; Buracco, S.; Cavalot, F.; Frascaroli, C.; Guerrasio, A.; Russo, I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thromb Haemost. 2017, 117, 1115–1128. [Google Scholar] [CrossRef]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S.; Dandona, P. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef]
- Vivas, D.; García-Rubira, J.C.; Bernardo, E.; Angiolillo, D.J.; Martín, P.; Calle-Pascual, A.; Núnez-Gil, I.; Macaya, C.; Fernández-Ortiz, A. Effects of intensive glucose control on platelet reactivity in patients with acute coronary syndromes. Results of the CHIPS Study (“Control de Hiperglucemia y Actividad Plaquetaria en Pacientes con Sindrome Coronario Agudo”). Heart 2011, 97, 803–809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucci, C.; Cosentino, N.; Genovese, S.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Rondinelli, M.; Riggio, D.; Biondi, M.L.; Rubino, M.; et al. Prognostic impact of admission high-sensitivity C-reactive protein in acute myocardial infarction patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2020, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Car-diovascular Inflammation Reduction Trial. Eur. Heart J. 2020, 41, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhi-bition in Patients With Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Tremblay, A.J.; Lamarche, B.; Deacon, C.F.; Weisnagel, S.J.; Couture, P. Effects of sitagliptin therapy on markers of low-grade in-flammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism 2014, 63, 1141–1148. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Simpson, R.W.; Dear, A.E. GLP-1-dependent and independent effects and molecular mechanisms of a dipeptidyl peptidase 4 inhibitor in vascular endothelial cells. Mol. Biol. Rep. 2013, 40, 2273–2279. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory potentials of incretin-based thera-pies used in the management of diabetes. Life Sci. 2020, 241, 117152. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. TECOS Study Group. Effect of Sitagliptin on Cardiovas-cular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Rosenstock, J.; Kahn, S.E.; Johansen, O.E.; Zinman, B.; Espeland, M.A.; Woerle, H.J.; Pfarr, E.; Keller, A.; Mattheus, M.; Baanstra, D.; et al. Effect of Linagliptin vs. Glimepiride on Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019, 322, 1155–1166. [Google Scholar] [CrossRef]
- McMurray, J.J.; Ponikowski, P.; Bolli, G.B.; Lukashevich, V.; Kozlovski, P.; Kothny, W.; Lewsey, J.D.; Krum, H.; VIVIDD Trial Committees and Investigators. Effects of Vildagliptin on Ventricular Function in Patients With Type 2 Diabetes Mellitus and Heart Failure: A Randomized Placebo-Controlled Trial. JACC Heart Fail. 2018, 6, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, A.E.; Katz, M.; Caixeta, A.; Makdisse, M.R.; Correia, A.G.; Pereira, C.; Franken, M.; Fava, A.N.; Serrano, C.V.; Jr. Prognostic value of serial brain natriuretic Peptide measurements in patients with acute myocardial infarction. Cardiology 2015, 131, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Jarolim, P.; White, W.B.; Cannon, C.P.; Gao, Q.; Morrow, D.A. Serial Measurement of Natriuretic Peptides and Cardiovascular Out-comes in Patients with Type 2 Diabetes in the EXAMINE Trial. Diabetes Care 2018, 41, 1510–1515. [Google Scholar] [CrossRef]
- Wolsk, E.; Claggett, B.; Pfeffer, M.A.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Lawson, F.C.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.; et al. Role of B-Type Natriuretic Peptide and N-Terminal Prohormone BNP as Predictors of Cardiovascular Morbidity and Mortality in Patients With a Recent Coronary Event and Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2017, 6, e004743. [Google Scholar] [CrossRef]
- Rabizadeh, S.; Tavakoli Ardakani, M.A.; Mouodi, M.; Bitaraf, M.; Shab-Bidar, S.; Esteghamati, A.; Nakhjavani, M. DPP4 Inhibitors in the Man-agement of Hospitalized Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Ther. 2020, 37, 3660–3675. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Adrion, C.; Grabmaier, U.; Theisen, D.; von Ziegler, F.; Leber, A.; Becker, A.; Sohn, H.Y.; Hoffmann, E.; Mansmann, U.; et al. Sitagliptin plus granulocyte-colony stimulating factor in patients suffering from acute myocardial infarction: A double-blinded randomized placebo-controlled trial of efficacy and safety (SITAGRAMI trial). Int. J. Cardiol. 2016, 205, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, S.; Miyauchi, K.; Yokoi, H.; Suwa, S.; Nishizaki, Y.; Yokoyama, T.; Nojiri, S.; Iwabuchi, M.; Shirai, S.; Ando, K.; et al. Effect of sitagliptin on plaque changes in coro-nary artery following acute coronary syndrome in diabetic patients: The ESPECIAL-ACS study. J. Cardiol. 2017, 69, 369–376. [Google Scholar] [CrossRef]

| Overall (n = 70) | Placebo (n = 35) | DPP-4i (n = 35) | p-Value | |
|---|---|---|---|---|
| Demographic characteristics and comorbidities | ||||
| Age (years), mean ± SD | 62.6 ± 8.8 | 61.7 ± 9.4 | 63.5 ± 8.2 | 0.41 a |
| Men, n (%) | 45 (64.3%) | 21 (60.0%) | 24 (68.6%) | 0.45 b |
| Caucasian, n (%) | 61 (87.1%) | 29 (60.0%) | 32 (91.4%) | 0.47 c |
| Weight (Kg), mean ± SD | 76.5 ± 14.5 | 78.1 ± 14.4 | 75.0 ± 14.7 | 0.36 a |
| BMI (kg/m2), mean ± SD | 28.0 ± 5.1 | 28.9 ±6.0 | 27.1 ± 3.8 | 0.13 a |
| Hypertension, n (%) | 59 (84.3%) | 31 (88.6%) | 28 (80.0%) | 0.32 b |
| Dyslipidemia, n (%) | 31 (44.3%) | 17 (48.6%) | 14 (40.0%) | 0.47 b |
| History of AMI, n (%) | 21 (30.0%) | 13 (37.1%) | 8 (22.9%) | 0.19 b |
| Known T2DM, n (%) | 65 (92.9%) | 33 (94.3%) | 32 (91.4%) | 1.00 c |
| Years since diagnosis of T2DM, median (IQR) | 9.0 (4.00:13.00) | 10.0 (4.00:13.00) | 5.0 (3.00:12.00) | 0.57 d |
| Current smoking, n (%) | 22 (31.4%) | 10 (28.6%) | 12 (34.3%) | 0.60 b |
| Index event | ||||
| STEMI, type I n (%) | 52 (74.3%) | 24 (68.6%) | 28 (80.0%) | 0.27 b |
| Killip 1, n (%) | 58 (82.8%) | 29 (82.8%) | 29 (82.8%) | 1.00 c |
| PCI + fibrinolitic, n (%) | 28 (40.0%) | 14 (40.0%) | 14 (40.0%) | 1.00 c |
| PCI, n (%) | 37 (52.9%) | 19 (54.2%) | 18 (51.4%) | 1.00 c |
| LVEF (%), mean ± SD | 50.2 ± 8.6 | 51.4 ± 8.9 | 49.1 ± 8.2 | 0.25 a |
| ∆t for randomization (hs), median (IIQ) | 56.5 (37.0:68.0) | 62.0 (36.0:70.0) | 55.0 (37.0:65.0) | 0,24 d |
| Laboratory parameters | ||||
| Hemoglobin (g/dL), mean ± SD | 13.3 ± 1.8 | 13.2 ± 1.8 | 13.4 ± 1.8 | 0.69 a |
| WBC (103/mm3), mean ± SD | 9.7 ± 3.1 | 9.6 ± 3.3 | 9.7 ± 2.9 | 0.91 a |
| Platelet count (103/mm3), mean ± SD | 224.6 ± 53.1 | 221.8 ± 59.7 | 227.4 ± 46.2 | 0.66 a |
| MPV (fL), mean ± SD | 10.8 ± 0.8 | 10.9 ± 0.8 | 10.7 ± 0.8 | 0.28 a |
| IPF(%) | 6.0 ± 3.0 | 6.2 ± 2.6 | 5.8 ± 3.4 | 0.59 a |
| Cholesterol (mg/dL), mean ± SD | 175.9 ± 42.6 | 162.9 ± 39.3 | 188.9 ± 42.2 | 0.01 a |
| LDL-C (mg/dL), mean ± SD | 104.5 ± 35.8 | 93.6 ± 32.4 | 115.4 ± 36.2 | 0.01 a |
| HDL-C (mg/dL), mean ± SD | 41.3 ± 10.7 | 43.0 ± 11.0 | 39.6 ± 10.2 | 0.18 a |
| TGL(mg/dL), mean ± SD | 157.1 ± 103.8 | 130.9 ± 63.3 | 183.2 ± 128.3 | 0.01 a |
| BNP (pg/mL), median (IQR) | 168.0 (68.0:291.0) | 148.0 (58.0:297.0) | 176.0 (83.0:291.0) | 0.97 d |
| hs-CRP (mg/dL), mean ± SD | 39.9 ± 48.4 | 29.7 ± 42.9 | 50.0 ± 52.0 | 0.10 a |
| e-GFR (mL/min/1.73 m2), mean ± SD | 61.8 ± 18.6 | 61.0 ± 17.7 | 62.7 ± 19.7 | 0.71 a |
| Hb1Ac (%), mean ± SD | 8.1 ± 1.6 | 7.9 ± 1.6 | 8.2 ± 1.6 | 0.58 a |
| Glucose (mg/dL), mean ± SD | 215.0 ± 86.1 | 205.1 ± 94.3 | 225.0 ± 77.1 | 0.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genestreti, P.R.R.; Furtado, R.H.M.; Salsoso, R.; Dalçóquio, T.F.; Franci, A.; Menezes, F.R.; Caporrino, C.; Ferrari, A.G.; Nakashima, C.A.K.; Scanavini Filho, M.A.; et al. Effects of DPP4 Inhibitor in Platelet Reactivity and Other Cardiac Risk Markers in Patients with Type 2 Diabetes and Acute Myocardial Infarction. J. Clin. Med. 2022, 11, 5776. https://doi.org/10.3390/jcm11195776
Genestreti PRR, Furtado RHM, Salsoso R, Dalçóquio TF, Franci A, Menezes FR, Caporrino C, Ferrari AG, Nakashima CAK, Scanavini Filho MA, et al. Effects of DPP4 Inhibitor in Platelet Reactivity and Other Cardiac Risk Markers in Patients with Type 2 Diabetes and Acute Myocardial Infarction. Journal of Clinical Medicine. 2022; 11(19):5776. https://doi.org/10.3390/jcm11195776
Chicago/Turabian StyleGenestreti, Paulo R. Rizzo, Remo H. M. Furtado, Rocio Salsoso, Talia F. Dalçóquio, Andre Franci, Fernando R. Menezes, Cesar Caporrino, Aline G. Ferrari, Carlos A. K. Nakashima, Marco A. Scanavini Filho, and et al. 2022. "Effects of DPP4 Inhibitor in Platelet Reactivity and Other Cardiac Risk Markers in Patients with Type 2 Diabetes and Acute Myocardial Infarction" Journal of Clinical Medicine 11, no. 19: 5776. https://doi.org/10.3390/jcm11195776
APA StyleGenestreti, P. R. R., Furtado, R. H. M., Salsoso, R., Dalçóquio, T. F., Franci, A., Menezes, F. R., Caporrino, C., Ferrari, A. G., Nakashima, C. A. K., Scanavini Filho, M. A., Lima, F. G., Giraldez, R. R. C. V., Baracioli, L. M., & Nicolau, J. C. (2022). Effects of DPP4 Inhibitor in Platelet Reactivity and Other Cardiac Risk Markers in Patients with Type 2 Diabetes and Acute Myocardial Infarction. Journal of Clinical Medicine, 11(19), 5776. https://doi.org/10.3390/jcm11195776






