Echocardiographic Probability of Pulmonary Hypertension in Cardiac Surgery Patients—Occurrence and Association with Respiratory Adverse Events—An Observational Prospective Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria of the Study
2.2. Echocardiographic Measurements
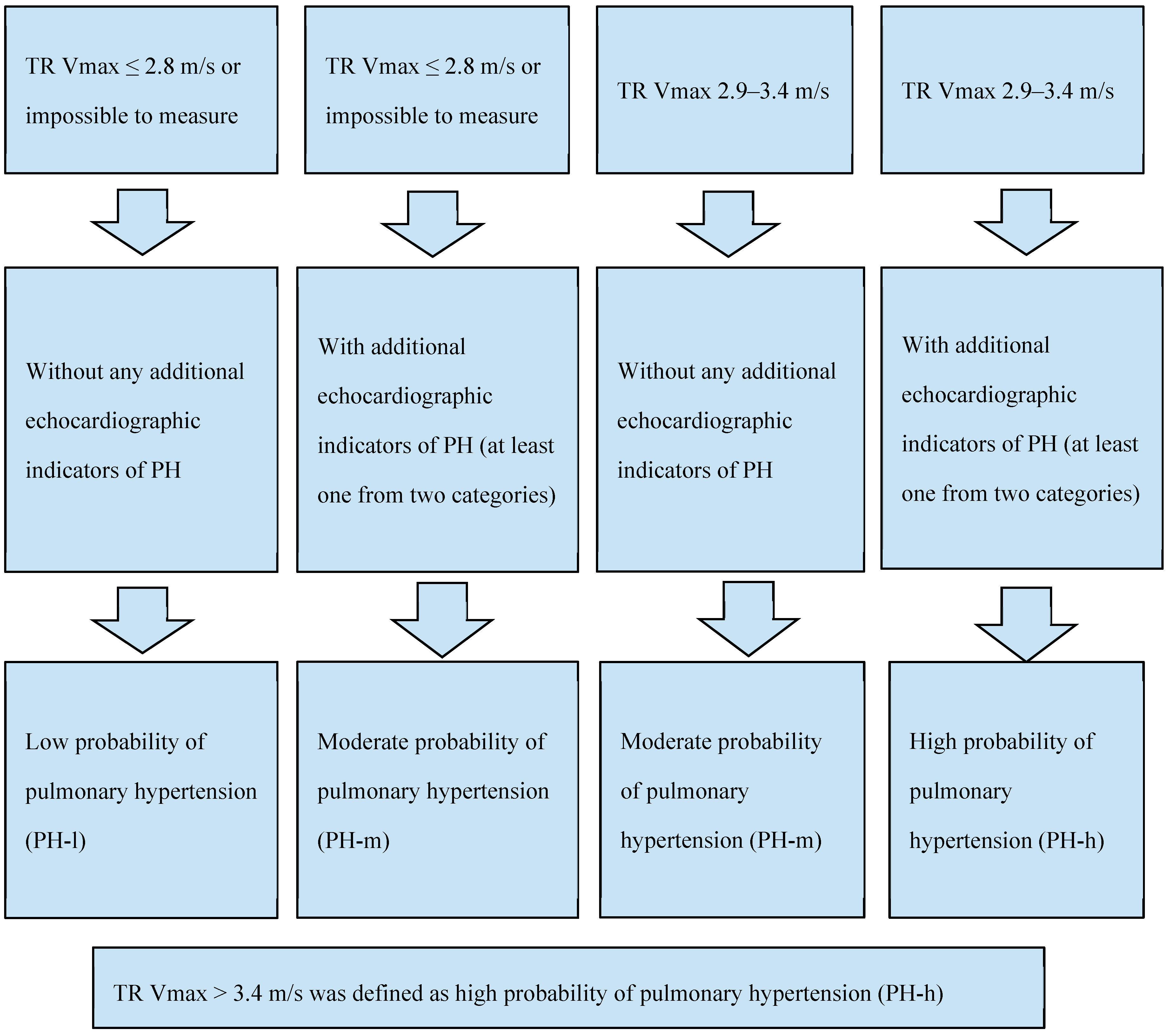
2.2.1. Probability of Pulmonary Hypertension Assessment
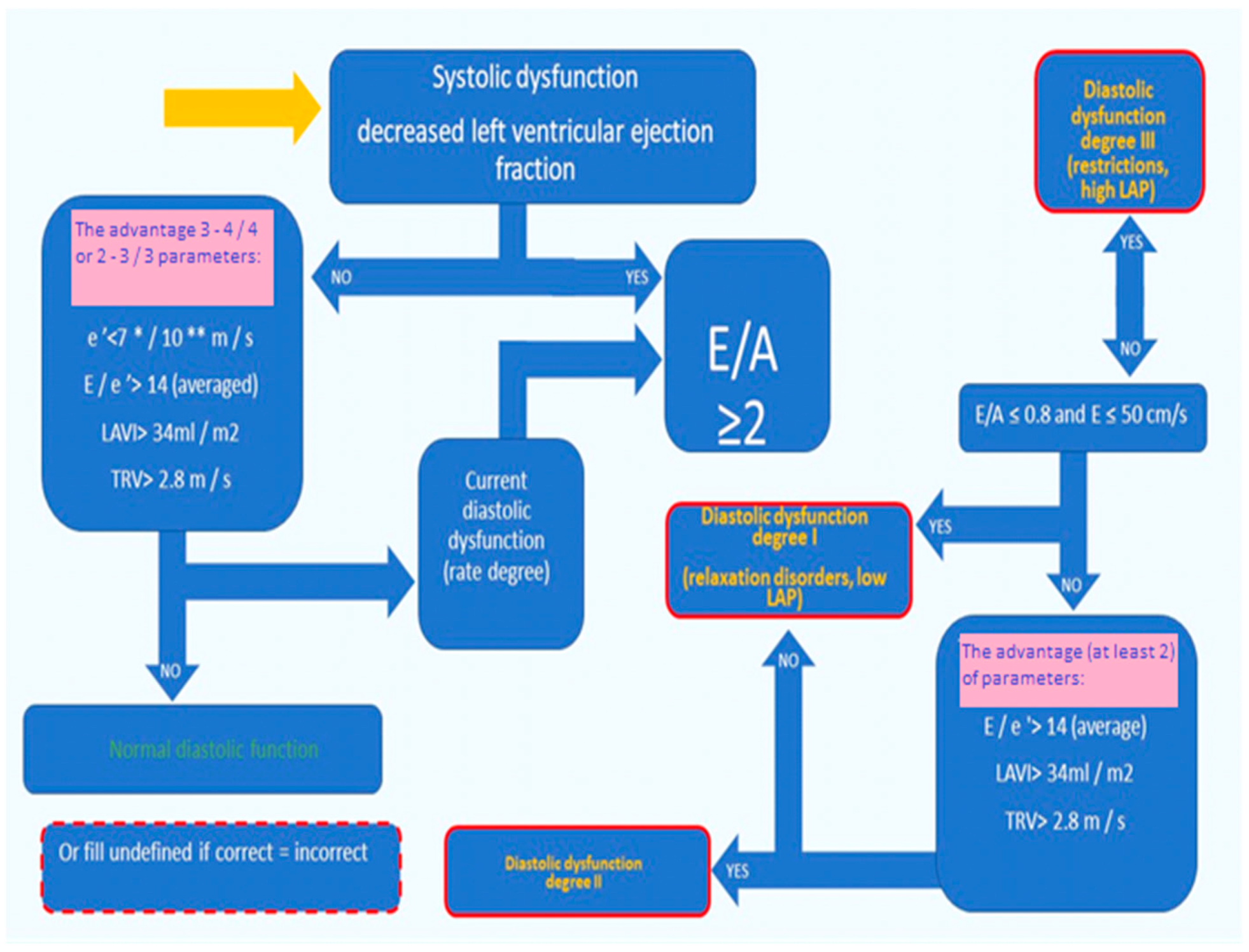
2.2.2. Left Ventricular Systolic and Diastolic Function Assessment
2.3. Perioperative Management and Surgery Procedures
2.3.1. General Anesthesia Procedures
2.3.2. Postoperative Management and Measurements
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristic
3.2. Primary and Secondary Endpoints
3.2.1. Primary Endpoints
3.2.2. Secondary Endpoints
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Galié, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015, 46, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Kipfmueller, F.; Akkas, S.; Pugnaloni, F.; Bo, B.; Lemloh, L.; Schroeder, L.; Gembruch, U.; Geipel, A.; Berg, C.; Heydweiller, A.; et al. Echocardiographic Assessment of Pulmonary Hypertension in Neonates with Congenital Diaphragmatic Hernia Using Pulmonary Artery Flow Characteristics. J. Clin. Med. 2022, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Topyła-Putowska, W.; Tomaszewski, M.; Wysokiński, A.; Tomaszewski, A. Echocardiography in pulmonary arterial hypertension: Comprehensive evaluation and technical considerations. J. Clin. Med. 2021, 10, 3229. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.D.; Parsonnet, V. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann. Thorac. Surg. 2000, 69, 823–828. [Google Scholar] [CrossRef]
- Denault, A.; Deschamps, A.; Tardif, J.-C.; Lambert, J.; Perrault, L. Pulmonary hypertension in cardiac surgery. Curr. Cardiol. Rev. 2010, 6. [Google Scholar] [CrossRef]
- Bharathi, K.S.; Kundra, T.S.; Nagaraja, P.S.; Kaur, P.; Manjunatha, N. Inhaled Levosimendan versus intravenous Levosimendan in patients with pulmonary hypertension undergoing mitral valve replacement. Ann. Card. Anaesth. 2018, 21, 328–332. [Google Scholar] [CrossRef]
- Gerges, M.; Gerges, C.; Pistritto, A.-M.; Lang, M.B.; Trip, P.; Jakowitsch, J.; Binder, T.; Lang, I.M. Pulmonary hypertension in heart failure. Epidemiology, Right Ventricular Function, and Survival. Am. J. Respir. Crit. Care Med. 2015, 192, 1234–1246. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, I.; AbouEzzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef]
- Metkus, T.S.; Suarez-Pierre, A.; Crawford, T.C.; Lawton, J.S.; Goeddel, L.; Dodd-O, J.; Mukherjee, M.; Abraham, T.P.; Whitman, G.J. Diastolic dysfunction is common and predicts outcome after cardiac surgery. J. Cardiothorac. Surg. 2018, 13, 67. [Google Scholar] [CrossRef]
- Nicoara, A.; Swaminathan, M. Diastolic dysfunction, diagnostic and perioperative management in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 60–66. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Pletz, M.W.; Golpon, H.; Welte, T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2007, 29, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef]
- Toy, P.; Lowell, C. TRALI-Definition, mechanisms, incidence and clinical relevance. Best Pr. Res. Clin. Anaesthesiol. 2007, 21, 183–193. [Google Scholar] [CrossRef]
- Lam, C.S.; Roger, V.L.; Rodeheffer, R.J.; Borlaug, B.A.; Enders, F.T.; Redfield, M.M. Pulmonary hypertension in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2009, 53, 1119–1126. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Lam, C.S.; Vachiery, J.-L.; Bauersachs, J.; Gerges, C.; Lang, I.M.; Bonderman, D.; Olsson, K.M.; Gibbs, J.S.R.; Dorfmüller, P.; et al. Pulmonary hypertension in heart failure with preserved ejection fraction: A plea for proper phenotyping and further research. Eur. Heart J. 2017, 38, 2869–2873. [Google Scholar] [CrossRef]
- Vachiéry, J.-L.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, J.S.R.; et al. Pulmonary hypertension due to left heart diseases. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D100–D108. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Wang, H.; Le, S.; Yang, H.; Wang, F.; Du, X. Risk factors for postoperative pneumonia after cardiac surgery: A prediction model. J. Thorac. Dis. 2021, 13, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Ewig, S.; Lode, H.; Carlet, J.; For The European HAP Working Group. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensiv. Care Med. 2009, 35, 9–29. [Google Scholar] [CrossRef]
- McAuley, P.A.; Blair, S.N. Obesity paradoxes. J. Sports Sci. 2011, 29, 773–782. [Google Scholar] [CrossRef]
- Johnson, A.P.; Parlow, J.L.; Whitehead, M.; Xu, J.; Rohland, S.; Milne, B. Body Mass Index, outcomes, and mortality following cardiac surgery in Ontario. Can. J. Am. Heart Assoc. 2015, 4, e002140. [Google Scholar] [CrossRef]
- Vlaar, A.P.J.; Hofstra, J.J.; Determann, R.M.; Veelo, D.P.; Paulus, F.; Kulik, W.; Korevaar, J.; de Mol, B.A.; Koopman, M.M.W.; Porcelijn, L.; et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: A prospective nested case-control study. Blood 2011, 117, 4218–4225. [Google Scholar] [CrossRef]
- Milot, J.; Perron, J.; Lacasse, Y.; Létourneau, L.; Cartier, P.C.; Maltais, F. Incidence and predictors of ARDS after cardiac surgery. Chest 2001, 119, 884–888. [Google Scholar] [CrossRef]
- Pinzani, A.; De Gevigney, G.; Pinzani, V.; Ninet, J.; Milon, H.; Delahaye, J.P. Pre- and postoperative right cardiac insufficiency in patients with mitral or mitral -aortic valve diseases. Arch. Mal. Coeur. Vaiss. 1993, 86, 27–34. [Google Scholar]
| Inclusion Criteria: |
|---|
|
Age > 18 years Coronary artery disease, qualified for coronary artery bypass grafting with use of cardiopulmonary bypass Elective surgery |
| Left ventricular ejection fraction (LVEF) ≥ 40% in echocardiography |
| Exclusion Criteria: |
|---|
| Pulmonary diseases with severe or moderate restrictive or obstructive disorder |
| Moderate or severe mitral, tricuspid, aortic or pulmonic valve insufficiency or stenosis, qualified for operation 1 |
| Non-elective surgery |
| Off—pump surgery |
| LVEF < 40% |
| Infective endocarditis |
| Hypertrophic cardiomyopathy |
| Atrial fibrillation or post pacemaker/cardioverter defibrillator implantation status |
| Pulmonary arterial hypertension treated with targeted treatment 2 |
| Perioperative myocardial infarction (MI type 5) 3 |
| Perioperative stroke |
| Postoperative hemorrhagic complications requiring surgical revision |
| PH-l | PH-m/h | p | |
|---|---|---|---|
| n | 27 | 29 | |
| Age | 65 (63–71) | 71 (66–73) | 0.017 |
| Sex (M, %) | 26 (96.3%) | 19 (65.52%) | 0.005 |
| Obesity | 12 (44.44%) | 14 (48.28%) | NS |
| Body mass index | 29 (28–31) | 29 (28–32) | NS |
| EuroSCORE II result | 0.76 (0.67–0.92) | 1.27 (0.93–1.67) | <0.001 |
| Smoking history (n, %) | 13 (48.15%) | 8 (27.59%) | NS |
| COPD n (%) | 1 (3.7%) | 2 (6.9%) | NS |
| Stroke n (%) | 1 (3.7%) | 3 (10.34%) | NS |
| Diabetes n (%) | 10 (37.04%) | 14 (48.28%) | NS |
| LIMA + RIMA n (%) | 4 (14.81%) | 3 (10.34%) | NS |
| 2 by-pass grafts n (%) | 12 (44.44%) | 15 (51.72%) | NS |
| 3 by-pass grafts n (%) | 6 (22.22%) | 10 (31.48%) | NS |
| 4 by-pass grafts n (%) | 7 (25.93%) | 4 (13.79%) | NS |
| 5 by-pass grafts n (%) | 2 (7.41%) | 0 | NS |
| LAD surgery n (%) | 27 (100%) | 29 (100%) | NS |
| LCx surgery n (%) | 12(44,4%) | 14 (48,27%) | NS |
| RCA surgery n (%) | 19 (70,37%) | 20 (68,96%) | NS |
| Arterial hypertension n (%) | 21 (77.78%) | 22 (75.86%) | NS |
| CKD stage 2 n (%) | 9 (33.3%) | 10 (34.48%) | NS |
| CKD stage 3, 4 or 5 | 0 | 0 | NS |
| Parameter | PH-l | PH-m/h | p |
|---|---|---|---|
| P Eff pre | 0 | 0 | NS |
| P Eff post | 0 | 0 | NS |
| TR V max pre | 1.5 (1.3–1.9) | 2.81 (2.2–2.9) | <0.001 |
| TR V max post | 1.6 (1.2–1.9) | 2.7 (2.0–3.0) | <0.001 |
| TAPSE pre | 22.89 (±4.21) | 21.83 (±3.7) | NS |
| TAPSE post | 24 (±3.2) | 22 (±2.4) | NS |
| PV AccT pre | 108 (92–120) | 85 (72–90) | <0.001 |
| PV AccT post | 103 (90–115) | 80 (71–87) | <0.001 |
| RV/LV pre | 0.8 (0.7–0.88) | 1.0 (0.9–1.1) | <0.001 |
| RV/LV post | 0.8 (0.6–0.9) | 1.1 (0.95–1.2) | <0.001 |
| IVC pre | 1.2 (1.1–1.7) | 2.2 (2.1–2.3) | <0.001 |
| IVC post | 1.4 (1.0–1.8) | 2.3 (2.0–2.5) | <0.001 |
| sPAP pre | 17 (13–21) | 47 (31–51) | <0.001 |
| sPAP post | 19 (15–22) | 48 (30–52) | <0.001 |
| RA area > 18 cm2 pre | 0 (0%) | 10 (34.48%) | <0.001 |
| RA area > 18 cm2 post | 0 (0%) | 11 (37.9%) | <0.001 |
| LVEF pre | 63 (55–66) | 61.2 (48–67.5) | NS |
| LVEF post | 66 (57–68) | 62 (46–69) | NS |
| LV DD stage II or III pre | 5 (18.5%) | 19 (65.5%) | <0.001 |
| LV DD stage II or III post | 4 (14.8%) | 19 (65.5%) | <0.001 |
| SBP pre | 147.7 (110–170) | 147.4 (105–170) | NS |
| SBP post | 141 (90–165) | 139.5 (85–159) | NS |
| DBP pre | 68.8 (60–80) | 69.3 (60–85) | NS |
| DBP post | 65 (55–80) | 63.4 (52–84) | NS |
| HR pre | 72 (64–90) | 76 (68–88) | NS |
| HR post | 79 (72–100) | 82 (74–104) | NS |
| CPB Parameters | PH-l | PH-m/h | p |
|---|---|---|---|
| Perfusion time (minutes) | 59 (52–71) | 51 (45–58) | 0.008 |
| Reperfusion time (minutes) | 21 (16–26) | 17 (14–22) | 0.09 |
| Aortic cross-clamp time (minutes) | 37 (32–45) | 32 (27–35) | 0.015 |
| Primary Endpoint | PH-l | PH-m/h | p |
|---|---|---|---|
| Pneumonia n (%) | 1 (3.7%) | 11 (37.93%) | 0.002 |
| Re-intubation | 0 | 0 | NS |
| Pulmonary congestion (%) | 12 (44.44%) | 26 (89.66%) | 0.0008 |
| Pulmonary edema | 0 | 0 | NS |
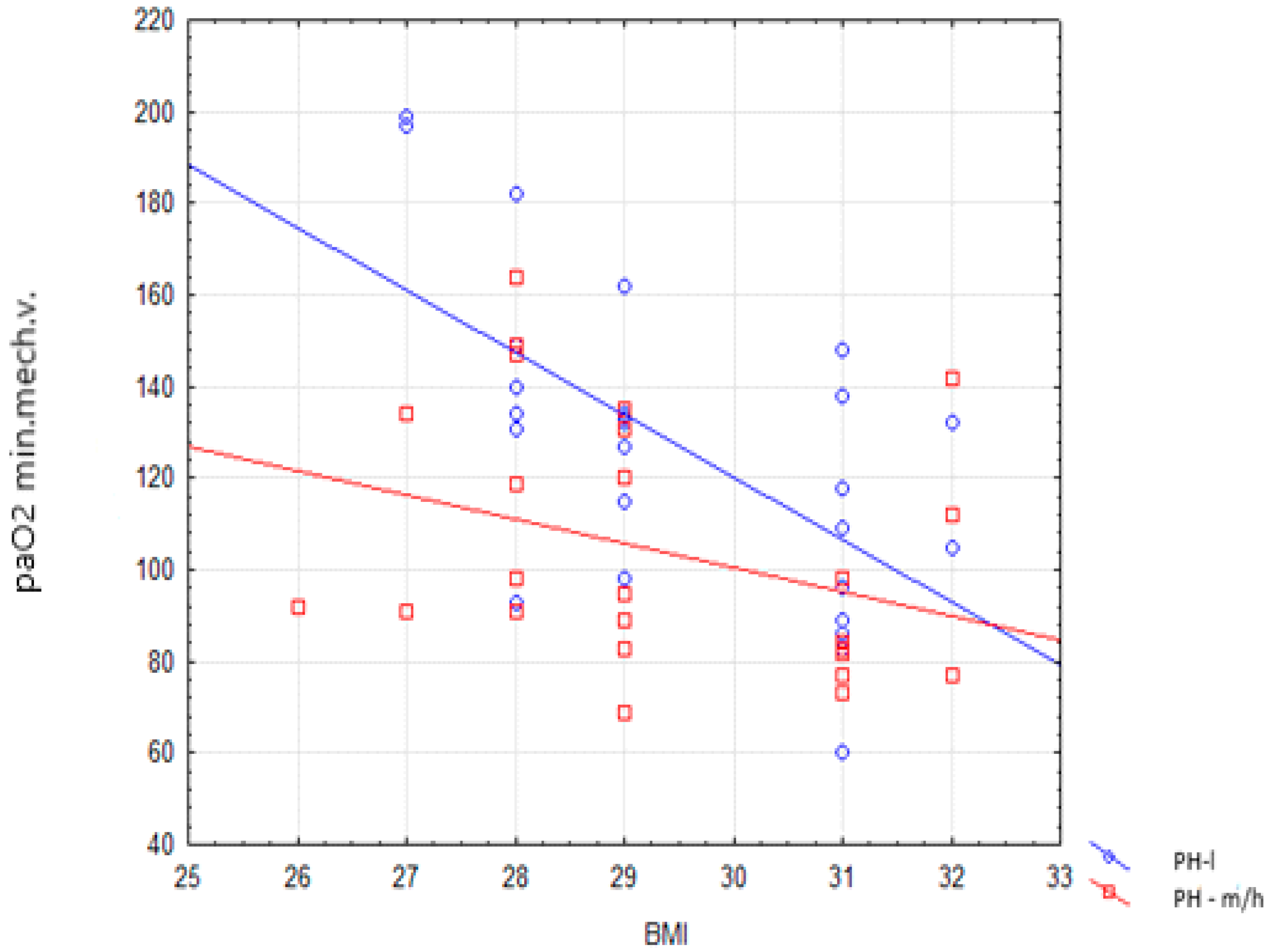
| PaO2 min during mechanical ventilation | 131 (98–140) | 95.0 (83.0–131.0) | 0.019 |
| paO2/FiO2 min | 298 (237–373) | 211 (190–291) | 0.005 |
| PaCO2 min during mechanical ventilation | 40 (37–44) | 40.5 (35.5–43.5) | NS |
| PaO2 min after tracheal extubation | 110 (93–135) | 100 (85–113) | NS |
| PaCO2 min after tracheal extubation | 42.0 (39–44) | 43 (39.5–44.5) | NS |
| Length of mechanical ventilation (hours) | 7.45 (5.00–9.05) | 8.45 (6.15–10.40) | NS |
| Variables | Co-Efficient (®) | 95% CI | p |
|---|---|---|---|
| Intercept | 229.85 | 84.8–374.88 | 0.002 |
| PH m-h | −171.11 | −291.08–−51.15 | 0.006 |
| LV EF | 1.38 | 0.68–2.08 | <0.001 |
| BMI | −6.67 | −10.96–−2.41 | 0.003 |
| PH and BMI interaction | 5.43 | 1.37–9.49 | 0.009 |
| Secondary Endpoint | PH-l | PH-m/h | p |
|---|---|---|---|
| Pneumothorax | 1 (3.7%) | 1 (3.45%) | NS |
| Pleural effusion | 1 (3.7%) | 2 (6.9%) | NS |
| ARDS | 0 | 0 | NS |
| TRALI | 0 | 0 | NS |
| Length of ICU stay (hours) | 44 (36–54) | 56 (40–72) | 0.016 |
| Length of hospitalization (days) | 7 (6–9) | 7 (6–8) | NS |
| In-hospital mortality | 0 | 0 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braksator, M.; Jachymek, M.; Witkiewicz, K.; Piątek, P.; Witkiewicz, W.; Peregud-Pogorzelska, M.; Kotfis, K.; Brykczyński, M. Echocardiographic Probability of Pulmonary Hypertension in Cardiac Surgery Patients—Occurrence and Association with Respiratory Adverse Events—An Observational Prospective Single-Center Study. J. Clin. Med. 2022, 11, 5749. https://doi.org/10.3390/jcm11195749
Braksator M, Jachymek M, Witkiewicz K, Piątek P, Witkiewicz W, Peregud-Pogorzelska M, Kotfis K, Brykczyński M. Echocardiographic Probability of Pulmonary Hypertension in Cardiac Surgery Patients—Occurrence and Association with Respiratory Adverse Events—An Observational Prospective Single-Center Study. Journal of Clinical Medicine. 2022; 11(19):5749. https://doi.org/10.3390/jcm11195749
Chicago/Turabian StyleBraksator, Marta, Magdalena Jachymek, Karina Witkiewicz, Patrycja Piątek, Wojciech Witkiewicz, Małgorzata Peregud-Pogorzelska, Katarzyna Kotfis, and Mirosław Brykczyński. 2022. "Echocardiographic Probability of Pulmonary Hypertension in Cardiac Surgery Patients—Occurrence and Association with Respiratory Adverse Events—An Observational Prospective Single-Center Study" Journal of Clinical Medicine 11, no. 19: 5749. https://doi.org/10.3390/jcm11195749
APA StyleBraksator, M., Jachymek, M., Witkiewicz, K., Piątek, P., Witkiewicz, W., Peregud-Pogorzelska, M., Kotfis, K., & Brykczyński, M. (2022). Echocardiographic Probability of Pulmonary Hypertension in Cardiac Surgery Patients—Occurrence and Association with Respiratory Adverse Events—An Observational Prospective Single-Center Study. Journal of Clinical Medicine, 11(19), 5749. https://doi.org/10.3390/jcm11195749







