Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Analysis of Data
3. Results
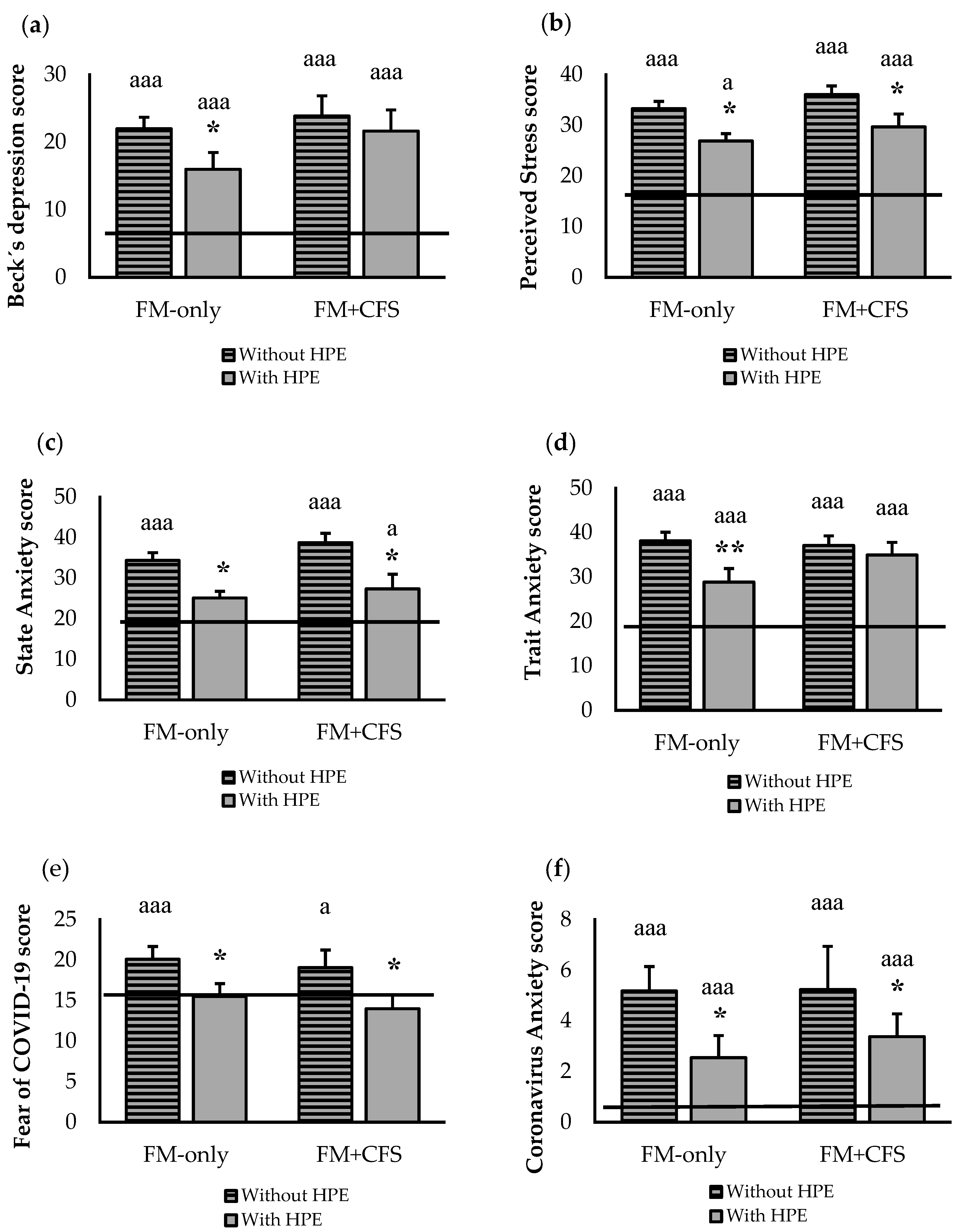
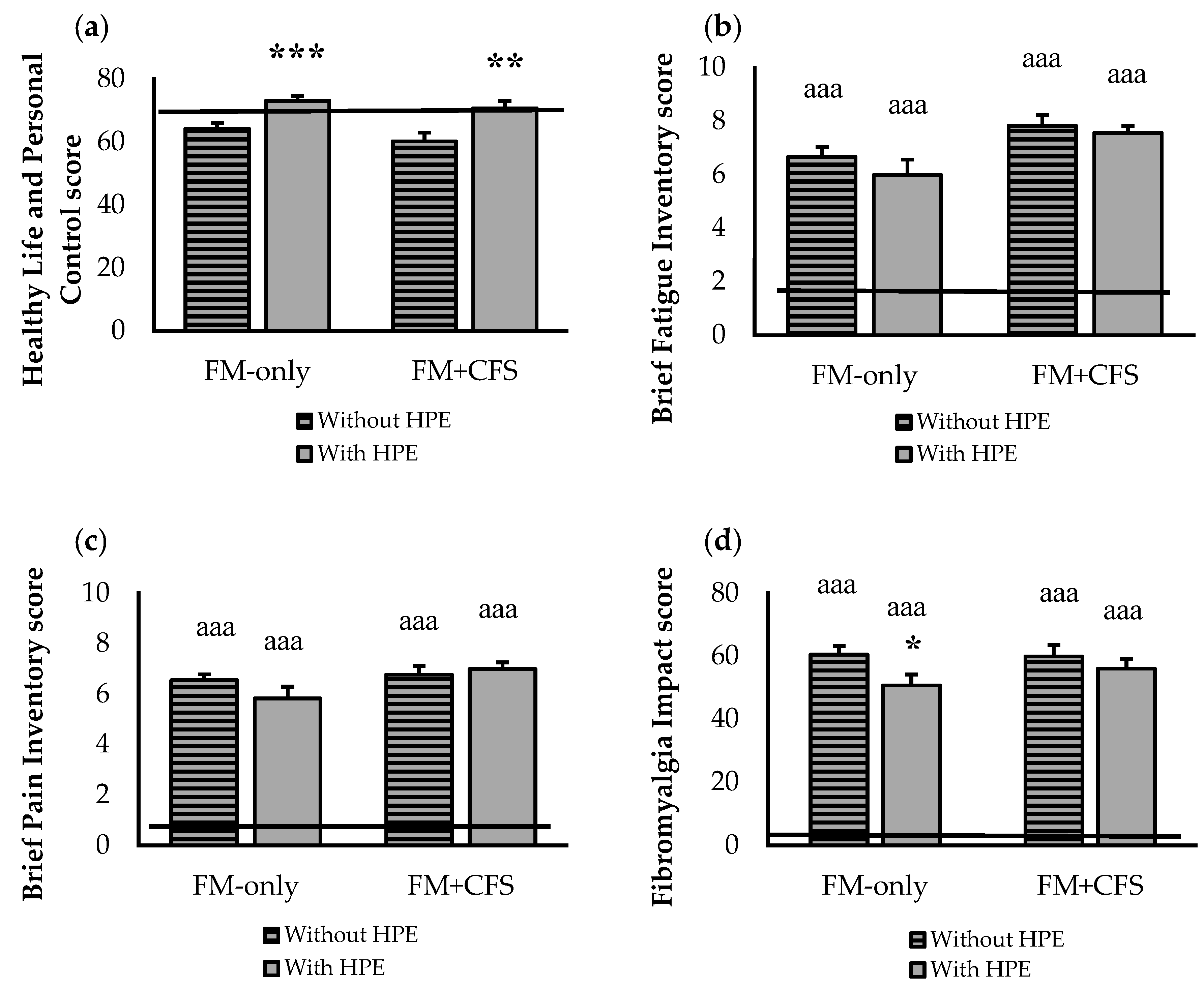
3.1. Psychological State and Quality of Life
3.2. Influence of Codiagnosis of CFS and Performance of HPE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Flub, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR Revised Recommendations for the Management of Fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, D.; Fassoulaki, A.; Tsoulas, C.; Siafaka, I.; Vadalouca, A. A Meta-Analysis to Determine the Effect of Pharmacological and Non-Pharmacological Treatments on Fibromyalgia Symptoms Comprising OMERACT-10 Response Criteria. Clin. Rheumatol. 2016, 35, 573–586. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Henriksen, M.; Segura-Jiménez, V.; Aparicio, V.A.; Carbonell-Baeza, A.; Delgado-Fernández, M.; Amris, K.; Ruiz, J.R. Association of Physical Fitness with Fibromyalgia Severity in Women: The Al-Ándalus Project. Arch. Phys. Med. Rehabil. 2015, 96, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Culos-Reed, S.N.; Brawley, L.R. Fibromyalgia, Physical Activity, and Daily Functioning: The Importance of Efficacy and Health-Related Quality of Life. Arthritis Care Res. 2000, 13, 343–351. [Google Scholar] [CrossRef]
- Ortega, E.; García, J.J.; Bote, M.E.; Martín-Cordero, L.; Escalante, Y.; Saavedra, J.M.; Northoff, H.; Giraldo, E. Exercise in Fibromyalgia and Related Inflammatory Disorders: Known Effects and Unknown Chances. Exerc. Immunol. Rev. 2009, 15, 42–65. [Google Scholar] [PubMed]
- Sañudo, B.; Galiano, D.; Carrasco, L.; de Hoyo, M. Evidence-Based Recommendations for Physical Activity in Women with Fibromyalgia. Rev. Andaluza Med. del Deport. 2010, 3, 159–160. [Google Scholar]
- Bote, M.E.; Garca, J.J.; Hinchado, M.D.; Ortega, E. Inflammatory/Stress Feedback Dysregulation in Women with Fibromyalgia. Neuroimmunomodulation 2012, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bote, M.E.; García, J.J.; Hinchado, M.D.; Ortega, E. An Exploratory Study of the Effect of Regular Aquatic Exercise on the Function of Neutrophils from Women with Fibromyalgia: Role of IL-8 and Noradrenaline. Brain. Behav. Immun. 2014, 39, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Dawes, J.; Stephenson, M.D. Training Individuals with Chronic Fatigue Syndrome. Strength Cond. J. 2008, 30, 55–57. [Google Scholar] [CrossRef]
- Aaron, L.A.; Burke, M.M.; Buchwald, D. Overlapping Conditions Among Patients With Chronic Fatigue Syndrome, Fibromyalgia, and Temporomandibular Disorder. Arch. Intern. Med. 2000, 160, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. The Prevalence of Fibromyalgia in Other Chronic Pain Conditions. Pain Res. Treat. 2012, 2012, 584573. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; Stegner, A.J.; Nagelkirk, P.R.; Meyer, J.D.; Togo, F.; Natelson, B.H. Responses to Exercise Differ for Chronic Fatigue Syndrome Patients with Fibromyalgia. Med. Sci. Sports Exerc. 2012, 44, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise Therapy for Chronic Fatigue Syndrome. Cochrane Database Syst. Rev. 2017, 2017, CD003200. [Google Scholar]
- Ortega, E.; Bote, M.E.; Giraldo, E.; García, J.J. Aquatic Exercise Improves the Monocyte Pro- and Anti-Inflammatory Cytokine Production Balance in Fibromyalgia Patients. Scand. J. Med. Sci. Sport. 2012, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre Benavent, R.; Alonso Arroyo, A.; Anguita Sánchez, M.; Bolaños Pizarro, M.; Heras, M.; González Alcalde, G.; Macaya Miguel, C.; Navarro Molina, C.; Castelló Cogollos, L.; Valderrama Zurián, J.C.; et al. Evolution and Scientific Impact of Research Grants From the Spanish Society of Cardiology and Spanish Heart Foundation (2000–2006). Rev. Española Cardiol. Engl. Ed. 2011, 64, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Félix-Redondo, F.J.; Fernández-Bergés, D.; Fernando Pérez, J.; Zaro, M.J.; García, A.; Lozano, L.; Sanz, H.; Grau, M.; Álvarez-Palacios, P.; Tejero, V. Prevalence, Awareness, Treatment and Control of Cardiovascular Risk Factors in the Extremadura Population (Spain). HERMEX Study. Aten. Primaria 2011, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.; International Chronic Fatigue Syndrome Study Group. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression, Archives of General. Psychiatry 1961, 4, 561. [Google Scholar]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. The Spanish Adaptation of Beck’s Depression Inventory-II (BDI-II): 2. Psychometric Properties in the General Population. Clin. y Salud 2003, 14, 249–280. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Remor, E. Psychometric Properties of a European Spanish Version of the Perceived Stress Scale (PSS). Span. J. Psychol. 2006, 9, 86–93. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory for AdultsTM; APA PsycTests: Washington, DC, USA, 1983. [Google Scholar]
- Buela-Casal, G.; Guillén-Riquelme, A. Short Form of the Spanish Adaptation of the State-Trait Anxiety Inventory. Int. J. Clin. Health Psychol. 2017, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.L.; Cleeland, C.S.; Flanery, R.C. Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases. Pain 1983, 17, 197–210. [Google Scholar] [CrossRef]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Muriel, C.; Gracia, A.; Núñez-Olarte, J.M.; Perulero, N.; Gálvez, R.; Carulla, J.; Cleeland, C.S.; Barutell, C.; Gómez, M.; et al. Validación Española Del Cuestionario Brief Pain Inventory En Pacientes Con Dolor de Causa Neoplásica. Med. Clin. 2003, 120, 52–59. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The Rapid Assessment of Fatigue Severity in Cancer Patients: Use of the Brief Fatigue Inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Valenzuela, J.O.; Gning, I.; Irarrázaval, M.E.; Fasce, G.; Marín, L.; Mendoza, T.R.; Palos, G.; Reynolds, R.; Wang, X.S.; Cleeland, C.S. Psychometric Validation of the Spanish Version of the Brief Fatigue Inventory [abstract] The University of Texas MD Anderson Cancer Center, Division of Internal Medicine Research Retreat, Houston, TX, USA, 24 May 2012. Available online: https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-fatigue-inventory.html (accessed on 24 January 2022).
- Darviri, C.; Alexopoulos, E.C.; Artemiadis, A.K.; Tigani, X.; Kraniotou, C.; Darvyri, P.; Chrousos, G.P. The Healthy Lifestyle and Personal Control Questionnaire (HLPCQ): A Novel Tool for Assessing Self-Empowerment through a Constellation of Daily Activities. BMC Public Health 2014, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The Fibromyalgia Impact Questionnaire: Development and Validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar] [PubMed]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A Validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar] [PubMed]
- Lee, S.A. Coronavirus Anxiety Scale: A Brief Mental Health Screener for COVID-19 Related Anxiety. Death Stud. 2020, 44, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Caycho-Rodríguez, T.; Vilca, L.W.; Peña-Calero, B.N.; Barboza-Palomino, M.; White, M.; Reyes-Bossio, M. Measurement of Coronaphobia in Older Adults: Validation of the Spanish Version of the Coronavirus Anxiety Scale. Rev. Esp. Geriatr. Gerontol. 2022, 57, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kwasi Ahorsu, D.; Lin, C.-Y.; Imani, V.; Saffari, M.; Griffiths, M.D.; Pakpour, A.H. The Fear of COVID-19 Scale: Development and Initial Validation. Int. J. Ment. Health Addict. 2022, 20, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Teruel, D.; Robles-Bello, M.A. The COVID-19 Fear Scale (FCV-19S): Psychometric Properties and Invariance of the Measure in the Spanish Version. Actas Esp. Psiquiatr. 2021, 49, 96–105. [Google Scholar] [PubMed]
- Bote, M.E.; Garcia, J.J.; Hinchado, M.D.; Ortega, E. Fibromyalgia: Anti-Inflammatory and Stress Responses after Acute Moderate Exercise. PLoS ONE 2013, 8, e74524. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Gershwin, M.E. Fibromyalgia: A Critical and Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 100–151. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.; Sáez-Francàs, N.; Castro-Marrero, J.; Aliste, L.; Collado, A.; Alegre, J. Impact of the Fibromyalgia in the Chronic Fatigue Syndrome. Med. Clin. 2014, 142, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cadenas-Sánchez, C.; Ruiz-Ruiz, J. Effect of a Physical Activity Programme in Patients with Fibromyalgia: A Systematic Review. Med. Clin. 2014, 143, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Dannaway, J.; New, C.C.; New, C.H.; Maher, C.G. Exercise Therapy Is a Beneficial Intervention for Chronic Fatigue Syndrome (PEDro Synthesis). Br. J. Sports Med. 2018, 52, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Mohabbat, A.B.; Mohabbat, N.M.L.; Wight, E.C. Fibromyalgia and Chronic Fatigue Syndrome in the Age of COVID-19. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Giorgi, V.; Sirotti, S.; Bongiovanni, S.; Farah, S.; Bazzichi, L.; Marotto, D.; Atzeni, F.; Rizzi, M.; Batticciotto, A.; et al. The Effect of Novel Coronavirus Disease-2019 (COVID-19) on Fibromyalgia Syndrome. Clin. Exp. Rheumatol. 2021, 39, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Cankurtaran, D.; Tezel, N.; Ercan, B.; Yildiz, S.Y.; Akyuz, E.U. The Effects of COVID-19 Fear and Anxiety on Symptom Severity, Sleep Quality, and Mood in Patients with Fibromyalgia: A Pilot Study. Adv. Rheumatol. 2021, 61, 41. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.P.M.; Bodenmuller, P.H.K.; de Oliveira Mello, D.B.; Skare, T.L. COVID-19 Pandemic and Exercising: A Cross-Sectional Study with 1156 Patients with Fibromyalgia. Rev. Assoc. Med. Bras. 2021, 67, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Merriwether, E.N.; Frey-Law, L.A.; Rakel, B.A.; Zimmerman, M.B.; Dailey, D.L.; Vance, C.G.T.; Golchha, M.; Geasland, K.M.; Chimenti, R.; Crofford, L.J.; et al. Physical Activity Is Related to Function and Fatigue but Not Pain in Women with Fibromyalgia: Baseline Analyses from the Fibromyalgia Activity Study with TENS (FAST). Arthritis Res. Ther. 2018, 20, 199. [Google Scholar] [CrossRef] [PubMed]
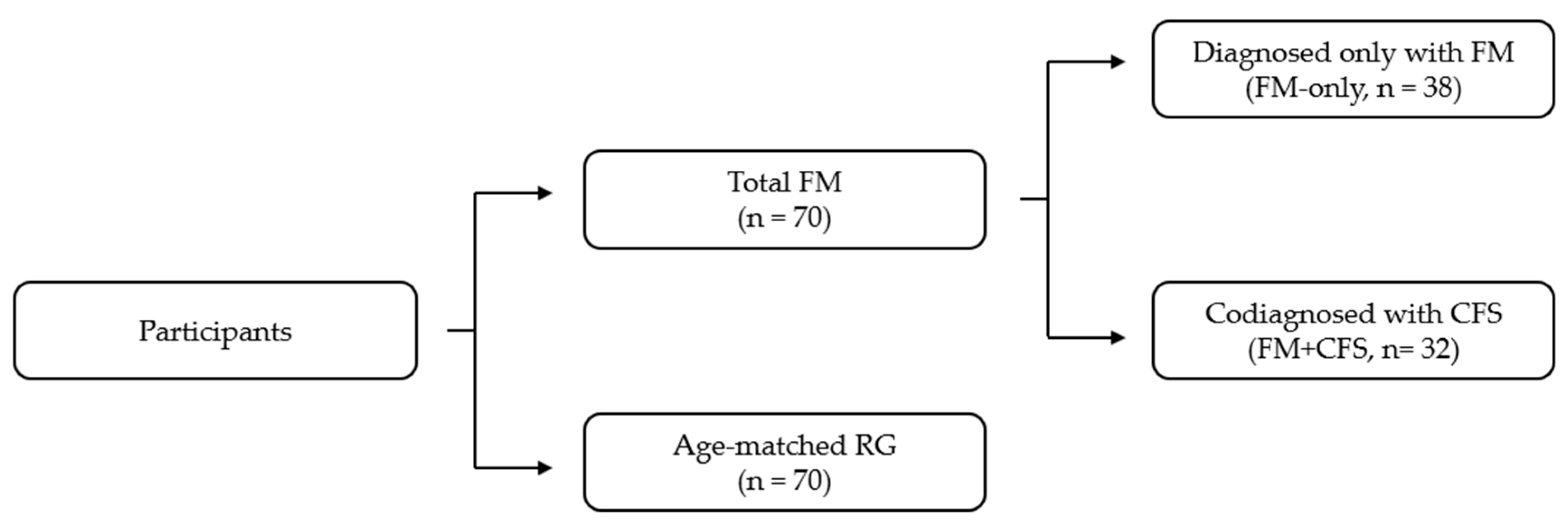


| RG (N = 70) | FM-Only (N = 38) | FM + CFS (N = 32) | Statistical Significance | |
|---|---|---|---|---|
| Gender (%) | Women (100%) | Women (100%) | Women (100%) | |
| Ethnic group (%) | Caucasian (100%) | Caucasian (100%) | Caucasian (100%) | |
| Age (years) | 53.93 ± 8.27 | 54.82 ± 8.52 | 57.31 ± 7.86 | p > 0.05 |
| BMI (kg/m2) | 25.72 ± 4.75 | 26.42 ± 5.25 | 26.82 ± 5.16 | p > 0.05 |
| Duration of FM or CFS diagnosed (years) | ___ | >2 | >2 | |
| Employment status: | Chi-Square (X2) p < 0.001 (X2 < 0.001) | |||
| 8.6 | 15.8 | 12.5 | |
| 77.1 | 28.9 * | 18.8 * | |
| 8.6 | 26.3 * | 18.8 | |
| ___ | 5.3 | 18.6 * | |
| 5.7 | 23.7 * | 31.3 * | |
| Common comorbidities | Chi-Square (X2) | |||
| 11.4 | 28.9 | 37.5 * | p < 0.01 (X2 = 0.007) |
| 8.6 | 13.2 | 18.8 | p > 0.05 (X2 = 0.332) |
| ___ | 28.9 * | 18.8 * | p < 0.001 (X2 < 0.001) |
| RG | FM-Only | FM + CFS Chi-Square (X2) | |
|---|---|---|---|
| Reporting HPE (%) | 54.29 | 28.95 * | 53.13 p < 0.05 (X2 = 0.03) |
| Type of reported HPE: | p > 0.05 (X2 = 0.32) | ||
| 42.1 | 63.6 | 58.8 |
| 15.8 | 18.2 | 23.5 |
| 10.5 | 9.1 | 11.8 |
| 5.3 | 9.1 | 5.9 |
| 26.3 | ___ | ___ |
| RG | FM-Only | FM + CFS | ||||
|---|---|---|---|---|---|---|
| Items HLPCQ | Without HPE | With HPE | Without HPE | With HPE | Without HPE | With HPE |
| Dietary Healthy Choices score | 14.89 ± 0.52 | 16.66 ± 0.61 ** | 16.48 ± 0.70 | 19.00 ± 0.90 * | 15.94 ± 0.94 | 18.00 ± 0.79 * |
| Dietary Harm Avoidance score | 9.43 ± 0.89 | 9.05 ± 0.35 | 10.56 ± 0.43 | 11.31 ± 0.49 | 9.75 ± 0.57 | 11.94 ± 0.58 ** |
| Daily Routine score | 24.26 ± 0.25 | 24.49 ± 0.66 | 22.89 ± 1.08 | 24.92 ± 1.11 | 21.06 ± 1.71 | 22.88 ± 1.18 |
| Organized Physical Exercise score | 3.37 ± 0.46 | 6.22 ± 0.25 *** | 3.56 ± 0.23 | 5.62 ± 0.58 ** | 3.75 ± 0.48 | 5.24 ± 0.49 ** |
| Social and Mental Balance score | 12.91 ± 1.61 | 13.54 ± 0.47 | 11.44 ± 0.49 | 11.69 ± 0.68 | 10.81 ± 0.56 | 12.24 ± 0.58 * |
| Total score | 65.53 ± 1.61 | 70.23 ± 1.42 ** | 64.89 ± 1.74 | 72.54 ± 1.74 *** | 61.31 ± 2.85 | 70.29 ± 2.41 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinchado, M.D.; Otero, E.; Navarro, M.d.C.; Martín-Cordero, L.; Gálvez, I.; Ortega, E. Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia. J. Clin. Med. 2022, 11, 5735. https://doi.org/10.3390/jcm11195735
Hinchado MD, Otero E, Navarro MdC, Martín-Cordero L, Gálvez I, Ortega E. Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia. Journal of Clinical Medicine. 2022; 11(19):5735. https://doi.org/10.3390/jcm11195735
Chicago/Turabian StyleHinchado, María Dolores, Eduardo Otero, María del Carmen Navarro, Leticia Martín-Cordero, Isabel Gálvez, and Eduardo Ortega. 2022. "Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia" Journal of Clinical Medicine 11, no. 19: 5735. https://doi.org/10.3390/jcm11195735
APA StyleHinchado, M. D., Otero, E., Navarro, M. d. C., Martín-Cordero, L., Gálvez, I., & Ortega, E. (2022). Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia. Journal of Clinical Medicine, 11(19), 5735. https://doi.org/10.3390/jcm11195735








