Impact of Treating Age-Related Macular Degeneration before Visual Function Is Impaired
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Eye Examinations
2.3. Treatments
2.4. Definition of Treatment Failure (Death)
2.5. Statistical Analyses
3. Results
3.1. Patients’ Demographics
3.2. Predictive Factors for Good Visual Outcome
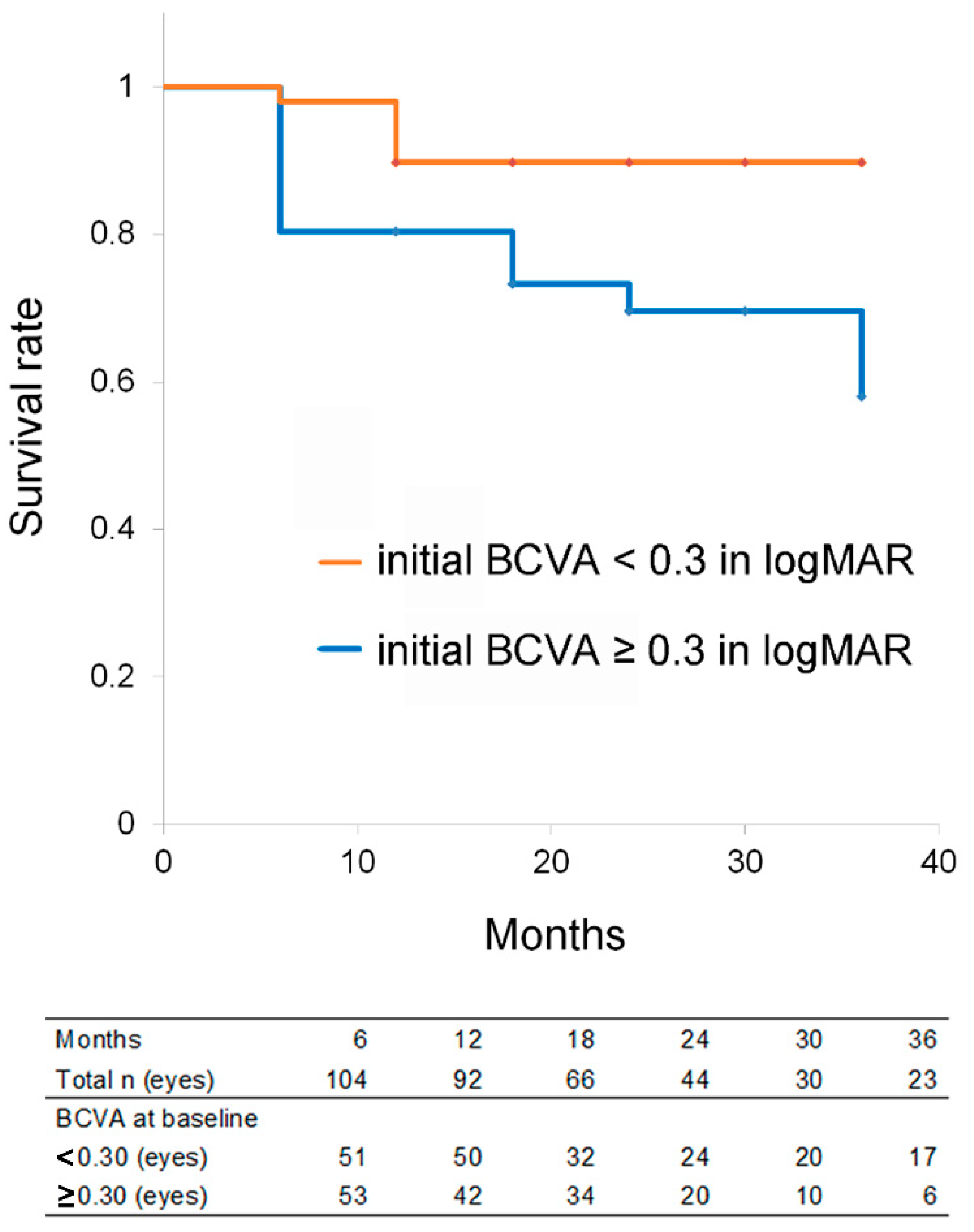
3.3. Kaplan–Meier Survival Analysis after Anti-Vascular Endothelial Growth Factor Treatment
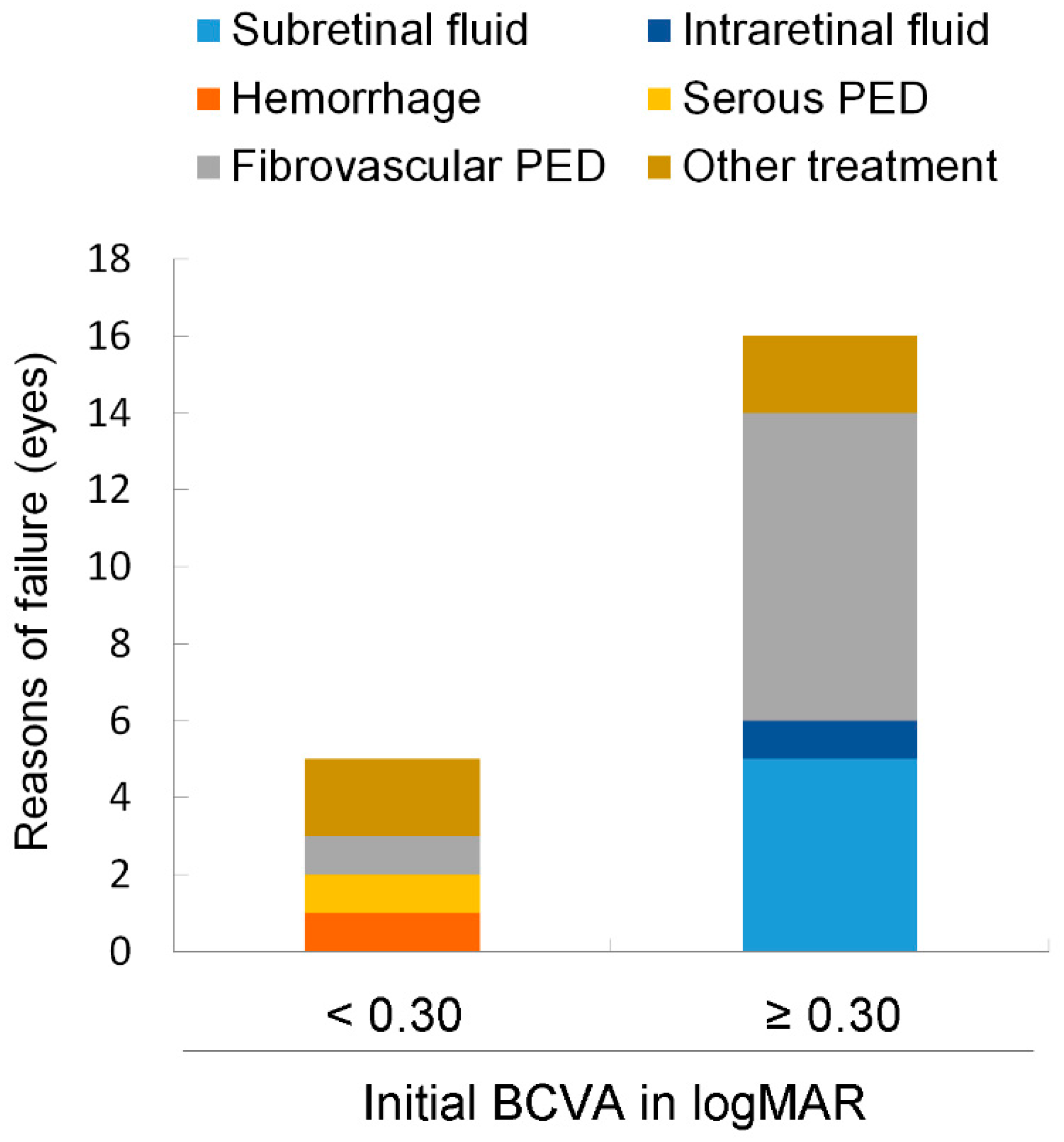
3.4. Conditions for Treatment Success
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lancet, T. Age-related macular degeneration: Treatment at what cost? Lancet 2018, 392, 1090. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R.; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 566–583. [Google Scholar] [CrossRef]
- Lalwani, G.A.; Rosenfeld, P.J.; Fung, A.E.; Dubovy, S.R.; Michels, S.; Feuer, W.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO Study. Am. J. Ophthalmol. 2009, 148, 43–58.e1. [Google Scholar] [CrossRef]
- Gupta, O.P.; Shienbaum, G.; Patel, A.H.; Fecarotta, C.; Kaiser, R.S.; Regillo, C.D. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 2010, 117, 2134–2140. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Minami, S.; Kurihara, T.; Kamoshita, M.; Sonobe, H.; Watanabe, K.; Uchida, A.; Shinoda, H.; Tsubota, K.; et al. Dynamic changes in choroidal conditions during anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Sci. Rep. 2019, 9, 11389. [Google Scholar] [CrossRef]
- Minami, S.; Nagai, N.; Suzuki, M.; Kurihara, T.; Sonobe, H.; Kamoshita, M.; Uchida, A.; Shinoda, H.; Takagi, H.; Sonoda, S.; et al. Benefits of aflibercept treatment for age-related macular degeneration patients with good best-corrected visual acuity at baseline. Sci. Rep. 2018, 8, 58. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagai, N.; Shinoda, H.; Uchida, A.; Kurihara, T.; Tomita, Y.; Kamoshita, M.; Iyama, C.; Tsubota, K.; Ozawa, Y. Distinct Responsiveness to Intravitreal Ranibizumab Therapy in Polypoidal Choroidal Vasculopathy With Single or Multiple Polyps. Am. J. Ophthalmol. 2016, 166, 52–59. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagai, N.; Izumi-Nagai, K.; Shinoda, H.; Koto, T.; Uchida, A.; Mochimaru, H.; Yuki, K.; Sasaki, M.; Tsubota, K.; et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br. J. Ophthalmol. 2014, 98, 1186–1191. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Uchida, A.; Kurihara, T.; Kamoshita, M.; Minami, S.; Shinoda, H.; Tsubota, K.; Ozawa, Y. Non-responsiveness to intravitreal aflibercept treatment in neovascular age-related macular degeneration: Implications of serous pigment epithelial detachment. Sci. Rep. 2016, 6, 29619. [Google Scholar] [CrossRef]
- Rayess, N.; Houston, S.K., 3rd; Gupta, O.P.; Ho, A.C.; Regillo, C.D. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am. J. Ophthalmol. 2015, 159, 3–8.e1. [Google Scholar] [CrossRef]
- Thoongsuwan, S.; Hanutsaha, P.; Chantarasorn, Y.; Ruamviboonsuk, P.; Vongkulsiri, S.; Kungwanpongpun, P. Treatment Outcome of Wet Age-Related Macular Degeneration Management in Thailand: A Retrospective Real-World Study (TOWER Study). Ophthalmol. Ther. 2022, 11, 739–757. [Google Scholar] [CrossRef]
- Jacob, J.; Brie, H.; Leys, A.; Levecq, L.; Mergaerts, F.; Denhaerynck, K.; Vancayzeele, S.; Van Craeyveld, E.; Abraham, I.; MacDonald, K. Six-year outcomes in neovascular age-related macular degeneration with ranibizumab. Int. J. Ophthalmol. 2017, 10, 81–90. [Google Scholar] [CrossRef]
- Mattson, M.P.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef]
- Nagai, N.; Suzuki, M.; Uchida, A.; Kurihara, T.; Ban, N.; Minami, S.; Shinoda, H.; Tsubota, K.; Ozawa, Y. The Area and Number of Intraretinal Cystoid Spaces Predict the Visual Outcome after Ranibizumab Monotherapy in Diabetic Macular Edema. J. Clin. Med. 2020, 9, 1391. [Google Scholar] [CrossRef]
- Glatt, H.; Machemer, R. Experimental subretinal hemorrhage in rabbits. Am. J. Ophthalmol. 1982, 94, 762–773. [Google Scholar] [CrossRef]
- Toth, C.A.; Morse, L.S.; Hjelmeland, L.M.; Landers, M.B., 3rd. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch. Ophthalmol. 1991, 109, 723–729. [Google Scholar] [CrossRef]
- Lee, K.; Park, Y.G.; Park, Y.H. Visual Prognosis after Pneumatic Displacement of Submacular Hemorrhage According to Age-Related Macular Degeneration Subtypes. Retina 2020, 40, 2304–2311. [Google Scholar] [CrossRef]
- Ohji, M.; Saito, Y.; Hayashi, A.; Lewis, J.M.; Tano, Y. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch. Ophthalmol. 1998, 116, 1326–1332. [Google Scholar] [CrossRef]
- Khanani, A.M.; Eichenbaum, D.; Schlottmann, P.G.; Tuomi, L.; Sarraf, D. Optimal Management of Pigment Epithelial Detachments in Eyes with Neovascular Age-Related Macular Degeneration. Retina 2018, 38, 2103–2117. [Google Scholar] [CrossRef]

| Eyes | 104 |
|---|---|
| Age (years) | 73.8 ± 1.1 (range, 50–93) |
| Sex (men; eyes (%)) | 69 (66.3%) |
| Subtypes of age-related macular degeneration (AMD) | |
| Polypoidal choroidal vasculopathy (PCV) | 84 (80.8%) |
| Typical AMD | 13 (12.5%) |
| Retinal angiomatous proliferation (RAP) | 7 (6.7%) |
| Best-corrected visual acuity (logMAR) | 0.444 ± 0.053 (−0.176–2.3) |
| Central Retinal Thickness (μm) | 356 ± 16 (102–911) |
| Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Sex (Male) | 0.791 | 0.274–2.279 | 0.664 |
| Best-corrected visual acuity <0.3 in logMAR | 3.172 | 1.029–9.783 | 0.045 * |
| Subtypes of age-related macular degeneration (AMD) | |||
| Polypoidal choroidal vasculopathy | 1.729 | 0.547–5.643 | 0.351 |
| Typical AMD | 0.723 | 0.169–3.106 | 0.663 |
| Retinal angiomatous proliferation | 0.500 | 0.098–2.554 | 0.405 |
| Central retinal thickness ≥ 300 μm | 1.231 | 0.457–3.313 | 0.681 |
| Intraretinal fluid (IRF) | 0.806 | 0.261–2.490 | 0.708 |
| Subretinal fluid (SRF) | 0.876 | 0.211–3.644 | 0.856 |
| All types of hemorrhages | 1.499 | 0.559–4.476 | 0.388 |
| Sub-macular hemorrhage (> 1 disc diameter) | 0.409 | 0.101–1.651 | 0.209 |
| Serous pigment epithelial detachment (PED) | 1.288 | 0.429–3.863 | 0.652 |
| Hemorrhagic PED | 0.871 | 0.246–3.087 | 0.831 |
| Fibrovascular PED | 0.222 | 0.078–0.637 | 0.005 ** |
| Subretinal hyper-reflective material (SHRM) | 0.871 | 0.246–3.087 | 0.831 |
| Dropped Out | Continued | p | |
|---|---|---|---|
| n (Eyes (%)) | 23 (22.1) | 81 (77.9) | |
| Age (mean ± standard deviation (range)) | 75.5 ± 2.5 (56–91) | 73.4 ± 1.2 (50–93) | 0.361 |
| Sex (male %) | 14 (60.8) | 55 (67.9) | 0.866 |
| BCVA at baseline >0.5 (Eyes (%)) | 13 (56.5) | 27 (33.3) | 0.039 * |
| BCVA at last visit >0.5 (Eyes (%)) | 11 (47.8) | 18 (22.2) | 0.025 * |
| Hazard Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Sex (Male) | 0.965 | 0.389–2.393 | 0.939 |
| Best-corrected visual acuity < 0.3 in logMAR | 2.947 | 1.047–8.289 | 0.041 * |
| Polypoidal choroidal vasculopathy (PCV) | 1.930 | 0.747–4.988 | 0.175 |
| Central retinal thickness ≥ 300 μm | 1.325 | 0.563–3.123 | 0.519 |
| Intraretinal fluid (IRF) | 0.841 | 0.321–2.204 | 0.724 |
| Sub-macular hemorrhage (> 1 disc diameter) | 0.480 | 0.161–1.432 | 0.188 |
| BCVA at Baseline (logMAR) | p | ||
|---|---|---|---|
| <0.30 | ≥0.30 | ||
| Eyes | 51 | 53 | |
| Age (years) | 70.9 ± 1.5 (50–93) | 76.6 ± 1.5 (51–92) | 0.008 ** |
| Sex (men; eyes (%)) | 37 (72.5) | 32 (60.4) | 0.134 |
| Subtypes of age-related macular degeneration (AMD) | |||
| Polypoidal choroidal vasculopathy | 43 (84.3) | 42 (77.4) | 0.135 |
| Typical AMD | 4 (13.7) | 6 (11.3) | |
| Retinal angiomatous proliferation | 1 (2.0) | 6 (11.3) | |
| Central Retinal Thickness (μm) | 342 ± 23 (102–883) | 370 ± 24 (153–911) | 0.309 |
| Subretinal fluid (SRF) (eyes (%)) | 49 (96.1) | 40 (75.5) | 0.003 ** |
| Intraretinal fluid (IRF) (eyes (%)) | 2 (3.9) | 21 (39.6) | <0.001 ** |
| All types of hemorrhages (eyes (%)) | 16 (31.4) | 26 (49.1) | 0.050 |
| Submacular hemorrhage (> 1 disc diameter) (eyes (%)) | 2 (3.9) | 9 (17.0) | 0.030 * |
| Hemorrhagic PED (eyes (%)) | 8 (15.7) | 12 (22.6) | 0.258 |
| Serous PED (eyes (%)) | 18 (35.3) | 12 (22.6) | 0.114 |
| Fibrovascular PED (eyes (%)) | 8 (15.7) | 19 (35.8) | 0.016 * |
| Subretinal hyper-reflective material (SHRM) (eyes (%)) | 17 (33.3) | 19 (35.8) | 0.475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aichi, R.; Nagai, N.; Ohkoshi, K.; Ozawa, Y. Impact of Treating Age-Related Macular Degeneration before Visual Function Is Impaired. J. Clin. Med. 2022, 11, 5726. https://doi.org/10.3390/jcm11195726
Aichi R, Nagai N, Ohkoshi K, Ozawa Y. Impact of Treating Age-Related Macular Degeneration before Visual Function Is Impaired. Journal of Clinical Medicine. 2022; 11(19):5726. https://doi.org/10.3390/jcm11195726
Chicago/Turabian StyleAichi, Risa, Norihiro Nagai, Kishiko Ohkoshi, and Yoko Ozawa. 2022. "Impact of Treating Age-Related Macular Degeneration before Visual Function Is Impaired" Journal of Clinical Medicine 11, no. 19: 5726. https://doi.org/10.3390/jcm11195726
APA StyleAichi, R., Nagai, N., Ohkoshi, K., & Ozawa, Y. (2022). Impact of Treating Age-Related Macular Degeneration before Visual Function Is Impaired. Journal of Clinical Medicine, 11(19), 5726. https://doi.org/10.3390/jcm11195726






