Complex Management of Nephrotic Syndrome and Kidney Failure during Pregnancy in a Type 1 Diabetes Patient: A Challenging Case
Abstract
1. Introduction
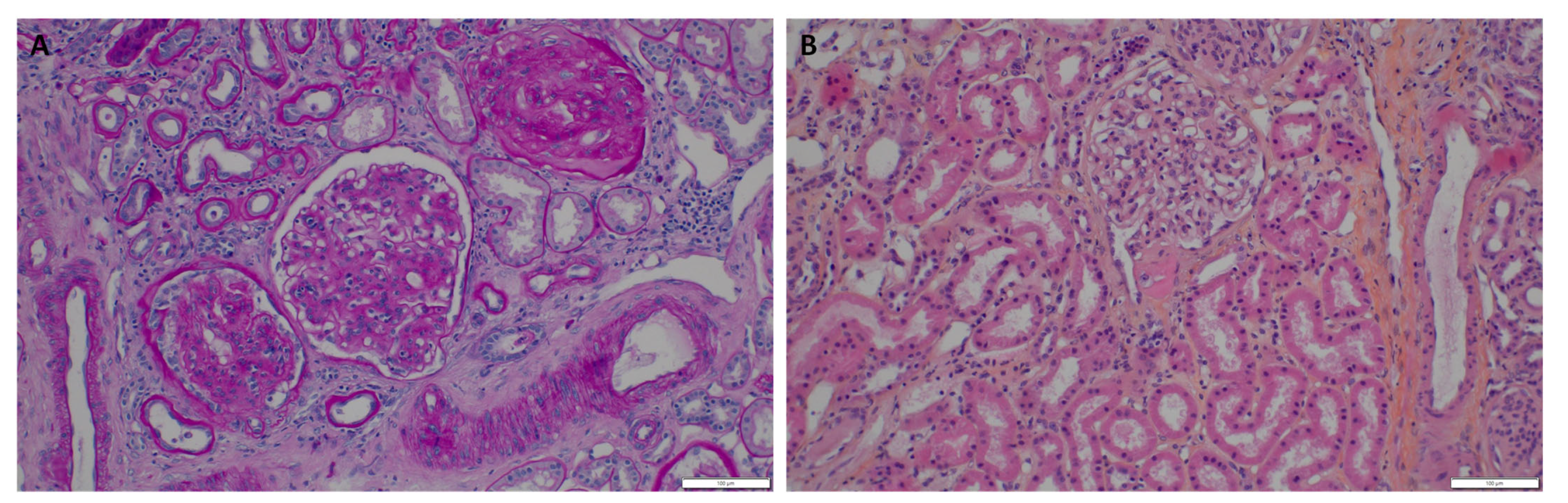
2. The Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egan, A.M.; Brassill, M.J.; Brosnan, E.; Carmody, L.; Clarke, H.; Coogan Kelly, C.; Culliney, L.; Durkan, M.; Fenlon, M.; Ferry, P.; et al. An Irish National Diabetes in Pregnancy Audit: Aiming for best outcomes for women with diabetes. Diabet. Med. 2020, 37, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Spotti, D. Pregnancy in women with diabetic nephropathy. J. Nephrol. 2019, 32, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, S.K.; Vestgaard, M.J.; Jørgensen, I.L.; Ásbjörnsdóttir, B.; Ringholm, L.; McIntyre, H.D.; Damm, P.; Mathiesen, E.R. Diastolic blood pressure is a potentially modifiable risk factor for preeclampsia in women with pre-existing diabetes. Diabetes Res. Clin. Pract. 2018, 138, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.M.; Marín Carrillo, L.F.; Arévalo Correa, C.M.; Muñoz Velandia, O.M.; Rondón Sepúlveda, M.A.; Silva Herrera, J.L.; Henao Carrillo, D.C. Maternal-Fetal Outcomes in 34 Pregnant Women with Type 1 Diabetes in Sensor-Augmented Insulin Pump Therapy. Diabetes Technol. Ther. 2017, 19, 417–422. [Google Scholar] [CrossRef]
- Bramham, K. Diabetic Nephropathy and Pregnancy. Semin. Nephrol. 2017, 37, 362–369. [Google Scholar] [CrossRef]
- Hernández Rivera, J.C.H.; Pérez López, M.J.; Corzo Bermúdez, C.H.; García Covarrubias, L.; Bermúdez Aceves, L.A.; Chucuan Castillo, C.A.; Salazar Mendoza, M.; Piccoli, G.B.; Paniagua Sierra, R. Delayed Initiation of Hemodialysis in Pregnant Women with Chronic Kidney Disease: Logistical Problems Impact Clinical Outcomes. An Experience from an Emerging Country. J. Clin. Med. 2019, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Tavassoli, E.; Melluzza, C.; Grassi, G.; Monzeglio, C.; Donvito, V.; Leone, F.; Attini, R.; Ghiotto, S.; Clari, R.; et al. Severe diabetic nephropathy in type 1 diabetes and pregnancy—A case series. Rev. Diabet. Stud. 2013, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, L.; Mathiesen, E.R.; Kelstrup, L.; Damm, P. Managing type 1 diabetes mellitus in pregnancy—From planning to breastfeeding. Nat. Rev. Endocrinol. 2012, 8, 659–667. [Google Scholar] [CrossRef]
- Kitzmiller, J.L.; Block, J.M.; Brown, F.M.; Catalano, P.M.; Conway, D.L.; Coustan, D.R.; Gunderson, E.P.; Herman, W.H.; Hoffman, L.D.; Inturrisi, M.; et al. Managing Preexisting Diabetes for Pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care 2008, 31, 1060–1079. [Google Scholar] [CrossRef]
- Rossing, K.; Jacobsen, P.; Hommel, E.; Mathiesen, E.; Svenningsen, A.; Rossing, P.; Parving, H.-H. Pregnancy and progression of diabetic nephropathy. Diabetologia 2002, 45, 36–41. [Google Scholar] [CrossRef]
- Sibai, B.M. Diabetic Nephropathy in Pregnancy. In A Practical Manual of Diabetes in Pregnancy; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 153–164. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and Perinatal Outcomes in Type 1 Diabetic Pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef]
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; Coleman, M.A.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: A 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164. [Google Scholar] [CrossRef]
- McGrath, R.T.; Glastras, S.J.; Seeho, S.K.; Scott, E.S.; Fulcher, G.R.; Hocking, S.L. Association Between Glycemic Variability, HbA1c, and Large-for-Gestational-Age Neonates in Women with Type 1 Diabetes. Diabetes Care 2017, 40, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, E.R.; Ali, N.; Alibegovic, A.C.; Anastasiou, E.; Cypryk, K.; De Valk, H.; Dores, J.; Dunne, F.; Gall, M.-A.; Garcia, S.D.; et al. Risk of Major Congenital Malformations or Perinatal or Neonatal Death with Insulin Detemir Versus Other Basal Insulins in Pregnant Women with Preexisting Diabetes: The Real-World EVOLVE Study. Diabetes Care 2021, 44, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Eidem, I.; Vangen, S.; Hanssen, K.F.; Vollset, S.E.; Henriksen, T.; Joner, G.; Stene, L.C. Perinatal and infant mortality in term and preterm births among women with type 1 diabetes. Diabetologia 2011, 54, 2771. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Damm, P.; Moelsted-Pedersen, L.; Ovesen, P.; Westergaard, J.G.; Moeller, M.; Beck-Nielsen, H. Outcomes in type 1 diabetic pregnancies: A nationwide, population-based study. Diabetes Care 2004, 27, 2819–2823. [Google Scholar] [CrossRef]
- Macintosh, M.C.M.; Fleming, K.M.; Bailey, J.A.; Doyle, P.; Modder, J.; Acolet, D.; Golightly, S.; Miller, A. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: Population based study. BMJ 2006, 333, 177. [Google Scholar] [CrossRef]
- Colstrup, M.; Mathiesen, E.R.; Damm, P.; Jensen, D.M.; Ringholm, L. Pregnancy in women with type 1 diabetes: Have the goals of St. Vincent declaration been met concerning foetal and neonatal complications? J. Matern. Fetal Neonatal Med. 2013, 26, 1682–1686. [Google Scholar] [CrossRef]
- De Castro, I.; Easterling, T.R.; Bansal, N.; Jefferson, J.A. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int. 2017, 91, 1464–1472. [Google Scholar] [CrossRef]
- Piccoli, G.; Daidola, G.; Attini, R.; Parisi, S.; Fassio, F.; Naretto, C.; Deagostini, M.; Castelluccia, N.; Ferraresi, M.; Roccatello, D.; et al. Kidney biopsy in pregnancy: Evidence for counselling? A systematic narrative review. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 412–427. [Google Scholar] [CrossRef]
- Wiles, K.; Chappell, L.; Clark, K.; Elman, L.; Hall, M.; Lightstone, L.; Mohamed, G.; Mukherjee, D.; Nelson-Piercy, C.; Webster, P.; et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 2019, 20, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Karge, A.; Beckert, L.; Moog, P.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Abel, K.; Flechsenhar, S.; Kuschel, B.; Graupner, O. Role of sFlt-1/PIGF ratio and uterine Doppler in pregnancies with chronic kidney disease suspected with Pre-eclampsia or HELLP syndrome. Pregnancy Hypertens. 2020, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Seed, P.T.; Lightstone, L.; Nelson-Piercy, C.; Gill, C.; Webster, P.; Poston, L.; Chappell, L.C. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int. 2016, 89, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Øyen, N.; Diaz, L.J.; Leirgul, E.; Boyd, H.A.; Priest, J.; Mathiesen, E.R.; Quertermous, T.; Wohlfahrt, J.; Melbye, M. Prepregnancy Diabetes and Offspring Risk of Congenital Heart Disease. Circulation 2016, 133, 2243–2253. [Google Scholar] [CrossRef]
- Fleiss, B.; Wong, F.; Brownfoot, F.; Shearer, I.K.; Baud, O.; Walker, D.W.; Gressens, P.; Tolcos, M. Knowledge Gaps and Emerging Research Areas in Intrauterine Growth Restriction-Associated Brain Injury. Front. Endocrinol. 2019, 10, 188. [Google Scholar] [CrossRef]
- Dashe, J.S.; Pressman, E.K.; Hibbard, J.U. SMFM Consult Series #46: Evaluation and management of polyhydramnios. Am. J. Obstet. Gynecol. 2018, 219, B2–B8. [Google Scholar] [CrossRef]
- Wang, M.L.; He, Y.D.; Yang, H.X.; Chen, Q. Successful pregnancy after protective hemodialysis for chronic kidney disease: A case report. World J. Clin. Cases 2020, 8, 4521–4526. [Google Scholar] [CrossRef]
- Jesudason, S.; Grace, B.S.; McDonald, S.P. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 143–149. [Google Scholar] [CrossRef]
- Ibarra-Hernández, M.; Orozco-Guillén, O.A.; De la Alcantar-Vallín, M.L.; Garrido-Roldan, R.; Jiménez-Alvarado, M.P.; Castro, K.B.; Villa-Villagrana, F.; Borbolla, M.; Gallardo-Gaona, J.M.; García-García, G.; et al. Acute kidney injury in pregnancy and the role of underlying CKD: A point of view from México. J. Nephrol. 2017, 30, 773–780. [Google Scholar] [CrossRef]
- Nava, J.; Moran, S.; Figueroa, V.; Salinas, A.; Lopez, M.; Urbina, R.; Gutierrez, A.; Lujan, J.L.; Orozco, A.; Montufar, R.; et al. Successful pregnancy in a CKD patient on a low-protein, supplemented diet: An opportunity to reflect on CKD and pregnancy in Mexico, an emerging country. J. Nephrol. 2017, 30, 877–882. [Google Scholar] [CrossRef]
- Attini, R.; Montersino, B.; Leone, F.; Minelli, F.; Fassio, F.; Rossetti, M.M.; Colla, L.; Masturzo, B.; Barreca, A.; Menato, G.; et al. Dialysis or a Plant-Based Diet in Advanced CKD in Pregnancy? A Case Report and Critical Appraisal of the Literature. J. Clin. Med. 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Guillien, A.O.; Muñoz-Manrique, C.; Reyes-López, M.A.; Perichat-Perera, O.; Miranda-Araujo, O.; D’Alessandro, C.; Piccoli, G.B. Quality or Quantity of Proteins in the Diet for CKD Patients: Does “Junk Food” Make a Difference? Lessons from a High-Risk Pregnancy. Kidney Blood Press Res. 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gramkow, A.M.; Aarup, M.; Andersen, L.L.T.; Tepel, M. Dialysis for twins. Clin Kidney J. 2014, 7, 57–58. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Yao, Q.; Zhou, L.; Fu, P. Successful management of twin pregnancy in a woman with advanced chronic kidney disease: A case report. Medicine 2019, 98, e16840. [Google Scholar] [CrossRef] [PubMed]
- Martimbianco, A.L.C.; De Fátima Carreira Moreira, R.; Pacheco, R.L.; De Oliveira Cruz Latorraca, C.; Dos Santos, A.P.P.; Logullo, P.; Riera, R. Efficacy and safety of hemodialysis strategies for pregnant women with chronic kidney disease: Systematic review. Semin. Dial. 2022. [Google Scholar] [CrossRef]
- Hladunewich, M.A.; Hou, S.; Odutayo, A.; Cornelis, T.; Pierratos, A.; Goldstein, M.; Tennankore, K.; Keunen, J.; Hui, D.; Chan, C.T. Intensive Hemodialysis Associates with Improved Pregnancy Outcomes: A Canadian and United States Cohort Comparison. J. Am. Soc. Nephrol. 2014, 25, 1103–1109. [Google Scholar] [CrossRef]
- Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Giacchino, F.; Credendino, O.; Daidone, G.; Gregorini, G.; Moroni, G.; Attini, R.; et al. Best practices on pregnancy on dialysis: The Italian Study Group on Kidney and Pregnancy. J. Nephrol. 2015, 28, 279–288. [Google Scholar] [CrossRef]
- Asamiya, Y.; Otsubo, S.; Matsuda, Y.; Kimata, N.; Kikuchi, K.A.N.; Miwa, N.; Uchida, K.; Mineshima, M.; Mitani, M.; Ohta, H.; et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009, 75, 1217–1222. [Google Scholar] [CrossRef]
- Attini, R.; Leone, F.; Parisi, S.; Fassio, F.; Capizzi, I.; Loi, V.; Colla, L.; Rossetti, M.; Gerbino, M.; Maxia, S.; et al. Vegan-vegetarian low-protein supplemented diets in pregnant CKD patients: Fifteen years of experience. BMC Nephrol. 2016, 17, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Suonio, S.; Saarikoski, S.; Tahvanainen, K.; Pääkkönen, A.; Olkkonen, H. Acute effects of dihydralazine mesylate, furosemide, and metoprolol on maternal hemodynamics in pregnancy-induced hypertension. Am. J. Obstet. Gynecol. 1986, 155, 122–125. [Google Scholar] [CrossRef]
- Wang, L.A.; Smith, P.B.; Laughon, M.; Goldberg, R.N.; Ku, L.C.; Zimmerman, K.O.; Balevic, S.; Clark, R.H.; Benjamin, D.K.; Greenberg, R.G. Prolonged furosemide exposure and risk of abnormal newborn hearing screen in premature infants. Early Hum. Dev. 2018, 125, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Pergialiotis, V.; Papapanagiotou, A.; Loutradis, D.; Daskalakis, G. Comparative efficacy and safety of oral antihypertensive agents in pregnant women with chronic hypertension: A network metaanalysis. Am. J. Obstet. Gynecol. 2020, 223, 525–537. [Google Scholar] [CrossRef]
- Ringholm Nielsen, L.; Damm, P.; Mathiesen, E.R. Improved Pregnancy Outcome in Type 1 Diabetic Women with Microalbuminuria or Diabetic Nephropathy: Effect of intensified antihypertensive therapy? Diabetes Care 2009, 32, 38–44. [Google Scholar] [CrossRef]
- Jesudason, S.; Hewawasam, E.; Moloney, B.; Tan, R.; Li, J.; Blakey, H.; Bramham, K.; Hall, M.; Juneja, R.; Jarvis, E.; et al. Comparison of catheters or new arteriovenous fistulas for commencement of haemodialysis in pregnant women with chronic kidney disease: An international observational study. J. Nephrol. 2022, 35, 1689–1698. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Gallieni, M. Patients’ wishes, pregnancy and vascular access: When one size does not fit all. J. Vasc. Access. 2018, 19, 518–520. [Google Scholar] [CrossRef] [PubMed]

| Pros | Cons | |
|---|---|---|
| Volume overload | Avoid loop diuretic use (risks of placental hypoperfusion and fetal growth impairment, ototoxicity) | Good effectiveness of loop diuretics on volume overload (slower depletion than during dialysis) Dialysis-induced volume shifts may be detrimental to placental perfusion |
| Metabolic disorders | Negative relationship between BUN level and birth weight | No threshold of BUN for dialysis start has been established during pregnancy |
| Other | Risk related to dialysis access placement (permanent central catheter) and permanence (infectious, thrombotic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drapeau, L.; Beaumier, M.; Esbelin, J.; Comoz, F.; Figueres, L.; Piccoli, G.B.; Kervella, D. Complex Management of Nephrotic Syndrome and Kidney Failure during Pregnancy in a Type 1 Diabetes Patient: A Challenging Case. J. Clin. Med. 2022, 11, 5725. https://doi.org/10.3390/jcm11195725
Drapeau L, Beaumier M, Esbelin J, Comoz F, Figueres L, Piccoli GB, Kervella D. Complex Management of Nephrotic Syndrome and Kidney Failure during Pregnancy in a Type 1 Diabetes Patient: A Challenging Case. Journal of Clinical Medicine. 2022; 11(19):5725. https://doi.org/10.3390/jcm11195725
Chicago/Turabian StyleDrapeau, Leo, Mathilde Beaumier, Julie Esbelin, François Comoz, Lucile Figueres, Giorgina Barbara Piccoli, and Delphine Kervella. 2022. "Complex Management of Nephrotic Syndrome and Kidney Failure during Pregnancy in a Type 1 Diabetes Patient: A Challenging Case" Journal of Clinical Medicine 11, no. 19: 5725. https://doi.org/10.3390/jcm11195725
APA StyleDrapeau, L., Beaumier, M., Esbelin, J., Comoz, F., Figueres, L., Piccoli, G. B., & Kervella, D. (2022). Complex Management of Nephrotic Syndrome and Kidney Failure during Pregnancy in a Type 1 Diabetes Patient: A Challenging Case. Journal of Clinical Medicine, 11(19), 5725. https://doi.org/10.3390/jcm11195725






