Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal
Abstract
1. Introduction
2. Materials and Methods
3. Results
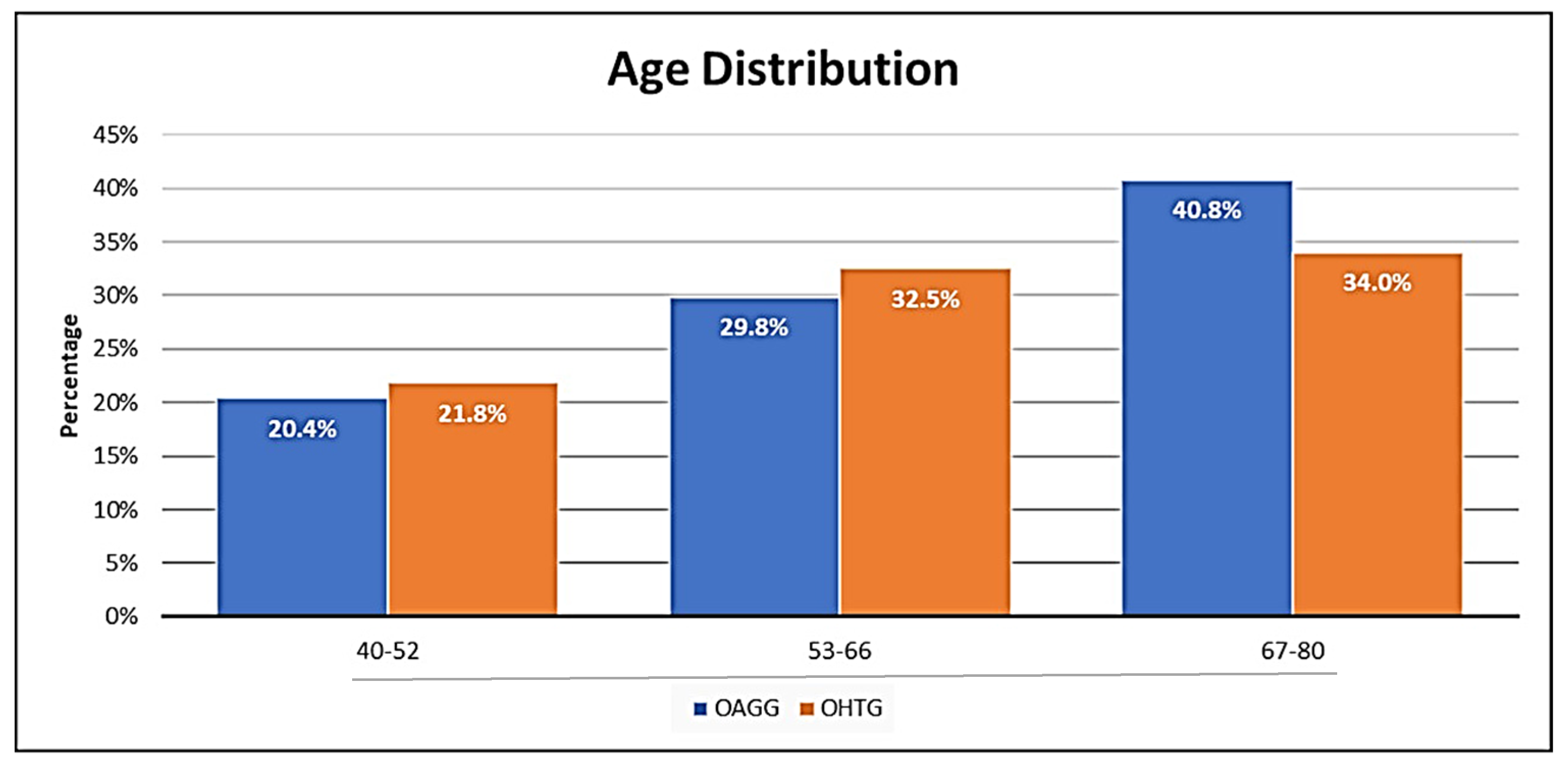
3.1. Demographic and Epidemiologic Data
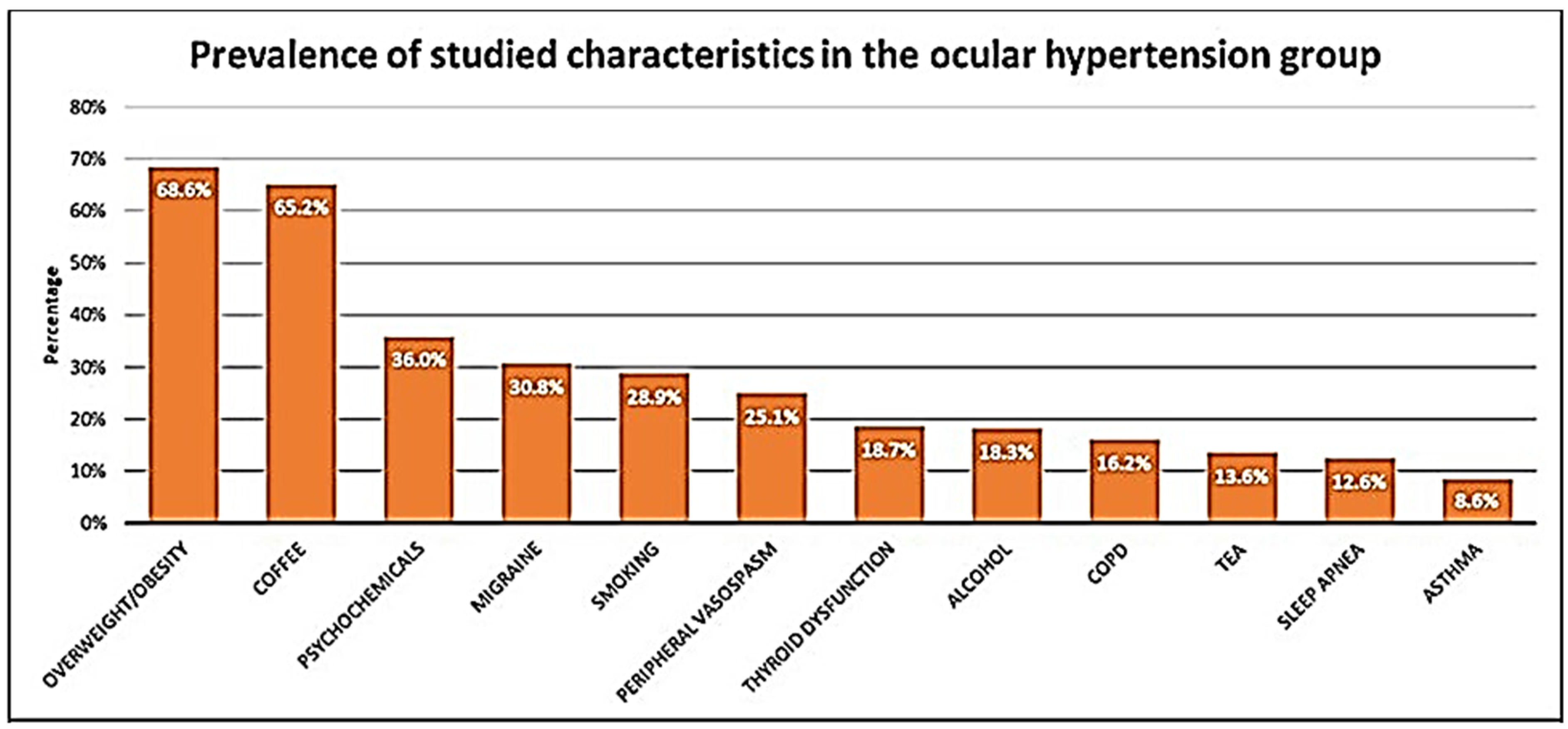
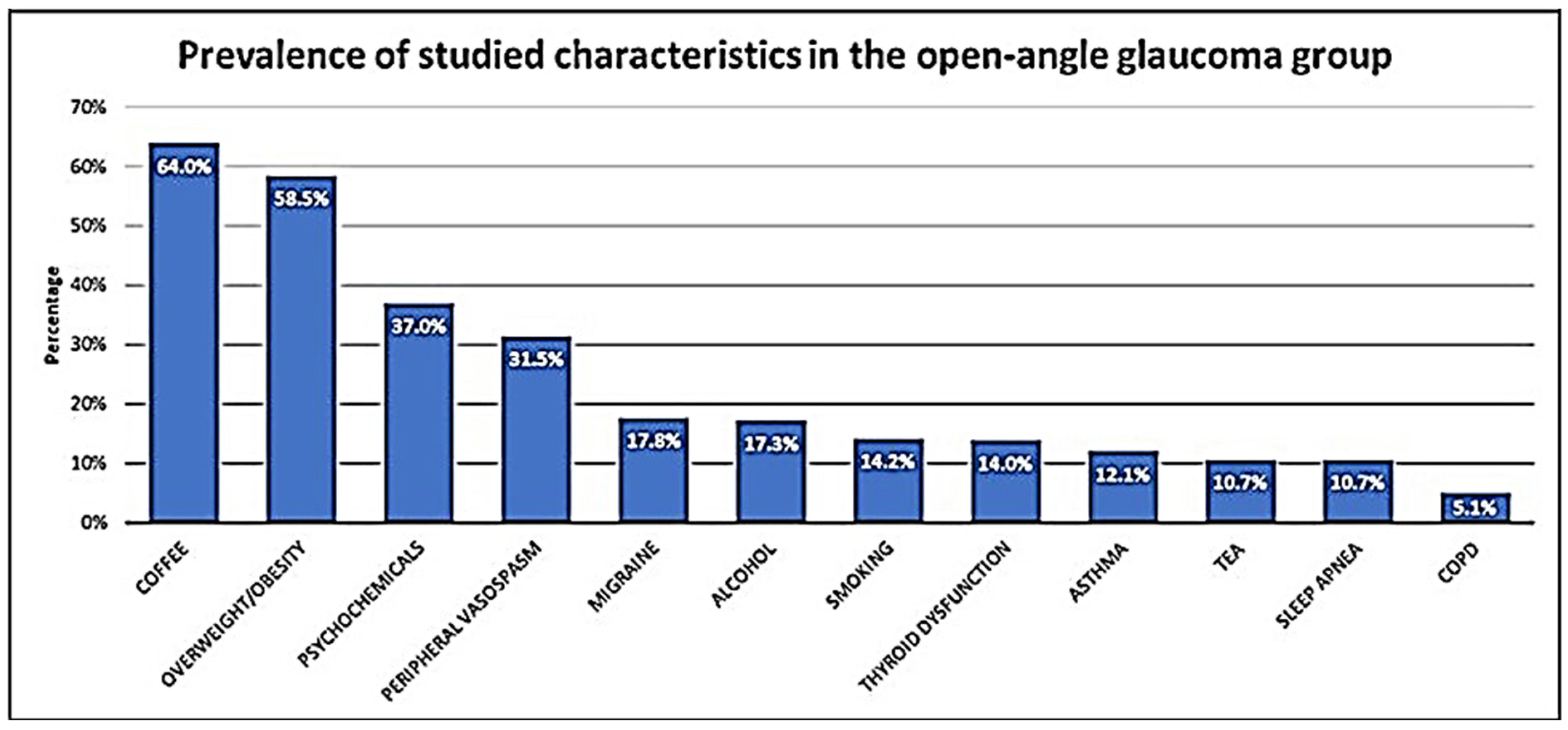
3.2. Clinical Characteristics
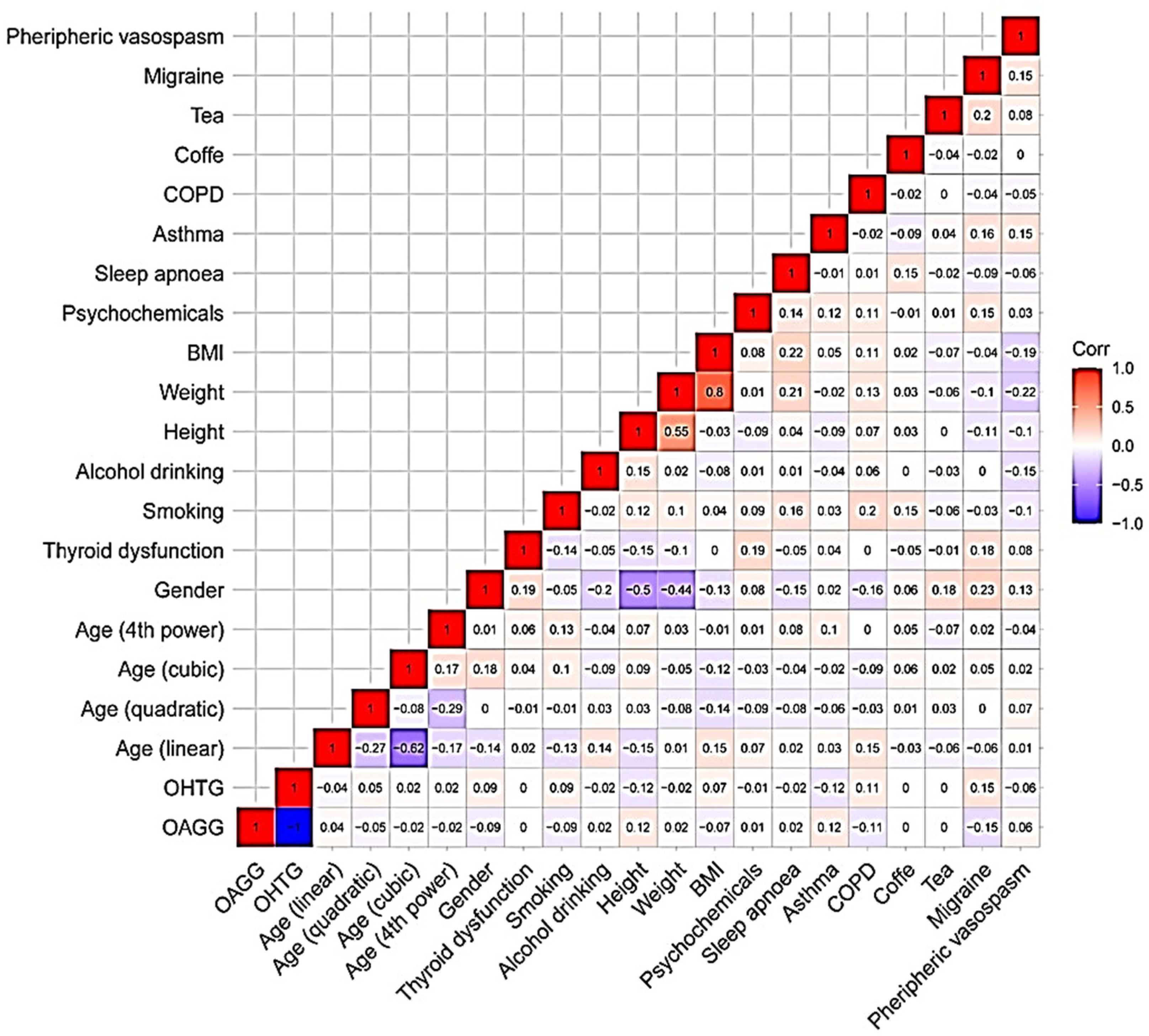
3.3. Statistical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ^4 | Fourth power |
| AH | Aqueous humor |
| AIC | Akaike information criterion |
| ANOVA | Analysis of variance |
| BCVA | Best correct visual acuity |
| BMI | Body mass index |
| C | Cubic |
| CCT | Central corneal thickness |
| COPD | Chronic obstruction pulmonary disease |
| DEMO | Register of sociodemographic data in the Microsoft Excel program |
| EGPS | European Glaucoma Prevention Study |
| GS | Glaucoma suspect |
| GWAS | Genome-wide association study |
| IOP | Intraocular pressure |
| L | Linear |
| LE | Left eye |
| MD | Mean deviation |
| OAG | Open-angle glaucoma |
| OAGG | Open-angle glaucoma group |
| OCPD | Obstructive chronic pulmonary disease |
| OCT | Optical coherence tomography |
| OFTARED | Ophthalmology network |
| OHT | Ocular hypertension |
| OHTG | Ocular hypertension group |
| OHTS | Ocular Hypertension Treatment Study |
| OND | Optic nerve degeneration |
| ONH | Optic nerve head |
| OPHTAL | Register of ophthalmologic data in the Microsoft Excel program |
| PARP-1 | Polyadenyl diphosphate ribose polymerase-1 |
| Q | Quadratic |
| QUEST | Register of survey data in the Microsoft Excel program |
| RE | Right eye |
| RGC | Retinal ganglion cell |
| RICORS | Cooperative Research Network of Results Oriented to Health |
| RNLF | Retinal Nerve Fiber Layer |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus 2 |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SNRI | Serotonin noradrenalin reuptake inhibitors |
| SSRI | Selective serotonin reuptake inhibitors |
| STAT | Statistical analysis register in the Microsoft Excel program |
| TM | Trabecular meshwork |
| VF | Visual field |
References
- Morrison, J.C.; Acott, T.S. Anatomy and physiology of aqueous humor outflow. In Glaucoma-Science and Practice; Thieme Medical Publishers Inc: New York, NY, USA, 2003; pp. 34–41. [Google Scholar]
- Åström, S.; Stenlund, H.; Lindén, C. Intraocular pressure changes over 21 years—A longitudinal age-cohort study in northern Sweden. Acta Ophthalmol. 2014, 92, 417–420. [Google Scholar] [CrossRef]
- Lee, M.K.; Cho, S.-I.; Kim, H.; Song, Y.-M.; Lee, K.; Kim, J.-I.; Kim, D.-M.; Chung, T.-Y.; Kim, Y.S.; Seo, J.-S.; et al. Epidemiologic Characteristics of Intraocular Pressure in the Korean and Mongolian Populations: The Healthy Twin and the GENDISCAN Study. Ophthalmology 2012, 119, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Connell, A.M.S.; Wu, S.; Hyman, L.G.; Schachat, A.P. Risk Factors for Open-angle Glaucoma: The Barbados Eye Study. Arch. Ophthalmol. 1995, 113, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Hollows, F.C.; Grahan, P.A. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br. J. Ophthalmol. 1966, 50, 570–586. [Google Scholar] [CrossRef]
- Shaffer, R. “Glaucoma Suspect” or “Ocular Hypertension”. Arch. Ophthalmol. 1977, 95, 588. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, T.; Zhang, X.; He, Y.; Jiang, B. Optical Coherence Tomography Evaluation of Peripapillary and Macular Structure Changes in Pre-perimetric Glaucoma, Early Perimetric Glaucoma, and Ocular Hypertension: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 696004. [Google Scholar] [CrossRef] [PubMed]
- Miglior, S.; Zeyen, T.; Pfeiffer, N.; Cunha-Vaz, J.; Torri, V.; Adamsons, I. European Glaucoma Prevention Study Group The European glaucoma prevention study design and baseline description of the participants. Ophthalmology 2002, 109, 1612–1621. [Google Scholar] [CrossRef]
- Miglior, S.; Zeyen, T.; Pfeiffer, N.; Cunha-Vaz, J.; Torri, V.; Adamsons, I. European Glaucoma Prevention Study (EGPS) Group. Ophthalmology 2005, 112, 366–375. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.E. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Parrish, R.K., 2nd. The European Glaucoma Prevention Study and the Ocular Hypertension Treatment Study: Why do two studies have different results? Curr. Opin. Ophthalmol. 2006, 17, 138–141. [Google Scholar] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamiri, A.Y.R.; Al-Mahweeti, B. Risk Factors for High Intraocular Pressure, Glaucoma and Retinal Detachment. N. Am. Acad. Res. 2020, 3, 167–183. [Google Scholar]
- Tham, Y.C.; Cheng, C.Y. Associations between chronic systemic diseases and primary open angle glaucoma: An epidemiological perspective. Clin. Exp. Ophthalmol. 2017, 45, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Och, M.; Kanclerz, P.; Leffler, C.; De Moraes, C.G. Primary Open Angle Glaucoma and Vascular Risk Factors: A Review of Population Based Studies from 1990 to 2019. J. Clin. Med. 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Meier-Gibbons, F.; Töteberg-Harms, M. Structure/function/treatment in glaucoma: Progress over the last 10 years. Ophthalmologe 2021, 118, 1216–1221. [Google Scholar] [CrossRef]
- Sullivan-Mee, M.; Tran, M.T.K.; Pensyl, D.; Tsan, G.; Katiyar, S. Prevalence, Features, and Severity of Glaucomatous Visual Field Loss Measured With the 10-2 Achromatic Threshold Visual Field Test. Am. J. Ophthalmol. 2016, 168, 40–51. [Google Scholar] [CrossRef]
- Casson, R.J. Medical therapy for glaucoma. A review. Clin. Exp. Ophthalmol. 2022, 50, 198–212. [Google Scholar] [CrossRef]
- Francis, B.A.; Varma, R.; Chopra, V.; Lai, M.-Y.; Shtir, C.; Azen, S.P. Intraocular Pressure, Central Corneal Thickness, and Prevalence of Open-Angle Glaucoma: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2008, 146, 741–746. [Google Scholar] [CrossRef]
- Moreno-Montañés, J.; Gándara, E.; Gutierrez-Ruiz, I.; Moreno-Galarraga, L.; Ruiz-Canela, M.; Bes-Rastrollo, M.; Martínez-González, M.; Fernandez-Montero, A. Healthy Lifestyle Score and Incidence of Glaucoma: The Sun Project. Nutrients 2022, 14, 779. [Google Scholar] [CrossRef]
- Hodapp, E.; Parrish, R.K., II; Anderson, D.R. Clinical Decisions in Glaucoma; The CV Mosby Comp: St. Louis, MO, USA, 1993; pp. 52–61. [Google Scholar]
- Akaike, H. This Week’s Citation Classic. Curr. Contents Eng. Technol. Appl. Sci. 1981, 12, 42. [Google Scholar]
- Saefken, B.; Kneib, T.; van Waveren, C.-S.; Greven, S. A unifying approach to the estimation of the conditional Akaike information in generalized linear mixed models. Electron. J. Stat. 2014, 8, 201–225. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef]
- Schell, G.J.; Lavieri, M.S.; Helm, J.E.; Liu, X.; Musch, D.C.; Van Oyen, M.P.; Stein, J.D. Using Filtered Forecasting Techniques to Determine Personalized Monitoring Schedules for Patients with Open-Angle Glaucoma. Ophthalmology 2014, 121, 1539–1546. [Google Scholar] [CrossRef]
- Camp, A.S.; Weinreb, R.N. Will perimetry be performed to monitor glaucoma in 2025? Ophthalmology 2017, 124, S71–S75. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Bowd, C.; Moghimi, S.; Tafreshi, A.; Rausch, S.; Zangwill, L.M. Ophthalmic Diagnostic Imaging: Glaucoma. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Bio-medical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; Chapter 5. [Google Scholar]
- Aksoy, F.; Altan, C.; Yılmaz, B.; Yılmaz, I.; Tunç, U.; Kesim, C.; Kocamaz, M.; Pasaoglu, I. A comparative evaluation of segmental analysis of macular layers in patients with early glaucoma, ocular hypertension, and healthy eyes. J. Fr. Ophtalmol. 2020, 43, 869–878. [Google Scholar] [CrossRef]
- Bak, E.; Kim, Y.W.; Ha, A.; Kim, Y.K.; Park, K.H.; Jeoung, J.W. Pre-perimetric Open Angle Glaucoma with Young Age of Onset: Natural Clinical Course and Risk Factors for Progression. Am. J. Ophthalmol. 2020, 216, 121–131. [Google Scholar] [CrossRef]
- Schuman, J.; Kostanyan, T.; Bussel, I. Review of Longitudinal Glaucoma Progression: 5 Years after the Shaffer Lecture. Ophthalmol. Glaucoma 2020, 3, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Ortega, A.; Norte-Muñoz, M.; Miralles de Imperial-Ollero, J.A.; Bernal-Garro, J.M.; Valiente-Soriano, F.J.; de la Villa Polo, P.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Functional and morphological alterations in a glaucoma model of acute ocular hypertension. Prog. Brain Res. 2020, 256, 1–29. [Google Scholar] [PubMed]
- Noailles, A.; Kutsyr, O.; Mayordomo-Febrer, A.; Lax, P.; López-Murcia, M.; Sanz-González, S.M.; Pinazo-Durán, M.D.; Cuenca, N. Sodium Hyaluronate-Induced Ocular Hypertension in Rats Damages the Direction-Selective Circuit and Inner/Outer Retinal Plexiform Layers. Investig. Opthalmol. Vis. Sci. 2022, 63, 2. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Duran, M.D.; Zanon-Moreno, V.; García-Medina, J.J.; Arévalo, J.F.; Gallego-Pinazo, R.; Nucci, C. Eclectic Ocular Comorbidities and Systemic Diseases with Eye Involvement: A Review. BioMed Res. Int. 2016, 2016, 6215745. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; Mitnitski, A. Changes in relative fitness and frailty across the adult lifespan: Evidence from the Canadian National Population Health Survey. Can. Med. Assoc. J. 2011, 183, E487–E494. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.; Gallego-Pinazo, R.; Medina, J.J.G.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef]
- Kumanyika, S.; Dietz, W.H. A Half-Century of Progress in Health: The National Academy of Medicine at 50: Solving Population-wide Obesity—Progress and Future Prospects. N. Engl. J. Med. 2020, 383, 2197–2200. [Google Scholar] [CrossRef]
- Ahmadi, M.N.; Lee, I.M.; Hamer, M.; Matthew, N.; del Pozo Cruz, B.; Chen, L.J.; Eroglu, E.; Lai, Y.L.; Ku, P.W.; Stamatakis, E. Changes in physical activity and adiposity with all-cause, cardiovascular disease, and cancer mortality. Int. J. Obes. 2022, 46, 1849–1858. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Wolfs, R.C.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Jansonius, N.M. Lifestyle and risk of developing open-angle glaucoma: The Rotterdam study. Arch. Ophthalmol. 2011, 129, 767–772. [Google Scholar] [CrossRef]
- Chen, W.-D.; Lai, L.-J.; Lee, K.-L.; Chen, T.-J.; Liu, C.-Y.; Yang, Y.-H. Is Obesity a Risk or Protective Factor for Open-Angle Glaucoma in Adults? A Two-Database, Asian, Matched-Cohort Study. J. Clin. Med. 2021, 10, 4021. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Han, K.; Park, H.-Y.L.; Lee, S.H.; Park, C.K. Metabolic Health, Obesity, and the Risk of Developing Open-Angle Glaucoma: Metabolically Healthy Obese Patients versus Metabolically Unhealthy but Normal Weight Patients. Diabetes Metab. J. 2020, 44, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, X.; Luo, W.; Jiang, B.; Lin, Q.; Tang, M.; Li, X.; Xie, L. The Causal Association Between Obesity and Primary Open-Angle Glaucoma: A Two-Sample Mendelian Randomization Study. Front. Genet. 2022, 13, 835524. [Google Scholar] [CrossRef] [PubMed]
- Euromonitor International for Trade Sources. Available online: https://firstwefeast.com/drink/2015/01/infographic-coffee-vs-tea-consumption-around-the-world (accessed on 15 August 2022).
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Tellone, E.; Galtieri, A.; Russo, A.; Ficarra, S. Protective Effects of the Caffeine Against Neurodegenerative Diseases. Curr. Med. Chem. 2019, 26, 5137–5151. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Hankinson, S.E.; Pasquale, L.R. Caffeine Consumption and the Risk of Primary Open-Angle Glaucoma: A Prospective Cohort Study. Investig. Opthalmol. Vis. Sci. 2008, 49, 1924–1931. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Cheng, J.; Wang, M.; Zhong, Y.; Yu, A.Y. Habitual Coffee Consumption Increases Risk of Primary Open-Angle Glaucoma: A Mendelian Randomization Study. Ophthalmology 2022, 129, 1014–1021. [Google Scholar] [CrossRef]
- Wu, C.M.; Wu, A.M.; Tseng, V.L.; Yu, F.; Coleman, A.L. Frequency of a diagnosis of glaucoma in individuals who consume coffee, tea and/or soft drinks. Br. J. Ophthalmol. 2018, 102, 1127–1133. [Google Scholar] [CrossRef]
- Gasiunas, K.; Galgauskas, S. Green tea-a new perspective of glaucoma prevention. Int. J. Ophthalmol. 2022, 15, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.C.; Ho, M.K.; Bharwani, A.A.; Cogo-Moreira, H.; Wang, Y.; Chow, M.S.C.; Fan, X.; Galea, S.; Leung, G.M.; Ni, M.Y. Mental disorders following COVID-19 and other epidemics: A systematic review and meta-analysis. Transl. Psychiatry 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Atalay, E.; Tamçelik, N.; Capar, O. High intraocular pressure after carbamazepine and gabapentin intake in a pseudoex-foliative patient. J. Glaucoma 2014, 23, 574–576. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Tseng, P.-T.; Stubbs, B.; Carvalho, A.F.; Li, D.-J.; Chen, T.-Y.; Lin, P.-Y.; Hsueh, Y.-T.; Chen, Y.-Z.; Chen, Y.-W.; et al. The risk of glaucoma and serotonergic antidepressants: A systematic review and meta-analysis. J. Affect. Disord. 2018, 241, 63–70. [Google Scholar] [CrossRef]
- Jain, V.; Jain, M.; Abdull, M.; Bastawrous, A. The association between cigarette smoking and primary open-angle glaucoma: A systematic review. Int. Ophthalmol. 2017, 37, 291–301. [Google Scholar] [CrossRef]
- Lund, I.; Moan, I.S.; Edvardsen, H.M.E. The relative impact of smoking, alcohol use and drug use on general sickness absence among Norwegian employees. BMC Public Health 2019, 19, 500–508. [Google Scholar] [CrossRef]
- Pérez-de-Arcelus, M.; Toledo, E.; Martínez-González, M.Á.; Martín-Calvo, N.; Fernández-Montero, A.; Moreno-Montañés, J. Smoking and incidence of glaucoma: The SUN Cohort. Medicine (Baltimore). Medicine 2017, 96, e5761. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Zanon-Viguer, V.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Smoking, an addi-tional risk factor in elder women with primary open-angle glaucoma. Mol. Vis. 2009, 15, 2953–2959. [Google Scholar]
- Stathatos, N. Thyroid physiology. Med. Clin. N. Am. 2012, 96, 165–173. [Google Scholar] [CrossRef]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, J.; Davies, D.M. Glaucoma and the thyroid. Br. J. Ophthalmol. 1965, 49, 441–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, A.J.; Rochtchina, E.; Wang, J.J.; Healey, P.R.; Mitchell, P. Open-angle glaucoma and systemic thyroid disease in an older population: The Blue Mountains Eye Study. Eye 2004, 18, 600–608. [Google Scholar] [CrossRef]
- Betzler, B.K.; Young, S.M.; Sundar, G. Intraocular Pressure and Glaucoma in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 219–225. [Google Scholar] [CrossRef]
- Silberstein, S.D. Migraine. Lancet 2004, 363, 381–391. [Google Scholar] [CrossRef]
- Donnet, A.; Ducros, A.; Radat, F.; Allaf, B.; Chouette, I.; Lanteri-Minet, M. Severe migraine and its control: A proposal for definitions and consequences for care. Rev. Neurol. 2021, 177, 924–934. [Google Scholar] [CrossRef]
- Xu, C.; Li, J.; Li, Z.; Mao, X. Migraine as a risk factor for primary open angle glaucoma: A systematic review and me-ta-analysis. Medicine (Baltimore). Medicine 2018, 97, e11377. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Su, C.C.; Wang, T.H.; Tsai, I.J. Migraine and increased risk of developing open angle glaucoma: A population-based cohort study. BMC Ophthalmol. 2019, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Pache, M.; Resink, T. Vasospasm, its Role in the Pathogenesis of Diseases with Particular Reference to the Eye. Prog. Retin. Eye Res. 2001, 20, 319–349. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Stana, D.; Nicolae, V.A.; Cirstoveanu, C.; Vancea, G.; Serban, D.; Socea, B. Association between vascular comorbidity and glaucoma progression: A four-year observational study. Exp. Ther. Med. 2021, 21, 283. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Mikelberg, F.S.; Balaszi, A.G.; LeBlanc, R.P.; Lesk, M.R.; Trope, G.E. Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch. Ophthalmol. 2008, 126, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Brigham, E.P.; McCormack, M.C. Asthma in the Primary Care Setting. Med. Clin. N. Am. 2019, 103, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Kwah, J.H.; Peters, A.T. Asthma in adults: Principles of treatment. Allergy Asthma Proc. 2019, 40, 396–402. [Google Scholar] [CrossRef]
- Soriano, J.B.; Visick, G.T.; Muellerova, H.; Payvandi, N.; Hansell, A.L. Patterns of Comorbidities in Newly Diagnosed COPD and Asthma in Primary Care. Chest 2005, 128, 2099–2107. [Google Scholar] [CrossRef]
- Adibi, A.; Sin, D.D.; Safari, A.; Johnson, K.M.; Aaron, S.D.; FitzGerald, J.M.; Sadatsafavi, M. The Acute COPD Exacerbation Prediction Tool (ACCEPT): A modelling study. Lancet Respir. Med. 2020, 8, 1013–1021. [Google Scholar] [CrossRef]
- Jassim, A.H.; Fan, Y.; Pappenhagen, N.; Nsiah, N.Y.; Inman, D.M. Oxidative Stress and Hypoxia Modify Mitochondrial Homeostasis During Glaucoma. Antioxid. Redox Signal. 2021, 35, 1341–1357. [Google Scholar] [CrossRef]
- Huerta, C.; Rodríguez, L.A.G.; Möller, C.S.; Arellano, F.M. The risk of obstructive airways disease in a glaucoma population. Pharmacoepidemiol. Drug Saf. 2001, 10, 157–163. [Google Scholar] [CrossRef]
- Cesareo, M.; Giannini, C.; Martucci, A.; Di Marino, M.; Pocobelli, G.; Aiello, F.; Mancino, R.; Nucci, C. Links between obstructive sleep apnea and glaucoma neurodegeneration. Prog. Brain Res. 2020, 257, 19–36. [Google Scholar]
| Inclusion Criteria | |
| OHTG | OAGG |
| Diagnosis of OHT without glaucoma OND signs | Diagnosis of OAG (early or moderate glaucoma stage) |
| Age 40–80 years | |
| Both sexes | |
| Capacity to understand and participate in the study | |
| Exclusion Criteria | |
| OHTG | OAGG |
| Glaucoma OND signs | Glaucoma type other than OAG |
| Other confounding ONH signs | Advanced glaucoma stage |
| <40 or >80 years old | |
| Other ocular diseases or systemic pathologies that may have interfered with study objectives | |
| Other treatments, ocular surgery, or laser treatment during last three months that may have interfered with study results | |
| Unable to participate in study | |
| Variable | OAGG | OHTG | p * | |
|---|---|---|---|---|
| Diagnostic | 214 (51.9) | 198 (48.1) | - | |
| Age | 62.5 | 60.8 | 0.665 | |
| Women | 108 (51.2) | 77 (39.1) | 0.019 | |
| IOP (mmHg) | RE | 15.8 ± 3.8 | 20.5 ± 2.3 | <0.001 |
| LE | 16.9 ± 3.9 | 20.1 ± 2.7 | <0.001 | |
| Thyroid dysfunction | 30 (14) | 37 (18.7) | 0.254 | |
| Smoking | 30 (14) | 56 (28.3) | <0.001 | |
| Alcohol drinking | 37 (17.3) | 36 (18.3) | 0.895 | |
| Height (cm) | 165 ± 10 | 163 ± 9 | 0.012 | |
| Weight (kg) | 72.8 ± 14.6 | 72.4 ± 12.9 | 0.774 | |
| BMI (kg/m2) | 26.5 ± 4.5 | 27.3 ± 4.5 | 0.095 | |
| Psychochemicals | 78 (36.4) | 74 (37.4) | 0.926 | |
| Sleep apnea | 23 (10.7) | 25 (12.6) | 0.660 | |
| Asthma | 26 (12.1) | 17 (8.6) | 0.307 | |
| COPD | 11 (5.1) | 32 (16.2) | <0.001 | |
| Coffee | 137 (64.0) | 129 (65.1) | 0.891 | |
| Tea | 23 (10.7) | 27 (13.6) | 0.456 | |
| Migraine | 38 (17.8) | 61 (30.8) | 0.003 | |
| Peripheral vasospasm | 67 (31.5) | 48 (25.1) | 0.195 | |
| Variable | Coefficient | SD | Z-Value | p * | OR |
|---|---|---|---|---|---|
| Intercept | −10.188 | 7.835 | −1.300 | 0.193 | - |
| Age (lineal) | −0.320 | 0.532 | −0.603 | 0.546 | 0.726 |
| Age (quadratic) | 0.571 | 0.449 | 1.271 | 0.203 | 1.771 |
| Age (cubic) | 0.023 | 0.319 | 0.075 | 0.940 | 1.024 |
| Age (4th power) | 0.196 | 0.234 | 0.839 | 0.401 | 1.217 |
| Sex | −0.050 | 0.307 | −0.165 | 0.869 | 0.951 |
| Thyroid dysfunction | 0.252 | 0.350 | 0.719 | 0.472 | 0.777 |
| Smoking | −0.501 | 0.329 | −1.524 | 0.127 | 1.651 |
| Alcohol drinking | 0.017 | 0.326 | 0.054 | 0.957 | 0.983 |
| Height (cm) | 5.601 | 4.825 | 1.161 | 0.245 | 270.816 |
| Weight (kg) | −0.115 | 0.060 | −1.923 | 0.054 | 0.891 |
| BMI (kg/m2) | 0.339 | 0.157 | 2.157 | 0.031 | 1.405 |
| Psychochemicals | 0.208 | 0.252 | 0.828 | 0.407 | 0.812 |
| Sleep apnea | 0.216 | 0.421 | 0.514 | 0.607 | 0.805 |
| Asthma | 1.365 | 0.465 | 2.934 | 0.003 | 0.255 |
| COPD | −0.938 | 0.446 | −2.103 | 0.035 | 2.555 |
| Coffee | 0.103 | 0.245 | 0.420 | 0.674 | 0.902 |
| Tea | 0.139 | 0.397 | 0.350 | 0.725 | 0.870 |
| Migraine | −0.946 | 0.293 | −3.226 | 0.001 | 2.577 |
| Peripheric vasospasm | 0.225 | 0.269 | 0.837 | 0.402 | 0.798 |
| Variable | Coefficient | SD | Z-Value | p * | OR |
|---|---|---|---|---|---|
| Intercept | −1.328 | 0.693 | −1.917 | 0.055 | - |
| Smoking | −0.557 | 0.305 | 1.822 | 0.069 | 1.745 |
| Weight | −0.041 | 0.014 | −2.869 | 0.004 | 0.959 |
| BMI | 0.139 | 0.044 | 3.140 | 0.001 | 1.149 |
| Asthma | −1.382 | 0.450 | −3.067 | 0.002 | 0.251 |
| COPD | 0.797 | 0.428 | 1.860 | 0.063 | 2.219 |
| Migraine | 0.864 | 0.272 | 3.173 | 0.001 | 2.372 |
| Variable | Coefficient | SD | Z-Value | p * | OR | 95% CI | GVIF | Df | GVIF1/(2*Df) |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | −1.341 | 0.751 | −1.785 | 0.074 | - | ||||
| Age (lineal) | −0.148 | 0.502 | −0.296 | 0.767 | 0.862 | 0.317–2.336 | 1.241785 | 4 | 1.027438 |
| Age (quadratic) | 0.528 | 0.432 | 1.221 | 0.222 | 1.696 | 0.720–4.011 | |||
| Age (cubic) | 0.051 | 0.311 | 0.165 | 0.869 | 1.053 | 0.569–1.942 | |||
| Age (4th power) | 0.171 | 0.229 | 0.750 | 0.453 | 1.187 | 0.759–1.860 | |||
| Gender | −0.103 | 0.288 | −0.356 | 0.727 | 0.902 | 0.510–1.584 | 1.651071 | 1 | 1.284940 |
| Smoking | 0.624 | 0.305 | 2.049 | 0.041 | 1.866 | 1.029–3.410 | 1.062069 | 1 | 1.030567 |
| Weight | −0.046 | 0.017 | −2.594 | 0.009 | 0.955 | 0.922–0.988 | 4.825147 | 1 | 2.196622 |
| BMI | 0.162 | 0.051 | 3.188 | 0.001 | 1.175 | 1.066–1.301 | 4.092805 | 1 | 2.023068 |
| Asthma | −1.399 | 0.453 | −3.103 | 0.002 | 0.247 | 0.096–0.572 | 1.079335 | 1 | 1.038910 |
| Migraine | 0.849 | 0.278 | 3.062 | 0.002 | 2.338 | 1.364–4.059 | 1.099779 | 1 | 1.048704 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Villanueva, C.; Milla, E.; Bolarin, J.M.; García-Medina, J.J.; Cruz-Espinosa, J.; Benítez-del-Castillo, J.; Salgado-Borges, J.; Hernández-Martínez, F.J.; Bendala-Tufanisco, E.; Andrés-Blasco, I.; et al. Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal. J. Clin. Med. 2022, 11, 5649. https://doi.org/10.3390/jcm11195649
Garcia-Villanueva C, Milla E, Bolarin JM, García-Medina JJ, Cruz-Espinosa J, Benítez-del-Castillo J, Salgado-Borges J, Hernández-Martínez FJ, Bendala-Tufanisco E, Andrés-Blasco I, et al. Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal. Journal of Clinical Medicine. 2022; 11(19):5649. https://doi.org/10.3390/jcm11195649
Chicago/Turabian StyleGarcia-Villanueva, Carolina, Elena Milla, José M. Bolarin, José J. García-Medina, Javier Cruz-Espinosa, Javier Benítez-del-Castillo, José Salgado-Borges, Francisco J. Hernández-Martínez, Elena Bendala-Tufanisco, Irene Andrés-Blasco, and et al. 2022. "Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal" Journal of Clinical Medicine 11, no. 19: 5649. https://doi.org/10.3390/jcm11195649
APA StyleGarcia-Villanueva, C., Milla, E., Bolarin, J. M., García-Medina, J. J., Cruz-Espinosa, J., Benítez-del-Castillo, J., Salgado-Borges, J., Hernández-Martínez, F. J., Bendala-Tufanisco, E., Andrés-Blasco, I., Gallego-Martinez, A., Zanón-Moreno, V. C., & Pinazo-Durán, M. D. (2022). Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal. Journal of Clinical Medicine, 11(19), 5649. https://doi.org/10.3390/jcm11195649







