Drivers of Radioresistance in Prostate Cancer
Abstract
1. Introduction
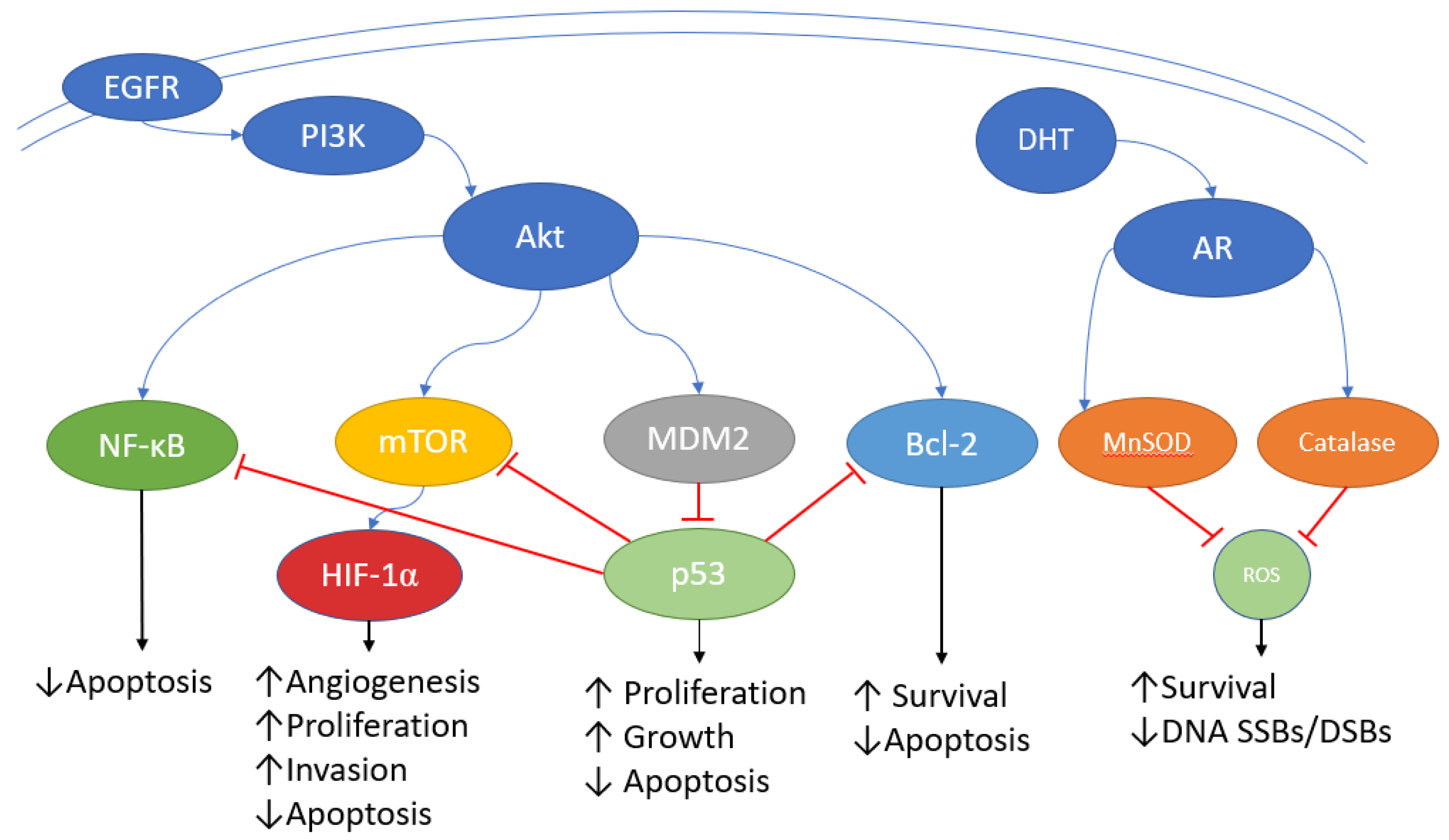
2. Androgen Receptors
3. Ataxia Telangiectasia Mutated Gene
4. PI3K/Akt/mTOR
5. Epidermal Growth Factor Receptor
6. Nuclear Factor Kappa B
7. Bcl-2
8. Hypoxia-Inducible Factor
9. miR-191
10. p53
11. Clinical Application of Pathway Inhibitors
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Australia 2021; Cancer Series No. 133; Cat. No. CAN 144. AIHW: Canberra, Australia, 2021.
- Nolsøe, A.B.; Jensen, C.F.S.; Østergren, P.B.; Fode, M. Neglected side effects to curative prostate cancer treatments. Int. J. Impot. Res. 2020, 33, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Y.; Xie, B.; Xu, J.; Wang, H.; Hassan, M.; Wang, R.; Hong, M.; Hong, Q.; Deng, Y. Indirect Effects of Radiation Induce Apoptosis and Neuroinflammation in Neuronal SH-SY5Y Cells. Neurochem. Res. 2014, 39, 2334–2342. [Google Scholar] [CrossRef]
- Manda, G.; Nechifor, M.T.; Neagu, T.M. Reactive Oxygen Species, Cancer and Anti-Cancer Therapies. Curr. Chem. Biol. 2009, 3, 22–46. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Li, J.J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastas. Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Li, L.; Story, M.; Legerski, R.J. Cellular responses to ionizing radiation damage. Int. J. Radiat. Oncol. 2001, 49, 1157–1162. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Coen, J.J.; Bae, K.; Zietman, A.L.; Patel, B.; Shipley, W.U.; Slater, J.D.; Rossi, C.J. Acute and Late Toxicity After Dose Escalation to 82 GyE Using Conformal Proton Radiation for Localized Prostate Cancer: Initial Report of American College of Radiology Phase II Study 03-12. Int. J. Radiat. Oncol. 2011, 81, 1005–1009. [Google Scholar] [CrossRef]
- Vora, S.A.; Wong, W.W.; Schild, S.; Ezzell, G.A.; Andrews, P.E.; Ferrigni, R.G.; Swanson, S.K. Outcome and Toxicity for Patients Treated with Intensity Modulated Radiation Therapy for Localized Prostate Cancer. J. Urol. 2013, 190, 521–526. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Tindall, D.J. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett. 2002, 187, 1–7. [Google Scholar] [CrossRef]
- E Knudsen, K.; Kelly, W.K. Outsmarting androgen receptor: Creative approaches for targeting aberrant androgen signaling in advanced prostate cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.-F.; Cai, L.; Zheng, D.; et al. Androgen Receptor Signaling Regulates DNA Repair in Prostate Cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.F.; Schiewer, M.J.; Dean, J.L.; Schrecengost, R.S.; de Leeuw, R.; Han, S.; Ma, T.; Den, R.B.; Dicker, A.P.; Feng, F.Y.; et al. A Hormone–DNA Repair Circuit Governs the Response to Genotoxic Insult. Cancer Discov. 2013, 3, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Pinthus, J.H.; Bryskin, I.; Trachtenberg, J.; Luz, J.-P.; Singh, G.; Fridman, E.; Wilson, B.C. Androgen Induces Adaptation to Oxidative Stress in Prostate Cancer: Implications for Treatment with Radiation Therapy. Neoplasia 2007, 9, 68–80. [Google Scholar] [CrossRef]
- Ghashghaei, M.; Niazi, T.M.; Aguilar-Mahecha, A.; Klein, K.O.; Greenwood, C.M.; Basik, M.; Muanza, T.M. Identification of a Radiosensitivity Molecular Signature Induced by Enzalutamide in Hor-mone-sensitive and Hormone-resistant Prostate Cancer Cells. Sci. Rep. 2019, 9, 8838. [Google Scholar] [CrossRef]
- Hoey, C.; Ray, J.; Jeon, J.; Huang, X.; Taeb, S.; Ylanko, J.; Andrews, D.W.; Boutros, P.C.; Liu, S.K. miRNA-106a and prostate cancer radioresistance: A novel role for LITAF in ATM regulation. Mol. Oncol. 2018, 12, 1324–1341. [Google Scholar] [CrossRef]
- Yan, D.; Ng, W.L.; Zhang, X.; Wang, P.; Zhang, Z.; Mo, Y.-Y.; Mao, H.; Hao, C.; Olson, J.J.; Curran, W.J.; et al. Targeting DNA-PKcs and ATM with miR-101 Sensitizes Tumors to Radiation. PLoS ONE 2010, 5, e11397. [Google Scholar] [CrossRef]
- Xie, X.; Xu, Z.; Wang, C.; Fang, C.; Zhao, J.; Xu, L.; Qian, X.; Dai, J.; Sun, F.; Xu, D.; et al. Tip60 is associated with resistance to X-ray irradiation in prostate cancer. FEBS Open Bio 2017, 8, 271–278. [Google Scholar] [CrossRef]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Reid, A.H.; Yap, T.A.; de Bono, J.S. Targeting the PI3K/AKT Pathway for the Treatment of Prostate Cancer. Clin. Cancer Res. 2009, 15, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of epithelial–mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014, 5, e1437. [Google Scholar] [CrossRef]
- Potiron, V.A.; Abderrhamani, R.; Giang, E.; Chiavassa, S.; Di Tomaso, E.; Maira, S.-M.; Paris, F.; Supiot, S. Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother. Oncol. 2013, 106, 138–146. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Tzeng, D.T.W.; Huang, Y.-P.; Lin, C.-J.; Lo, U.-G.; Wu, C.-L.; Lin, H.; Hsieh, J.-T.; Tang, C.-H.; Lai, C.-H. Antrocin Sensitizes Prostate Cancer Cells to Radiotherapy through Inhibiting PI3K/AKT and MAPK Signaling Pathways. Cancers 2018, 11, 34. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Day, K.C.; Hiles, G.L.; Kozminsky, M.; Dawsey, S.J.; Paul, A.; Broses, L.J.; Shah, R.; Kunja, L.P.; Hall, C.; Palanisamy, N.; et al. HER2 and EGFR Overexpression Support Metastatic Progression of Prostate Cancer to Bone. Cancer Res. 2017, 77, 74–85. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M. Targeting the epidermal growth factor receptor in radiotherapy: Radiobiological mech-anisms, preclinical and clinical results. Radiother. Oncol. 2004, 72, 257–266. [Google Scholar] [CrossRef]
- Skvortsova, I.; Skvortsov, S.; Stasyk, T.; Raju, U.; Popper, B.-A.; Schiestl, B.; von Guggenberg, E.; Neher, A.; Bonn, G.K.; Huber, L.A.; et al. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. PROTEOMICS 2008, 8, 4521–4533. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.; Singh, R.; Singh, N.; Singh, R.P. EGFR-mediated Rad51 expression potentiates intrinsic resistance in prostate cancer via EMT and DNA repair pathways. Life Sci. 2021, 286, 120031. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.; Lessard, L.; Mes-Masson, A.M.; Saad, F. Expression of NF-kappaB in prostate cancer lymph node metastases. Prostate 2004, 58, 308–313. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, L.; Creighton, C.J.; Zhang, Y.; Xin, L.; Ittmann, M.; Wang, J. Function of phosphorylation of NF-kB p65 ser536 in prostate cancer oncogenesis. Oncotarget 2015, 6, 6281. [Google Scholar] [CrossRef]
- E Hallahan, D.; Spriggs, D.R.; A Beckett, M.; Kufe, D.W.; Weichselbaum, R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA 1989, 86, 10104–10107. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, T.; Chen, G.; Brown, S.A.; Yu, Z.; Mattson, M.P.; Thompson, J.S. Effects of NF-kappaB1 (p50) targeted gene disruption on ionizing radiation-induced NF-kappaB ac-tivation and TNFalpha, IL-1alpha, IL-1beta and IL-6 mRNA expression in vivo. Int. J. Radiat. Biol. 2001, 77, 763–772. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Lessard, L.; Begin, L.R.; Gleave, M.E.; Mes-Masson, A.M.; Saad, F. Nuclear localisation of nuclear factor-kappaB transcription factors in prostate cancer: An immuno-histochemical study. Br. J. Cancer 2005, 93, 1019–1023. [Google Scholar] [CrossRef]
- Josson, S.; Xu, Y.; Fang, F.; Dhar, S.K.; Clair, D.K.S.; Clair, W.H.S. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene 2005, 25, 1554–1559. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, F.; St. Clair, D.K.; Sompol, P.; Josson, S.; St. Clair, W.H. SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol. Cancer Ther. 2008, 7, 2367–2376. [Google Scholar] [CrossRef]
- Holley, A.K.; Xu, Y.; Clair, D.K.S.; Clair, W.H.S. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Ann. N. Y. Acad. Sci. 2010, 1201, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Ding, J.; Hu, W.; Tan, C.; Wang, M.; Tang, J.; Xu, Y. miR-17-3p Downregulates Mitochondrial Antioxidant Enzymes and Enhances the Radiosensitivity of Prostate Cancer Cells. Mol. Ther. Nucleic Acids 2018, 13, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gupta, D.; Arora, R. NF-kB as a key player in regulation of cellular radiation responses and identification of radiation countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer 2006, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Catz, S.D.; Johnson, J.L. BCL-2 in prostate cancer: A minireview. Apoptosis 2003, 8, 29–37. [Google Scholar] [CrossRef]
- Mackey, T.J.; Borkowski, A.; Amin, P.; Jacobs, S.C.; Kyprianou, N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 1998. 52, 1085–1090. [CrossRef]
- Rosser, C.J.; O Reyes, A.; Vakar-Lopez, F.; Levy, L.B.; A Kuban, D.; Hoover, D.C.; Lee, A.K.; Pisters, L.L. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int. J. Radiat. Oncol. 2003, 56, 1–6. [Google Scholar] [CrossRef]
- Inayat, M.S.; Chendil, D.; Mohiuddin, M.; Elford, H.L.; Gallicchio, V.S.; Ahmed, M.M. Didox (A Novel Ribonucleotide Reductase Inhibitor) Overcomes bcl-2 Mediated Radiation Resistance in Prostate Cancer Cell Line PC-3. Cancer Biol. Ther. 2002, 1, 539–545. [Google Scholar] [CrossRef]
- An, J.; Chervin, A.S.; Nie, A.; Ducoff, H.S.; Huang, Z. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene 2006, 26, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ezekwudo, D.; Shashidharamurthy, R.; Devineni, D.; Bozeman, E.; Palaniappan, R.; Selvaraj, P. Inhibition of expression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett. 2008, 270, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Anai, S.; Shiverick, K.; Medrano, T.; Nakamura, K.; Goodison, S.; Brown, B.D.; Rosser, C.J. Downregulation of BCL-2 Induces Downregulation of Carbonic Anhydrase IX, Vascular Endothelial Growth Factor, and pAkt and Induces Radiation Sensitization. Urology 2007, 70, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, D.; Martin, L.M.; Atzberger, A.; Lynch, T.H.; Hollywood, D.; Marignol, L. Exposure to hypoxia following irradiation increases radioresistance in prostate cancer cells. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1106–1116. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.-S.; Kim, H.-J.; Lee, E.; Jeong, J.-H.; Park, I.; Jeong, Y.K.; Jang, W.I. Role of HIF-1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int. J. Hyperth. 2017, 34, 276–283. [Google Scholar] [CrossRef]
- Kimbro, K.S.; Simons, J.W. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr. Relat. Cancer 2006, 13, 739–749. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Zuo, X.; Basourakos, S.P.; Zhang, J.; Zhao, J.; Han, Y.; Lin, Y.; Wang, Y.; Jiang, Y.; et al. β-catenin nuclear translocation induced by HIF1alpha overexpression leads to the radioresistance of prostate cancer. Int. J. Oncol. 2018, 52, 1827–1840. [Google Scholar]
- Vergis, R.; Corbishley, C.M.; Norman, A.R.; Bartlett, J.; Jhavar, S.; Borre, M.; Heebøll, S.; Horwich, A.; Huddart, R.; Khoo, V.; et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: A retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008, 9, 342–351. [Google Scholar] [CrossRef]
- Nagpal, N.; Kulshreshtha, R. miR-191: An emerging player in disease biology. Front. Genet. 2014, 5, 99. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Chaudhry, M.A.; Omaruddin, R.A.; Brumbaugh, C.D.; Tariq, M.A.; Pourmand, N. Identification of radiation-induced microRNA transcriptome by next-generation massively parallel sequencing. J. Radiat. Res. 2013, 54, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Haughey, C.; Hoey, C.; Jeon, J.; Murphy, R.; Dura-Perez, L.; McCabe, N.; Downes, M.; Jain, S.; Boutros, P.C.; et al. miR-191 promotes radiation resistance of prostate cancer through interaction with RXRA. Cancer Lett. 2020, 473, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Kastan, M.B. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes Dev. 1998, 12, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gish, K.; Murphy, M.; Yin, Y.; Notterman, D.; Hoffman, W.H.; Tom, E.; Mack, D.H.; Levine, A.J. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000, 14, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Teodoro, J.G.; Evans, S.K.; Green, M.R. Inhibition of tumor angiogenesis by p53: A new role for the guardian of the genome. Klin. Wochenschr. 2007, 85, 1175–1186. [Google Scholar] [CrossRef]
- Rakozy, C.; Grignon, D.J.; Li, Y.; Gheiler, E.; Gururajanna, B.; Pontes, J.E.; Sakr, W.; Wood, D.P.; Sarkar, F.H. p53 Gene Alterations in Prostate Cancer After Radiation Failure and Their Association with Clinical Outcome: A Molecular and Immunohistochemical Analysis. Pathol. Res. Pract. 1999, 195, 129–135. [Google Scholar] [CrossRef]
- Stattin, P.; Damber, J.-E.; Modig, H.; Bergh, A. Pretreatment p53 immunoreactivity does not infer radioresistance in prostate cancer patients. Int. J. Radiat. Oncol. 1996, 35, 885–889. [Google Scholar] [CrossRef]
- Ritter, M.A.; Gilchrist, K.W.; Voytovich, M.; Chappell, R.J.; Verhoven, B.M. The role of p53 in radiation therapy outcomes for favorable-to-intermediate-risk prostate cancer. Int. J. Radiat. Oncol. 2002, 53, 574–580. [Google Scholar] [CrossRef]
- Sasaki, R.; Shirakawa, T.; Zhang, Z.J.; Tamekane, A.; Matsumoto, A.; Sugimura, K.; Matsuo, M.; Kamidono, S.; Gotoh, A. Additional gene therapy with Ad5CMV-p53 enhanced the efficacy of radiotherapy in human prostate cancer cells. Int. J. Radiat. Oncol. 2001, 51, 1336–1345. [Google Scholar] [CrossRef]
- Scott, S.L.; Earle, J.D.; Gumerlock, P.H. Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res. 2003, 63, 7190–7196. [Google Scholar] [PubMed]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. N. Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef]
- Denham, J.W.; Steigler, A.; Lamb, D.S.; Joseph, D.; Turner, S.; Matthews, J.; Atkinson, C.; North, J.; Christie, D.; Spry, N.A.; et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011, 12, 451–459. [Google Scholar] [CrossRef]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; San Segundo, C.G.; Rodríguez, M.A.C.; Macias, V.; Olive, A.P.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Cho, E.; Mostaghel, E.A.; Russell, K.J.; Liao, J.J.; Konodi, M.A.; Kurland, B.F.; Marck, B.T.; Matsumoto, A.M.; Dalkin, B.L.; Montgomery, R.B. External Beam Radiation Therapy and Abiraterone in Men with Localized Prostate Cancer: Safety and Effect on Tissue Androgens. Int. J. Radiat. Oncol. 2015, 92, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.; Bubley, G.J.; Bhatt, R.S.; Taplin, M.-E.; Dowling, S.; Mahoney, K.; Werner, E.; Nguyen, P. Enzalutamide wtith Radiation Therapy for Intermediate-Risk Prostate Cancer: A Phase 2 Study. Int. J. Radiat. Oncol. 2021, 110, 1416–1422. [Google Scholar] [CrossRef]
- McGowan, D.R.; Skwarski, M.; Bradley, K.M.; Campo, L.; Fenwick, J.D.; Gleeson, F.V.; Green, M.; Horne, A.; Maughan, T.S.; McCole, M.G.; et al. Buparlisib with thoracic radiotherapy and its effect on tumour hypoxia: A phase I study in patients with advanced non-small cell lung carcinoma. Eur. J. Cancer 2019, 113, 87–95. [Google Scholar] [CrossRef]
- Hill, E.J.; Roberts, C.; Franklin, J.M.; Enescu, M.; West, N.; MacGregor, T.P.; Chu, K.-Y.; Boyle, L.; Blesing, C.; Wang, L.-M.; et al. Clinical Trial of Oral Nelfinavir before and during Radiation Therapy for Advanced Rectal Cancer. Clin. Cancer Res. 2016, 22, 1922–1931. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Werner-Wasik, M.; Shih, H.A.; Ashby, L.S.; Yu, H.-H.M.; Stieber, V.W.; Malone, S.C.; et al. A randomized phase II study of everolimus in combination with chemoradiation in newly di-agnosed glio-blastoma: Results of NRG Oncology RTOG 0913. Neuro-Oncol. 2018, 20, 666–673. [Google Scholar] [CrossRef]
- Ma, D.J.; Galanis, E.; Anderson, S.K.; Schiff, D.; Kaufmann, T.J.; Peller, P.J.; Giannini, C.; Brown, P.D.; Uhm, J.H.; McGraw, S.; et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glio-blastoma: NCCTG N057K. Neuro-Oncol. 2015, 17, 1261–1269. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Xu, Z.; Yang, Q.; Zhu, G.; Liao, X.-Y.; Chen, X.; Zhu, B.; Duan, Y.; Sun, J. Concurrent EGFR-TKI and Thoracic Radiotherapy as First-Line Treatment for Stage IV Non-Small Cell Lung Cancer Harboring EGFR Active Mutations. Oncologist 2019, 24, 1031.e612. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Giralt, J.; Trigo, J.; Nuyts, S.; Ozsahin, M.; Skladowski, K.; Hatoum, G.; Daisne, J.-F.; Ancona, A.C.Y.; Cmelak, A.; Hatoum, G.; et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally ad-vanced squa-mous-cell carcinoma of the head and neck (CONCERT-2): A randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015, 16, 221–232. [Google Scholar] [CrossRef]
- Mesía, R.; Henke, M.; Fortin, A.; Minn, H.; Ancona, A.C.Y.; Cmelak, A.; Markowitz, A.B.; Hotte, S.J.; Singh, S.; Chan, A.T.C.; et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): A randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015, 16, 208–220. [Google Scholar] [CrossRef]
- Mardjuadi, F.I.; Carrasco, J.; Coche, J.-C.; Sempoux, C.; Jouret-Mourin, A.; Scalliet, P.; Goeminne, J.-C.; Daisne, J.-F.; Delaunoit, T.; Vuylsteke, P.; et al. Panitumumab as a radiosensitizing agent in KRAS wild-type locally advanced rectal cancer. Target. Oncol. 2014, 10, 375–383. [Google Scholar] [CrossRef]
- Pinto, C.; Di Bisceglie, M.; Di Fabio, F.; Bochicchio, A.; Latiano, T.; Cordio, S.; Rosati, G.; Aschele, C.; Marino, A.; Bergamo, F.; et al. Phase II Study of Preoperative Treatment with External Radiotherapy Plus Panitumumab in Low-Risk, Locally Advanced Rectal Cancer (RaP Study/STAR-03). Oncologist 2018, 23, 912–918. [Google Scholar] [CrossRef]
- Zhou, S.; Ye, W.; Shao, Q.; Zhang, M.; Liang, J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit. Rev. Oncol. 2013, 88, 706–715. [Google Scholar] [CrossRef]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. mTOR-Mediated Antioxidant Activation in Solid Tumor Radioresistance. J. Oncol. 2019, 2019, 5956867. [Google Scholar] [CrossRef]
| Target | Inhibitor | Approved Indications |
|---|---|---|
| AR | Abiraterone | mPCa |
| Enzalutamide | CRPCa | |
| Darolutamide | nmCRPCa | |
| Biclutamide, flutamide, cyproterone | CSPCa | |
| Apalutamide | mCSPCa, nmCRPCa | |
| PI3K | Idelalisib | Chronic lymphocytic leukaemia, small lymphocytic leukaemia, follicular lymphoma |
| mTOR | Everolimus | HR+/HER- negative breast cancer, neuroendocrine tumours, renal cell carcinoma |
| Sirolimus | Prevention of organ rejection following transplant | |
| EGFR | Panitumumab | Metastatic colorectal cancer |
| Cetuximab | Metastatic colorectal cancer, head and neck cancer in combination with radiotherapy | |
| Erlotinib, osemertinib, gefitinib, lapatinib | Non-small cell lung cancer | |
| Nf-κB | Bortezomib | Multiple myeloma, mantle cell lymphoma |
| Bcl-2 | Venetoclax | Chronic lymphocytic leukaemia, small lymphocytic leukaemia, follicular lymphoma, acute myeloid leukaemia, |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, L.; Bernaitis, N.; Christie, D.; Chess-Williams, R.; Sellers, D.; McDermott, C.; Dare, W.; Anoopkumar-Dukie, S. Drivers of Radioresistance in Prostate Cancer. J. Clin. Med. 2022, 11, 5637. https://doi.org/10.3390/jcm11195637
King L, Bernaitis N, Christie D, Chess-Williams R, Sellers D, McDermott C, Dare W, Anoopkumar-Dukie S. Drivers of Radioresistance in Prostate Cancer. Journal of Clinical Medicine. 2022; 11(19):5637. https://doi.org/10.3390/jcm11195637
Chicago/Turabian StyleKing, Liam, Nijole Bernaitis, David Christie, Russ Chess-Williams, Donna Sellers, Catherine McDermott, Wendy Dare, and Shailendra Anoopkumar-Dukie. 2022. "Drivers of Radioresistance in Prostate Cancer" Journal of Clinical Medicine 11, no. 19: 5637. https://doi.org/10.3390/jcm11195637
APA StyleKing, L., Bernaitis, N., Christie, D., Chess-Williams, R., Sellers, D., McDermott, C., Dare, W., & Anoopkumar-Dukie, S. (2022). Drivers of Radioresistance in Prostate Cancer. Journal of Clinical Medicine, 11(19), 5637. https://doi.org/10.3390/jcm11195637






