Initial Experience of Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Baltic Country Center
Abstract
:1. Background
2. Materials and Methods
2.1. Ethics
2.2. Patients and Data Collection
2.3. Study Outcomes
2.4. CRS and HIPEC Regimens
- For ovarian and gastric cancer, the HIPEC protocol consisted of Cisplatin at 75 mg/m2 with 4 L of 0.9% saline at 42 °C for 60 min;
- For colorectal cancer, the HIPEC protocol consisted of Oxaliplatin at 460/m2 with 4 L of 5% glucose at 42 °C for 45 min until 2017; later, it was changed to Mytomicin C at 30 mg + 10 mg, with 5 L of 1.5% dextrose at 42 °C for 90 min;
- For primary PSMs (pseudomyxoma peritonei, appendiceal cancer), the HIPEC protocol consisted of Mytomicin C 30 mg + 10 mg, with 5-L 1.5% dextrose at 42 °C for 90 min.
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Morbidity
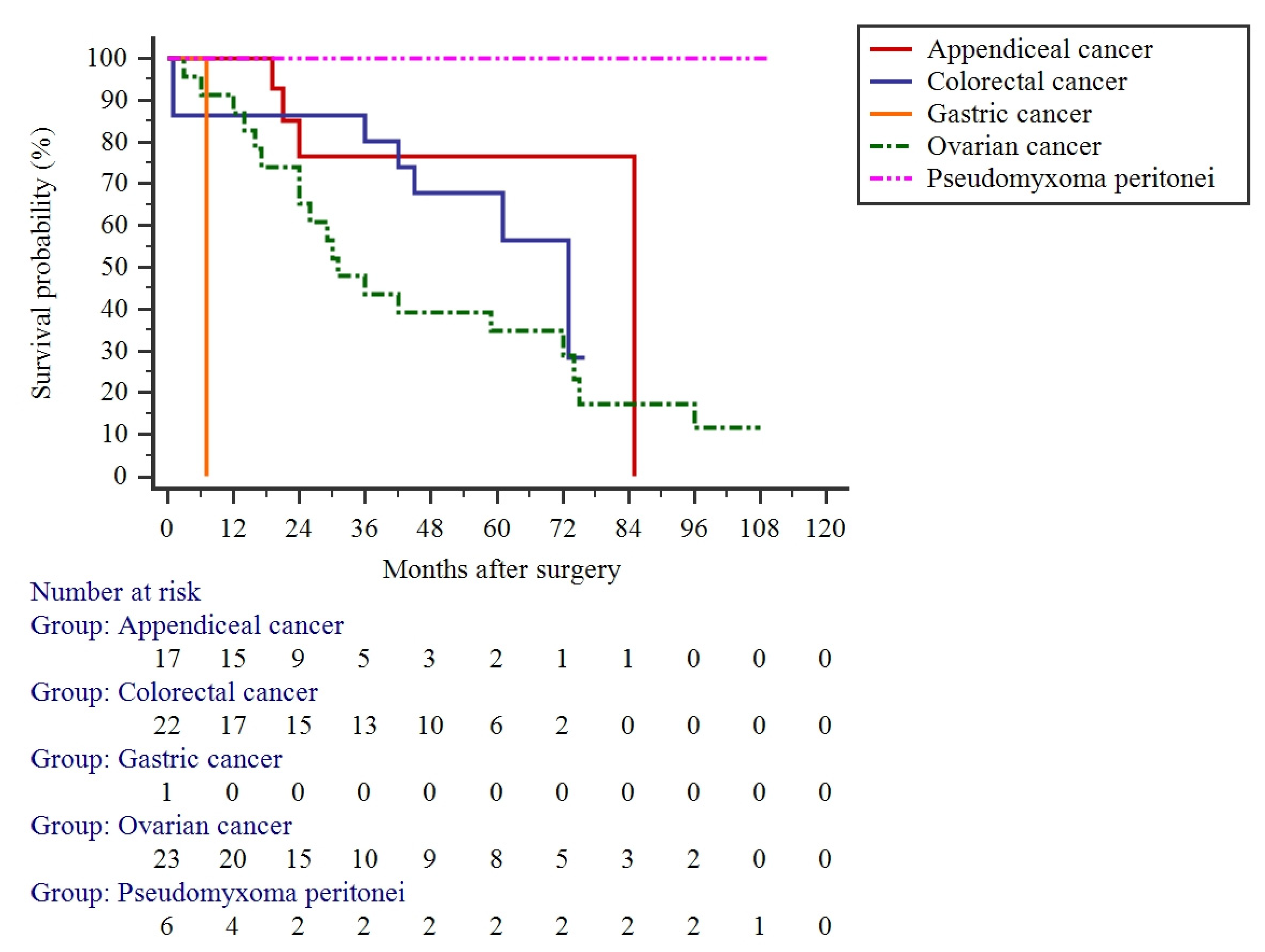
3.3. Long-Term Outcomes
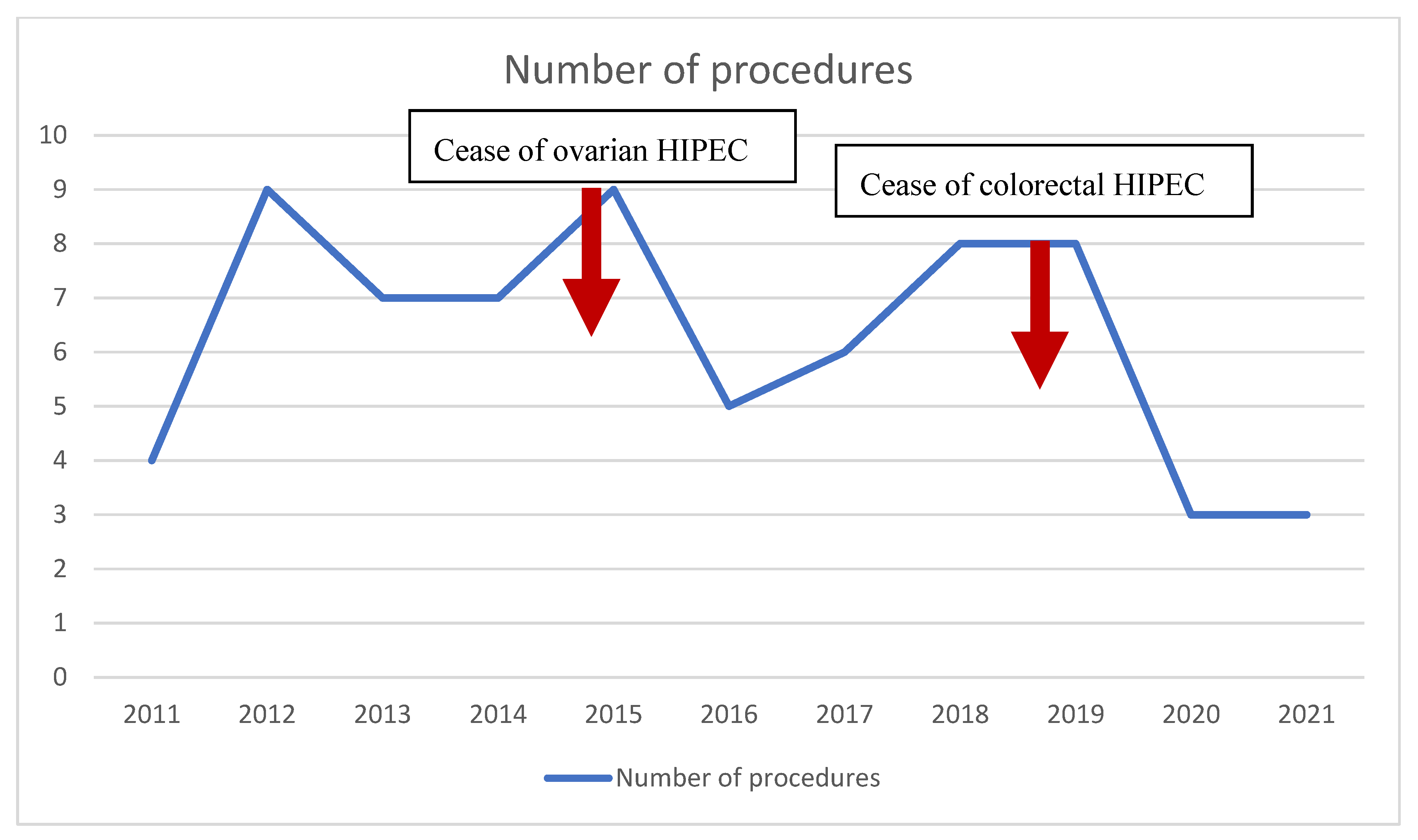
3.4. CRS and HIPEC Development in the Study Center
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSM | primary surface malignancy |
| CC | completeness of cytoreduction |
| CRS | cytoreduction |
| OS | overall survival |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| LAMN | low-grade appendiceal mucinous neoplasm |
| HAMN | high-grade appendiceal mucinous neoplasm |
References
- Pamela, K.; Matthias, Z.; Reinhold, K.-R.; Julia, P.; Peter, M.; Alexander, P.; Dietmar, Ö. Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Single-Center Experience in Austria. J. Gastrointest. Surg. 2018, 22, 884–893. [Google Scholar] [CrossRef]
- Yap, D.R.Y.; Wong, J.S.M.; Tan, Q.X.; Tan, J.W.-S.; Chia, C.S.; Ong, C.-A.J. Effect of HIPEC on Peritoneal Recurrence in Peritoneal Metastasis Treated with Cytoreductive Surgery: A Systematic Review. Front. Oncol. 2021, 11, 795390. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Koopman, M.; Teerenstra, S.; Bleichrodt, R.P.; De Hingh, I.H.J.T.; Punt, C.J.A. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 617–623. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest. Oncol. 2010, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.D.; Dhall, K. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the management of peritoneal surface malignancies—An evidence-based review. Curr. Probl. Cancer 2021, 45, 100737. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Bonnot, P.-E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.-M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.J. PROPHYLOCHIP: No benefit of second-look surgery plus HIPEC for colorectal peritoneal metastases. Lancet Oncol. 2020, 21, 1124–1125. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Surgical management of peritoneal carcinosis: Diagnosis, prevention and treatment. Langenbecks Arch. Für Chir. 1988, 373, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. J. Br. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Bruin, S.; Boot, H.; Van Slooten, G.; Van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- van Stein, R.M.; Aalbers, A.G.J.; Sonke, G.S.; van Driel, W.J. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. 2021, 7, 1231–1238. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.-J.; Yoo, H.J.; Nam, B.-H.; Bristow, R.; Park, S.-Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J. Clin. Oncol. 2017, 35, 5520. [Google Scholar] [CrossRef]
- Ansaloni, L.; Agnoletti, V.; Amadori, A.; Catena, F.; Cavaliere, D.; Coccolini, F.; De Iaco, P.; Di Battista, M.; Framarini, M.; Gazzotti, F.; et al. Evaluation of Extensive Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients With Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2012, 22, 778. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Inoue, D.; Kurokawa, T.; Yoshida, Y. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J. Obstet. Gynaecol. Res. 2020, 46, 1661–1671. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei after Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Järvinen, P.; Ristimäki, A.; Kantonen, J.; Aronen, M.; Huuhtanen, R.; Järvinen, H.; Lepistö, A. Comparison of serial debulking and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei of appendiceal origin. Int. J. Colorectal Dis. 2014, 29, 999–1007. [Google Scholar] [CrossRef]
- Sinukumar, S.; Mehta, S.; As, R.; Damodaran, D.; Ray, M.; Zaveri, S.; Kammar, P.; Bhatt, A. Analysis of Clinical Outcomes of Pseudomyxoma Peritonei from Appendicular Origin Following Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy—A Retrospective Study from INDEPSO. Indian J. Surg. Oncol. 2019, 10, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A clinical practice guideline. Curr. Oncol. 2020, 27, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; de Aretxabala, X.; Fujimura, T.; Fushida, S.; Katayama, K.; Bandou, E.; Sugiyama, K.; Kawamura, T.; Kinoshita, K.; Endou, Y.; et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepato-Gastroenterology 2001, 48, 1776–1782. [Google Scholar] [PubMed]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
- Seshadri, R.A.; Glehen, O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J. Gastroenterol. 2016, 22, 1114–1130. [Google Scholar] [CrossRef]
- Gasser, E.; Kogler, P.; Lorenz, A.; Kafka-Ritsch, R.; Öfner, D.; Perathoner, A. Do we still need CRS and HIPEC in colorectal cancer in times of modern chemotherapy and immunotherapy? MEMO—Mag. Eur. Med. Oncol. 2020, 13, 430–433. [Google Scholar] [CrossRef]
- Gronau, F.; Feldbruegge, L.; Oberwittler, F.; Gonzalez-Moreno, S.; Villeneuve, L.; Eveno, C.; Glehen, O.; Kusamura, S.; Rau, B. HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques. J. Clin. Med. 2022, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, L.; Crusellas, O.; Ramos, I.; Van der Speeten, K.; Barrios, P.; Sabia, D. Designing HIPEC regimens for colon cancer: Is the available evidence being appropriately considered? Surg. Open Dig. Adv. 2021, 3, 100019. [Google Scholar] [CrossRef]
- Gabriel, E.; Singla, S.; Kim, M.; Fisher, D.; Powers, C.; Visioni, A.; Attwood, K.; Skitzki, J. Water lavage as an adjunct to cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Am. J. Surg. 2017, 214, 462–467. [Google Scholar] [CrossRef]
- Almerey, T.; Gabriel, E.M.; Torp, K.D.; Bagaria, S.P. Intraoperative fluid restriction in hyperthermic intraperitoneal chemotherapy. J. Surg. Res. 2018, 231, 77–82. [Google Scholar] [CrossRef]
| Characteristics of HIPEC Patients | ||
|---|---|---|
| Sex | Female (n; %) | 56 (81.2%) |
| Male (n; %) | 13 (18.8%) | |
| Mean age ± SD (Q1; Q3), years | 57.2 ± 10.89 (49; 67) | |
| Mean hospitalization ± SD (Q1; Q3), days | 20.19 ± 14.69 (12; 23.5) | |
| Peritoneal histology (n; %): | ||
| Colorectal | 22 (31.9%) | |
| Ovarian | 23 (33.3%) | |
| Appendiceal: | 17 (24.6%) | |
| - LAMN | 13 (18.8%) | |
| - HAMN | 4 (5.8%) | |
| Pseudomyxoma peritonei | 6 (8.7%) | |
| Gastric | 1 (1.4%) | |
| Mean PCI score ± SD (Q1; Q3) | 12.2 ± 7.6 (6.25; 15.75) | |
| Mean operation time ± SD (Q1; Q3), min | 447.5 ± 152.7 (310; 540) | |
| Mean blood loss ± SD (Q1; Q3), mL | 350.7 ± 284.3 (200.0; 500.0) | |
| Cytoreduction completeness: | ||
|
| |
| Complications C–D: | ||
| 36 (52.2%) 21 (30.4%) 5 (7.2%) 6 (8.7%) 1 (1.4%) | |
| Complication | Number of Patients | C–D |
|---|---|---|
| Atrial fibrillation | 2 | 1 |
| Wound infection | 6 | 2 |
| Urinary tract infection | 3 | 2 |
| Renal insufficiency | 3 | 1 |
| Pancytopenia | 2 | 2 |
| Hydrothorax | 5 | 2 |
| Pneumonia | 3 | 2 |
| Intraabdominal abscess | 4 | 3a |
| Intraabdominal bleeding | 1 | 3b |
| Pancreatic fistula | 2 | 3a |
| Ileus | 3 | 1 |
| Anastomotic leakage | 5 | 3b |
| Compartment syndrome | 1 | 3b |
| Postoperative myocardial infarction | 1 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Račkauskas, R.; Baušys, A.; Jurgaitis, J.; Paškonis, M.; Strupas, K. Initial Experience of Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Baltic Country Center. J. Clin. Med. 2022, 11, 5554. https://doi.org/10.3390/jcm11195554
Račkauskas R, Baušys A, Jurgaitis J, Paškonis M, Strupas K. Initial Experience of Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Baltic Country Center. Journal of Clinical Medicine. 2022; 11(19):5554. https://doi.org/10.3390/jcm11195554
Chicago/Turabian StyleRačkauskas, Rokas, Augustinas Baušys, Jonas Jurgaitis, Marius Paškonis, and Kęstutis Strupas. 2022. "Initial Experience of Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Baltic Country Center" Journal of Clinical Medicine 11, no. 19: 5554. https://doi.org/10.3390/jcm11195554
APA StyleRačkauskas, R., Baušys, A., Jurgaitis, J., Paškonis, M., & Strupas, K. (2022). Initial Experience of Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Baltic Country Center. Journal of Clinical Medicine, 11(19), 5554. https://doi.org/10.3390/jcm11195554






