Successful Treatment of Acute Uric Acid Nephropathy with Rasburicase in a Primary Central Nervous System Lymphoma Patient Showing a Dramatic Response to Methotrexate—Case Report
Abstract
1. Introduction
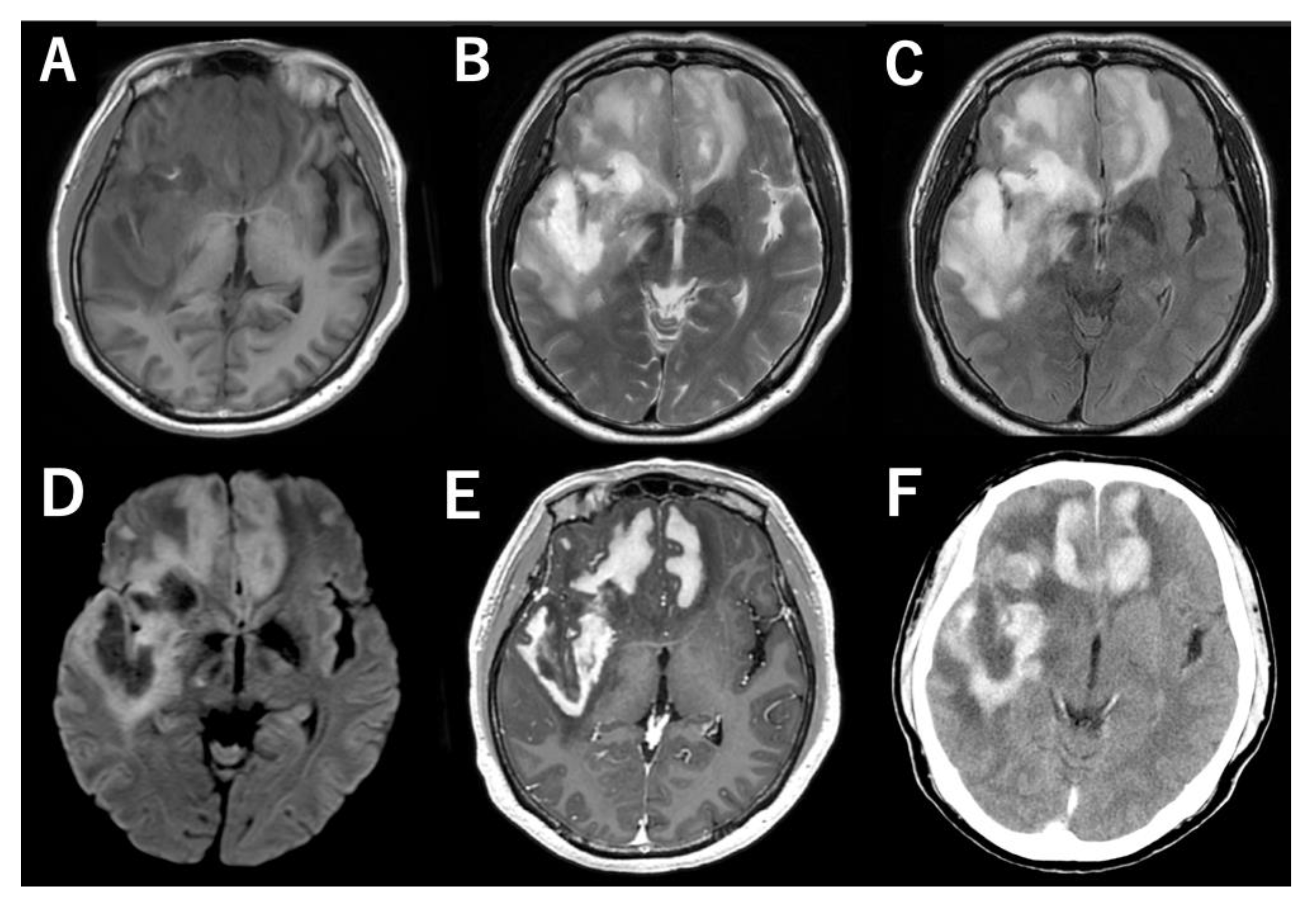
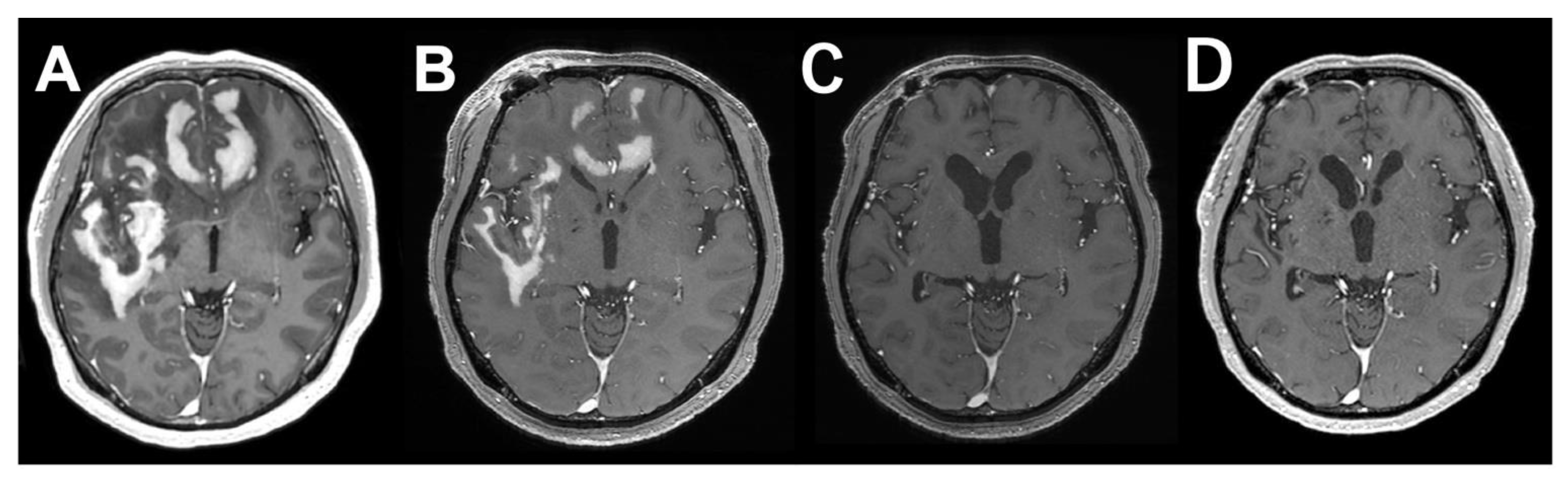
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Citterio, G.; Reni, M.; Gatta, G.; Ferreri, A.J.M. Primary central nervous system lymphoma. Crit. Rev. Oncol. 2017, 113, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Grommes, C. Updates on Primary Central Nervous System Lymphoma. Curr. Oncol. Rep. 2018, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Ogura, R.; Tsukamoto, Y.; Okada, M.; Natsumeda, M.; Isogawa, M.; Yoshida, S.; Fujii, Y. Advantages of Dose-dense Methotrexate Protocol for Primary Central Nervous System Lymphoma: Comparison of Two Different Protocols at a Single Institution. Neurol. Med. Chir. 2013, 53, 797–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Wang, X.; Tian, J.; Wang, Z. Renal Function and Plasma Methotrexate Concentrations Predict Toxicities in Adults Receiving High-Dose Methotrexate. Med. Sci. Monit. 2018, 24, 7719–7726. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Altman, A.; Pui, C.-H.; Younes, A.; Cairo, M.S. Guidelines for the Management of Pediatric and Adult Tumor Lysis Syndrome: An Evidence-Based Review. J. Clin. Oncol. 2008, 26, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A.; Panel, O.B.O.T.T.E. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: An expert TLS panel consensus. Br. J. Haematol. 2010, 149, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Zhou, Y.; Pauley, J.L.; Howard, S.C.; Jeha, S.; Relling, M.V.; Pui, C.-H. Effect of allopurinol versus urate oxidase on methotrexate pharmacokinetics in children with newly diagnosed acute lymphoblastic leukemia. Cancer 2010, 116, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Alakel, N.; Middeke, J.M.; Schetelig, J.; Bornhäuser, M. Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. Onco Targets Ther. 2017, 10, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.D.; Khosravan, R.; Vernillet, L.; Wu, J.-T.; Joseph-Ridge, N.; Mulford, D.J. Pharmacokinetics and Pharmacodynamics of Febuxostat, a New Non-purine Selective Inhibitor of Xanthine Oxidase in Subjects with Renal Impairment. Am. J. Ther. 2005, 12, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Kontzoglou, K.; Psyrri, A.; Pergialiotis, V. Febuxostat administration for the prevention of tumour lysis syndrome: A meta-analysis. J. Clin. Pharm. Ther. 2019, 44, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pinder, E.M.; Atwal, G.S.S.; Ayantunde, A.A.; Khan, S.; Sokal, M.; McCulloch, T.; Parsons, S.L. Tumour Lysis Syndrome Occurring in a Patient with Metastatic Gastrointestinal Stromal Tumour Treated with Glivec (Imatinib Mesylate, Gleevec, STI571). Sarcoma 2007, 2007, 82012. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, H.-S. A case of gouty arthritis following percutaneous radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Zoppoli, G.; Regairaz, M.; Leo, E.; Reinhold, W.C.; Varma, S.; Ballestrero, A.; Doroshow, J.H.; Pommier, Y. Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc. Natl. Acad. Sci. USA 2012, 109, 15030–15035. [Google Scholar] [CrossRef] [PubMed]
- Moribe, F.; Nishikori, M.; Sasanuma, H.; Akagawa, R.; Arima, H.; Takeda, S.; Takaori-Kondo, A.; Murai, J. Epigenetic suppression of SLFN11 in germinal center B cells in the process of the dynamic expression change during B-cell development. bioRxiv 2020, 228650. [Google Scholar] [CrossRef]
- Moribe, F.; Nishikori, M.; Takashima, T.; Taniyama, D.; Onishi, N.; Arima, H.; Sasanuma, H.; Akagawa, R.; Elloumi, F.; Takeda, S.; et al. Epigenetic suppression of SLFN11 in germinal center B-cells during B-cell development. PLoS ONE 2021, 16, e0237554. [Google Scholar] [CrossRef] [PubMed]
- Canet, E.; Zafrani, L.; Lambert, J.; Thieblemont, C.; Galicier, L.; Schnell, D.; Raffoux, E.; Lengline, E.; Chevret, S.; Darmon, M.; et al. Acute Kidney Injury in Patients with Newly Diagnosed High-Grade Hematological Malignancies: Impact on Remission and Survival. PLoS ONE 2013, 8, e55870. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouri, Y.; Natsumeda, M.; Okubo, N.; Sato, T.; Saito, T.; Shibuya, K.; Yamada, S.; On, J.; Tsukamoto, Y.; Okada, M.; et al. Successful Treatment of Acute Uric Acid Nephropathy with Rasburicase in a Primary Central Nervous System Lymphoma Patient Showing a Dramatic Response to Methotrexate—Case Report. J. Clin. Med. 2022, 11, 5548. https://doi.org/10.3390/jcm11195548
Mouri Y, Natsumeda M, Okubo N, Sato T, Saito T, Shibuya K, Yamada S, On J, Tsukamoto Y, Okada M, et al. Successful Treatment of Acute Uric Acid Nephropathy with Rasburicase in a Primary Central Nervous System Lymphoma Patient Showing a Dramatic Response to Methotrexate—Case Report. Journal of Clinical Medicine. 2022; 11(19):5548. https://doi.org/10.3390/jcm11195548
Chicago/Turabian StyleMouri, Yoshihiro, Manabu Natsumeda, Noritaka Okubo, Taro Sato, Taiki Saito, Kohei Shibuya, Shiori Yamada, Jotaro On, Yoshihiro Tsukamoto, Masayasu Okada, and et al. 2022. "Successful Treatment of Acute Uric Acid Nephropathy with Rasburicase in a Primary Central Nervous System Lymphoma Patient Showing a Dramatic Response to Methotrexate—Case Report" Journal of Clinical Medicine 11, no. 19: 5548. https://doi.org/10.3390/jcm11195548
APA StyleMouri, Y., Natsumeda, M., Okubo, N., Sato, T., Saito, T., Shibuya, K., Yamada, S., On, J., Tsukamoto, Y., Okada, M., Oishi, M., Eda, T., Murai, J., Shimizu, H., Kakita, A., & Fujii, Y. (2022). Successful Treatment of Acute Uric Acid Nephropathy with Rasburicase in a Primary Central Nervous System Lymphoma Patient Showing a Dramatic Response to Methotrexate—Case Report. Journal of Clinical Medicine, 11(19), 5548. https://doi.org/10.3390/jcm11195548






