Abstract
Background: Most of the drugs approved and registered for use in heart failure (HF) therapy were examined in randomized clinical trials (RCTs) with the primary composite endpoint of death or hospital readmission. This study aimed to analyze the rates of the newly calculated event: death without prior hospital readmission, in HFrEF patients in large RCTs to show that the newly defined endpoint probably delivers additional data on the structure of the composite endpoint and helps to interpret the results of interventional studies. Methods: This study included RCTs on therapeutic interventions in HF patients. A literature search was performed, and 31 trials in which death without hospital admission could be calculated were included in the analyses. The death without a prior hospital admission endpoint was calculated as the difference between the composite endpoint rate (death or hospital readmission) and the readmission rate. The differences in the new endpoint between the study groups were calculated. Result: The death rates without prior hospital admission were lower in the intervention groups in five trials. In the SENIORS study, significant differences were found in the primary (composite) and death without previous hospital admission endpoints. In the ACCLAIM, VEST, and GISSI-HF STATIN trials, death without previous hospital admission was the only endpoint with a significant difference between the study groups. Moreover, the new endpoint rates were higher in the intervention group in the latter two studies. Conclusions: The new endpoint describing patients who died without prior hospital admission might be useful in previous and future interventional studies to provide additional data on the structure of the composite endpoint. Some therapies might reduce death without previous hospital admission rates, which could be beneficial, even without a reduction in overall long-term mortality.
1. Introduction
Poor outcomes characterize heart failure with reduced ejection fraction (HFrEF). The one-year all-cause mortality may exceed 20% while rates of one-year hospital readmission for heart failure and any cause reach 17% and 48%, respectively [1]. Modern drug and device therapies may improve prognosis, but even optimal medical treatment (OMT) does not provide satisfying results. Most of the drugs recommended for HFrEF patients were examined in large randomized clinical trials (RCTs), mainly with mortality and hospital readmission endpoints. The composite endpoint of mortality and hospital readmission assumes that both events worsen prognosis and should be avoided [2]. However, acute HF should be considered an urgent indication for hospital admission to provide adequate treatment and improve prognosis [3]. It was proven that an excessive reduction in hospital readmission might increase mortality in HFrEF patients [4]. For this reason, not all hospitalizations in HF should be avoided. The endpoint describing overall mortality rates includes patients who might have hospital readmissions before death. However, there is a group of HF patients who died without hospital admission in the whole follow-up. It may be calculated as a difference between the composite endpoint rate (death or hospital readmission) and hospital readmission rate. In our opinion, the results of some interventional trials have not been fully explored and described. Large clinical trials are designed to examine the primary endpoint. However, both positive and negative studies might require a more detailed outcomes analysis, as some therapies might reduce the death rate without previous hospital admission, which could be beneficial, even without reducing the overall long-term mortality. On the other hand, reducing hospital readmissions might not be beneficial when it is associated with high death rates without prior hospital admission. Therefore, we aimed to analyze the rates of the newly calculated event (death without prior hospital readmission) in HFrEF patients in large RCTs to show that the newly defined endpoint might deliver additional data on the structure of the composite endpoint and helps to interpret the results of interventional studies.
2. Methods
This study included RCTs on drugs, non-drug interventions, and device therapies in patients with HFrEF. As some trials were designed before the current definition of HFrEF, the inclusion criteria regarding the left ventricular ejection fraction (LVEF) were not the same. Thus, all trials with decreased LVEF, regardless of the cut-off threshold, were included.
A literature search was performed in September and October 2020 to select RCTs fulfilling the eligibility criteria. Two authors searched the MEDLINE database independently to screen titles and abstracts using the predefined protocol with the search query:
“(heart failure [Title]) AND ((death [Title/Abstract]) or (mortality [Title/Abstract]) or (composite endpoint [Title/Abstract]))”.
The search results included 19,157 studies evaluated according to the flowchart (Figure 1).
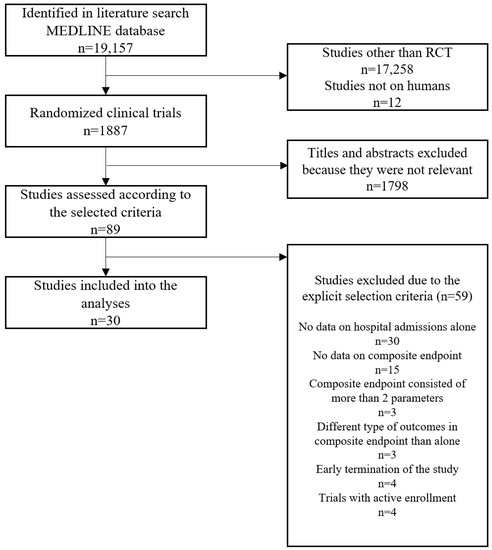
Figure 1.
The summary of the evidence search and selection—flowchart. RCT–randomized clinical trial.
This study included RCTs that enrolled patients with HF/HF with decreased EF with drug or non-drug intervention or device therapy with the following endpoints: composite endpoint consisting of death or hospital admission and hospital admission; cause of hospital admission had to be the same as in the composite endpoint (e.g., all-cause death or hospital admission due to HF; hospital admission due to HF).
The analysis excluded trials with follow-up shorter than 30 days or trials discontinued early or suspended or with more than two parameters in the composite endpoint (in such a case, there is no possibility of calculating the rate of deaths without a rehospitalization event), or trials enrolling patients with asymptomatic left ventricular dysfunction instead of HF.
All disagreements were referred to as the third researcher, who made a final decision (and did not fulfill the criteria for being an author).
The full texts of all selected articles were obtained. The data was extracted to the five predefined templates:
- The list of included HF trials contains the trial name, study drug/device/intervention, year of publication, enrolment dates, number of participants, follow-up, cause of death, and cause of hospital admission
- The characteristics of patients included in the analyzed HF trials, including age, sex, HF etiology, HF duration, percent NYHA III/IV class, LVEF, heart rate, percent of implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy with defibrillator (CRT-D), diuretics, beta-blockers, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blockers (ARBs)/angiotensin receptor-neprilysin inhibitor (ARNI), and mineralocorticoid receptor antagonist (MRA) use.
- Tables with different endpoints’ combinations with trial name, study groups, follow-up, composite endpoint rates, death, and hospital admission. Based on the extracted data on events: death or hospitalization and death alone, the new endpoint—death without rehospitalization—was calculated as the difference between the two events (Figure 2). Depending on the combinations of the causes of death and hospital admissions, the following endpoints were calculated (Supplementary Tables S2–S8):
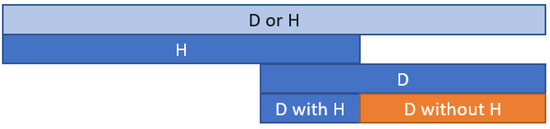 Figure 2. The death calculation method diagram without hospitalization (D without H) is based on the composite endpoint death or hospitalization (D or H). D—death; H—hospitalization.
Figure 2. The death calculation method diagram without hospitalization (D without H) is based on the composite endpoint death or hospitalization (D or H). D—death; H—hospitalization.- Death from CV causes without hospital admission for HF/worsening HF;
- Death for any reason without hospital admission;
- Death for any reason without CV hospital admission;
- Death for any reason without CV hospital admission;
- Death for any reason without any hospital admission;
- Death for CV reason without any hospital admission;
- Death for HF worsening without any hospital admission for HF worsening.
As no raw data is available regarding the mortality and follow-up (time to death), the difference between the death without hospital readmission rates was calculated using a 2 × 2 chi-square test (Supplementary Tables S2–S8).
3. Results
The analysis included 31 trials, of which 18 were progressive-positive and 13 progressive-negative trials presented in Table 1. The baseline characteristics and treatment of patients in selected trials are shown in Table S1.

Table 1.
The characteristics of trials included in the analysis.
The detailed rates of events are presented in Tables S2–S8.
The number of trials and death rates without previous hospital admission, depending on their cause, are presented in Table 2.

Table 2.
Summary of the analyses of death without previous hospital admission.
The detailed data on the endpoint rates in trials with a significant difference between groups regarding the death without hospital admission endpoint is presented in Table 3. In five trials, better outcomes in the intervention group were described using the new endpoint. In the PARADIGM-HF trial, the reduction of CV death without previous HF hospitalization was observed while in the MERIT-HF study, the intervention reduced all-cause death without prior HF hospitalization. In EPHESUS and SENIOR trials, a reduction in CV death without previous CV hospitalization was achieved. Moreover, in the EPHESUS study, a reduction in all-cause mortality without previous hospitalization for any reason was observed. In the ACCLAIM trial, death without previous hospital admission was the only endpoint with a significant difference between the study groups. In VEST and GISSI-HF STATIN trials, which did not show any essential differences between study groups in conventional endpoints, the death rates without previous hospital admission were higher in the intervention group (Table 3).

Table 3.
Trials with a significant difference between groups regarding death without hospital.
4. Discussion
Death without previous hospital admission is an easy to calculate endpoint in clinical trials as a difference between the rate of a composite endpoint of death or hospital admission and the rehospitalization rate. Depending on the cause of death and hospital admission in the composite endpoint, death rates without previous hospital admission may have different clinical interpretations and significance (Table 2). For example, deaths due to HF without HF hospitalization represent HF decompensation events without admission to the hospital. For the highest rates, greater hospitalization due to HF occurred, resulting in HF death. This parameter may indicate the quality of care and probably inappropriate HF treatment without needful hospital admission. The most prevalent (17 studies) composite endpoint of all-cause death without HF hospitalization represents all deaths without previous HF hospitalization. It also includes deaths not associated with HF or CV diseases, which may dilute the study intervention’s real effect on events. For that reason, it may have less clinical significance in terms of HF treatment effectiveness. However, a significant reduction in CV deaths is expected to influence all-cause mortality. Moreover, the difference between all-cause and cardiac-specific endpoints may be interpreted as a measure of significant adverse events [36].
Seven studies showed significant differences between the trial groups regarding death rates without prior hospital admission. In the PARADIGM-HF trial, a reduction in CV death without prior HF hospitalization was observed. Assuming that all acute HF cases were hospitalized, it may suggest that the trial drug reduced the number of CV deaths due to non-HF reasons or reduced the number of sudden cardiac deaths. In the MERIT-HF study, the intervention reduced all-cause death without prior HF hospitalization, suggesting the study drug’s positive impact on deaths other than HF-caused deaths. In EPHESUS and SENIOR trials, reduced CV mortality without previous CV hospitalization was achieved, which may describe the sudden cardiac death or death in the terminal phase of the disease when hospitalization might be intentionally avoided. Patients with CV diseases and a substantial risk of CV death are supposed to be hospitalized before death. In the SENIORS trial, the rates of CV death and separate hospital admission due to CV reasons did not differ between groups, and the only significant differences were found in the primary (composite) and death without previous hospital admission endpoints. Thus, the whole trial’s effect was probably driven by the differences in death rates without prior hospital admission.
In the ACCLAIM trial, death without previous hospital admission was the only endpoint with a significant difference between the study groups, as immune therapy reduced mortality without previous hospital admission with no effect on conventional endpoints. Using the new endpoint, the trial conclusions could influence future scientific directions regarding immune therapy in HF and possibly clinical recommendations. In another two trials without positive results in HF patients (VEST and GISSI-HF STATIN), death without previous hospital admission was the only endpoint with a significant difference between the study groups. Moreover, in both studies, the death rates without previous hospital admission were higher in the intervention group, meaning that patients in the placebo groups died more often in the hospital than in the intervention groups. It might be indirect proof of higher rates of sudden cardiac death in the intervention groups, as there is no reason for higher hospital mortality rates in patients in the placebo groups. It could also explain why the primary trial endpoints did not reach statistical significance (GISSI-STATIN and VEST trials).
Deaths without previous HF hospitalization may be a valuable clinical outcome and RCT event. However, contrary to hospital admissions due to myocardial infarction, not all HF hospitalizations are urgent. Hospital admissions due to HF may include acute (worsening) and non-acute hospitalizations (e.g., CIED implantations), leading to wrong conclusions. There is a clinical difference between hospitalization due to HF and worsening HF. Unfortunately, no different ICD-10 codes are available for chronic and acute HF provided for chronic and acute coronary syndromes. Thus, differentiation between urgent and non-urgent HF hospitalization may be difficult or impossible in retrospective studies. Moreover, in studies on myocardial infarction, non-fatal myocardial infarction was widely used as an endpoint and an element of a composite endpoint of death or non-fatal myocardial infarction. Such an endpoint probably cannot be used in HF, as it may be difficult to distinguish between a non-fatal acute HF and non-fatal chronic HF hospitalization.
In real-life settings, excessive reduction in HF rehospitalizations may increase mortality. Such an observation was made in the USA’s Medicare population, where hospitals with the worst hospital readmission rates were penalized. Consequently, the reduction in hospital admissions and economic profits was obscured by the higher mortality, as patients died outside the hospital [4]. Moreover, the endpoint “non-fatal HF hospital readmission” implies the assumption that fatal and non-fatal events are different regarding the clinical or pathophysiological mechanism [37].
On the other hand, the composite endpoint “death or non-fatal hospital readmission” favors mortality over rehospitalization as the composite endpoint’s element compared to the “death or hospital readmission”. According to the composite endpoint definition, the first registered event of a composite endpoint is considered the endpoint. In consequence, when death occurs during the hospital stay, the first endpoint (“death or non-fatal hospital readmission”) will be counted as death and the second (“death or hospital readmission”) as hospital readmission. The second issue is a time-to-event difference, which is supposed to be longer in the “death or non-fatal hospital readmission” endpoint, as patients who die in a hospital will be censored later. According to the Heart Failure Association of ESC statement, the preferred outcome in terms of mortality is cardiovascular mortality [36]. Among 30 analyzed trials, only 13 included CV mortality, which raises a question about the clinical interpretation and significance of trial outcomes.
In our opinion, the newly defined endpoint may have two practical applications. Our study showed that it could be easily calculated in most published trials. In such a case, it might explain the mechanism of death, as patients without rehospitalization before death most likely died of sudden cardiac death or were palliative patients who were not hospitalized and died at home. For that reason, it might help to interpret the outcomes of both positive and negative trials and potentially explain why some trials did not reach their goals in terms of the composite endpoints or hospital readmission. The conventional endpoints might not reveal all aspects of patient outcomes. Some therapies might reduce the death rate without previous hospital admission, which could be beneficial, even without reducing overall long-term mortality. Therefore, the second potential application of the newly defined endpoint may be in future clinical trials in different populations, including patients with heart failure, myocardial infarction, or after ICD/CRT-D implantation, to provide additional data on the structure of the composite endpoint. We do not intend to change or replace conventional hard endpoints with the newly defined endpoint as a primary endpoint but to deliver more detailed information on the outcome.
Our study has some limitations. Trials included in the analysis had different enrolment criteria and were conducted between 1986 and 2020, which affected individuals’ baseline characteristics, including drugs and device therapies (Supplementary Table S1). Moreover, follow-up varied between 1 and 57 months, which also influenced the rates of events. For these reasons, conducting a meta-analysis was methodologically impossible. The statistical methods for calculating the difference in death rates without previous hospital admission endpoints between groups were suboptimal. Unfortunately, the raw data on the trial events was unavailable, making it impossible to use optimal statistical tests. The most important limitation is that our retrospective calculation of the new endpoint rates cannot be interpreted as the prospective trial result, as all analyzed trials were designed for the endpoints used in their methodology, including calculating the power of statistical tests and sample size.
To sum up, the definition of endpoints in clinical trials plays a pivotal role in interpreting trial outcomes, affecting the clinical guidelines and recommendations. The new endpoint describing patients who died without prior hospital admission might be useful in previous and future interventional studies to provide additional data on the structure of the composite endpoint. Some therapies might reduce death without previous hospital admission rates, which could be beneficial, even without a reduction in overall long-term mortality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11195518/s1, Table S1. Supplement. Characteristics of patients group included in the heart failure trials–baseline characteristics and selected drugs. Table S2. The death from CV causes or HF hospitalization/HF worsening endpoint–death from CV causes without hospital admission for HF/worsening HF. Table S3. The all-cause death or HF hospitalization/HF worsening endpoint–death for any reason without hospital admission. Table S4. The all-cause death or CV hospitalization–death for any reason without CV hospital admission. Table S5. The CV death or CV hospitalization–death for any reason without CV hospital admission. Table S6. The all-cause death or all-cause hospitalization–death for any reason without any hospital admission. Table S7. The CV death or all-cause hospitalization–death for CV reason without any hospital admission. Table S8. The death related to HF/HF worsening or hospitalization due to HF/HF worsening–death for HF worsening without any hospital admission for HF worsening.
Author Contributions
Conceptualization, J.T.N.; Data curation, J.T.N.; Formal analysis, J.T.N.; Investigation, J.T.N.; Methodology, J.T.N.; Project administration, M.G.; Software, J.T.N.; Supervision, J.T.N. and M.G.; Validation, J.T.N.; Writing—original draft, J.T.N.; Writing—review & editing, J.T.N. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study reports data which is generally available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niedziela, J.T.; Parma, Z.; Pawlowski, T.; Rozentryt, P.; Gasior, M.; Wojakowski, W. Secular trends in first-time hospitalization for heart failure with following one-year readmission and mortality rates in the 3.8 million adult population of Silesia, Poland between 2010 and 2016. The SILCARD database. Int. J. Cardiol. 2018, 271, 146–151. [Google Scholar] [CrossRef]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for heart failure: Predict or prevent? Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Joynt Maddox, K.E.; Wasfy, J.H.; Haneuse, S.; Shen, C.; Yeh, R.W. Association of the Hospital Readmissions Reduction Program with Mortality among Medicare Beneficiaries Hospitalized for Heart Failure, Acute Myocardial Infarction, and Pneumonia. J. Am. Med. Assoc. 2018, 320, 2542–2552. [Google Scholar] [CrossRef]
- The SOLVD Investigators. Effect of Enalapril on Mortality and the Development of Heart Failure in Asymptomatic Patients with Reduced Left Ventricular Ejection Fractions. N. Engl. J. Med. 1992, 327, 685–691. [Google Scholar] [CrossRef]
- He, T.; Igitalis, D.; Nvestigation, I.; Roup, G. The Effect of Digoxin on Mortality and Morbidity in Patients with Heart Failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar] [CrossRef]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Granger, C.B.; McMurray, J.J.V.; Yusuf, S.; Held, P.; Michelson, E.L.; Olofsson, B.; Östergren, J.; Pfeffer, M.A.; Swedberg, K.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-alternative trial. Lancet 2003, 362, 772–776. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Östergren, J.; Swedberg, K.; Granger, C.B.; Held, P.; Michelson, E.L.; Olofsson, B.; Yusuf, S.; Pfeffer, M.A.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-added trial. Lancet 2003, 362, 767–771. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Young, J.B.; Dunlap, M.E.; Pfeffer, M.A.; Probstfield, J.L.; Cohen-Solal, A.; Dietz, R.; Granger, C.B.; Hradec, J.; Kuch, J.; McKelvie, R.S.; et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004, 110, 2618–2626. [Google Scholar] [CrossRef]
- Flather, M.D. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2004, 26, 215–225. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The effect of cardiac resynchronization on morbidity and mortality in heart failure: Comments. Indian Heart J. 2005, 57, 186. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and safety of exercise training in patients with chronic heart failure HF-ACTION randomized controlled trial. J. Am. Med. Assoc. 2009, 301, 1439–1450. [Google Scholar] [CrossRef]
- Konstam, M.A.; Neaton, J.D.; Dickstein, K.; Drexler, H.; Komajda, M.; Martinez, F.A.; Riegger, G.A.; Malbecq, W.; Smith, R.D.; Guptha, S.; et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet 2009, 374, 1840–1848. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.M., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Pitt, B.; Segal, R.; Martinez, F.A.; Meurers, G.; Cowley, A.J.; Thomas, I.; Deedwania, P.C.; Ney, D.E.; Snavely, D.B.; Chang, P.I.; et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997, 349, 747–752. [Google Scholar] [CrossRef]
- Cohn, J.N.; Goldstein, S.O.; Greenberg, B.H.; Lorell, B.H.; Bourge, R.C.; Jaski, B.E.; Gottlieb, S.O.; McGrew, F.; DeMets, D.L.; White, B.G. A Dose-Dependent Increase in Mortality with Vesnarinone among Patients with Severe Heart Failure. N. Engl. J. Med. 1998, 339, 1810–1816. [Google Scholar] [CrossRef]
- Pitt, B.; Poole-Wilson, P.A.; Segal, R.; Martinez, F.A.; Dickstein, K.; Camm, A.J.; Konstam, M.A.; Riegger, G.; Klinger, G.H.; Neaton, J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial—The Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef]
- GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- GISSI-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef]
- Torp-Pedersen, C.; Køber, L.; Carlsen, J.E.; Akkan, D.; Bruun, N.E.; Dacoronias, D.; Dickstein, K.; Haghfelt, T.; Öhlin, H.; McMurray, J.J. A randomised trial of a pre-synaptic stimulator of DA2-dopaminergic and α2-adrenergic receptors on morbidity and mortality in patients with heart failure. Eur. J. Heart Fail. 2008, 10, 89–95. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Anker, S.D.; Bourge, R.C.; Colucci, W.S.; Greenberg, B.H.; Hildebrandt, P.; Keren, A.; Motro, M.; Moyé, L.A.; Otterstad, J.E.; et al. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): A placebo-controlled randomised trial. Lancet 2008, 371, 228–236. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.; et al. Effect of Nesiritide in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J., III; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Swedberg, K.; Young, J.B.; Anand, I.S.; Cheng, S.; Desai, A.S.; Diaz, R.; Maggioni, A.P.; McMurray, J.J.; O’Connor, C.; Pfeffer, M.A.; et al. Treatment of Anemia with Darbepoetin Alfa in Systolic Heart Failure. N. Engl. J. Med. 2013, 368, 1210–1219. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Böhm, M.; Greene, S.J.; Fonarow, G.C.; Lewis, E.F.; Zannad, F.; Solomon, S.D.; Baschiera, F.; Botha, J.; Hua, T.A.; et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: The ASTRONAUT randomized trial. J. Am. Med. Assoc. 2013, 309, 1125–1135. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Krum, H.; Abraham, W.T.; Dickstein, K.; Køber, L.V.; Desai, A.S.; Solomon, S.D.; Greenlaw, N.; Ali, M.A.; Chiang, Y.; et al. Aliskiren, Enalapril, or Aliskiren and Enalapril in Heart Failure. N. Engl. J. Med. 2016, 374, 1521–1532. [Google Scholar] [CrossRef]
- Zannad, F.; Anker, S.D.; Byra, W.M.; Cleland, J.G.; Fu, M.; Gheorghiade, M.; Lam, C.S.; Mehra, M.R.; Neaton, J.D.; Nessel, C.C.; et al. Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N. Engl. J. Med. 2018, 379, 1332–1342. [Google Scholar] [CrossRef]
- Zannad, F.; Garcia, A.A.; Anker, S.D.; Armstrong, P.W.; Calvo, G.; Cleland, J.G.F.; Cohn, J.N.; Dickstein, K.; Domanski, M.J.; Ekman, I.; et al. Clinical outcome endpoints in heart failure trials: A European Society of Cardiology Heart Failure Association consensus document. Eur. J. Heart Fail. 2013, 15, 1082–1094. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Bigger, J.T.; McDermott, M.; Miller, J.P.; Moon, T.; Moss, A.J.; Oakes, D.; Rolnitzky, L.M.; Therneau, T.M. Nonfatal myocardial infarction is, by itself, an inappropriate end point in clinical trials in cardiology. Circulation 1990, 81, 684–685. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).