Nephrolithiasis: A Red Flag for Cardiovascular Risk
Abstract
1. Introduction
2. Nephrolithiasis and Cardiovascular Disease Are Associated: The Evidence
3. The Associated Conditions
3.1. Smoking
3.2. Hypertension
3.3. Diabetes Mellitus
3.4. Obesity
3.5. Dyslipidaemia
3.6. Metabolic Syndrome
4. The Logical Connection between Nephrolithiasis and CV Disease
5. Osteoporosis and the ‘Calcification Paradox’: The Missing Link between Nephrolithiasis and CV Disease?
6. Other Hypothesis Linking Nutrition, Nephrolithiasis and CV Disease
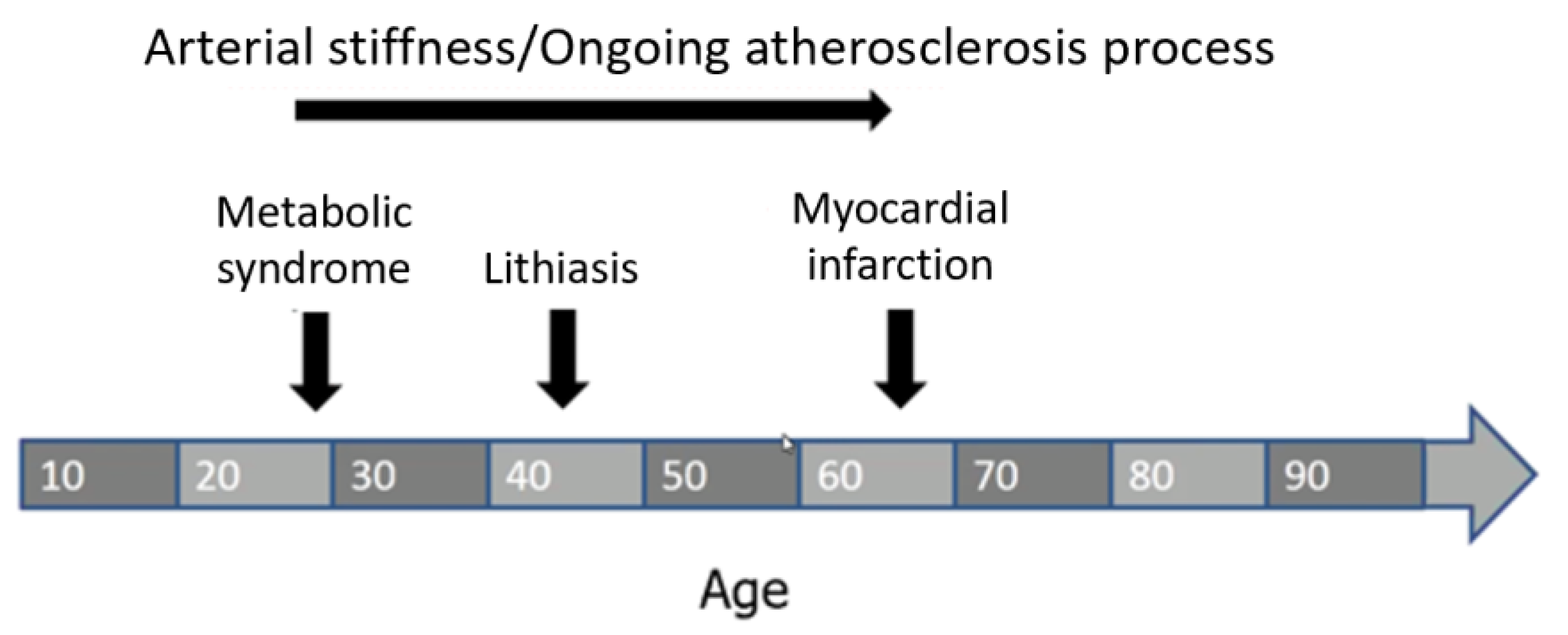
7. Practical Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geraghty, R.M.; Jones, P.; Somani, B.K. Worldwide Trends of Urinary Stone Disease Treatment Over the Last Two Decades: A Systematic Review. J. Endourol. 2017, 31, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Xu, T.; Cao, C.; Waldhoer, T.; Seitz, C.; D’Andrea, D.; Siyam, A.; Tarawneh, R.; Fajkovic, H.; Schernhammer, E.; et al. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007–2018 Data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Obesity, Weight Gain, and the Risk of Kidney Stones. JAMA 2005, 293, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Diabetes Mellitus and the Risk of Nephrolithiasis. Kidney Int. 2005, 68, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Luke, A.; Durazo-Arvizu, R.A.; Cao, G.; Shoham, D.; Kramer, H. Metabolic Syndrome and Self-Reported History of Kidney Stones: The National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am. J. Kidney Dis. 2008, 51, 741–747. [Google Scholar] [CrossRef]
- Rendina, D.; Mossetti, G.; De Filippo, G.; Benvenuto, D.; Vivona, C.L.; Imbroinise, A.; Zampa, G.; Ricchio, S.; Strazzullo, P. Association between Metabolic Syndrome and Nephrolithiasis in an Inpatient Population in Southern Italy: Role of Gender, Hypertension and Abdominal Obesity. Nephrol. Dial. Transplant. 2009, 24, 900–906. [Google Scholar] [CrossRef]
- Jeong, I.G.; Kang, T.; Bang, J.K.; Park, J.; Kim, W.; Hwang, S.S.; Kim, H.K.; Park, H.K. Association between Metabolic Syndrome and the Presence of Kidney Stones in a Screened Population. Am. J. Kidney Dis. 2011, 58, 383–388. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Penniston, K.L.; Antonelli, J.A.; Viprakasit, D.P.; Averch, T.D.; Sivalingam, S.; Sur, R.L.; Pais, V.M.; Chew, B.H.; Bird, V.G.; Nakada, S.Y. Validation and Reliability of the Wisconsin Stone Quality of Life Questionnaire. J. Urol. 2017, 197, 1280–1288. [Google Scholar] [CrossRef]
- Patel, N.; Brown, R.D.; Sarkissian, C.; De, S.; Monga, M. Quality of Life and Urolithiasis: The Patient—Reported Outcomes Measurement Information System (PROMIS). Int. Braz. J. Urol. 2017, 43, 880–886. [Google Scholar] [CrossRef]
- Gambaro, G.; Croppi, E.; Bushinsky, D.; Jaeger, P.; Cupisti, A.; Ticinesi, A.; Mazzaferro, S.; D’Addessi, A.; Ferraro, P.M. The Risk of Chronic Kidney Disease Associated with Urolithiasis and Its Urological Treatments: A Review. J. Urol. 2017, 198, 268–273. [Google Scholar] [CrossRef]
- Melton, L.J.; Crowson, C.S.; Khosla, S.; Wilson, D.M.; O’Fallon, W.M. Fracture Risk among Patients with Urolithiasis: A Population-Based Cohort Study. Kidney Int. 1998, 53, 459–464. [Google Scholar] [CrossRef]
- Taylor, E.N.; Feskanich, D.; Paik, J.M.; Curhan, G.C. Nephrolithiasis and Risk of Incident Bone Fracture. J. Urol. 2016, 195, 1482–1486. [Google Scholar] [CrossRef]
- Peng, J.-P.; Zheng, H. Kidney Stones May Increase the Risk of Coronary Heart Disease and Stroke: A PRISMA-Compliant Meta-Analysis. Medicine 2017, 96, e7898. [Google Scholar] [CrossRef]
- Chung, S.-D.; Liu, S.-P.; Keller, J.J.; Lin, H.-C. Urinary Calculi and an Increased Risk of Stroke: A Population-Based Follow-up Study. BJU Int. 2012, 110, E1053–E1059. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, C.M.; Bang, W.; Lim, J.-S.; Park, B.; Choi, H.G. Nephrolithiasis Predicts Ischemic Stroke: A Longitudinal Follow-up Study Using a National Sample Cohort. Int. J. Med. Sci. 2019, 16, 1050–1056. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Eisner, B.H.; Gambaro, G.; Rimm, E.B.; Mukamal, K.J.; Curhan, G.C. History of Kidney Stones and the Risk of Coronary Heart Disease. JAMA 2013, 310, 408–415. [Google Scholar] [CrossRef]
- Alexander, R.T.; Hemmelgarn, B.R.; Wiebe, N.; Bello, A.; Samuel, S.; Klarenbach, S.W.; Curhan, G.C.; Tonelli, M.; Alberta Kidney Disease Network. Kidney Stones and Cardiovascular Events: A Cohort Study. Clin. J. Am. Soc. Nephrol. 2014, 9, 506–512. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Lin, C.-L.; Chang, Y.-J.; Hsu, W.-H.; Lin, C.-C.; Wang, I.-K.; Chang, C.-T.; Chang, C.-H.; Lin, M.-C.; Kao, C.-H. Association Between Kidney Stones and Risk of Stroke: A Nationwide Population-Based Cohort Study. Medicine 2016, 95, e2847. [Google Scholar] [CrossRef]
- Reiner, A.P.; Kahn, A.; Eisner, B.H.; Pletcher, M.J.; Sadetsky, N.; Williams, O.D.; Polak, J.F.; Jacobs, D.R.; Stoller, M.L. Kidney Stones and Subclinical Atherosclerosis in Young Adults: The CARDIA Study. J. Urol. 2011, 185, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chang, Y.; Sung, E.; Kang, J.G.; Yun, K.E.; Jung, H.-S.; Hyun, Y.Y.; Lee, K.-B.; Joo, K.J.; Shin, H.; et al. Association Between Sonographically Diagnosed Nephrolithiasis and Subclinical Coronary Artery Calcification in Adults. Am. J. Kidney Dis. 2018, 71, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Ke, H.-L.; Huang, J.-C.; Huang, S.-P.; Geng, J.-H. Obesity-Related Indices and Its Association with Kidney Stone Disease: A Cross-Sectional and Longitudinal Cohort Study. Urolithiasis 2022, 50, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive Oxygen Species, Inflammation and Calcium Oxalate Nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar] [CrossRef]
- Staessen, J.; Amery, A.; Bernard, A.; Bruaux, P.; Buchet, J.P.; Claeys, F.; De Plaen, P.; Ducoffre, G.; Fagard, R.; Lauwerys, R.R. Effects of Exposure to Cadmium on Calcium Metabolism: A Population Study. Br. J. Ind. Med. 1991, 48, 710–714. [Google Scholar] [CrossRef][Green Version]
- Ferraro, P.M.; Bonello, M.; Frigo, A.C.; D’Addessi, A.; Sturniolo, A.; Gambaro, G. Cadmium Exposure and Kidney Stone Formation in the General Population—An Analysis of the National Health and Nutrition Examination Survey III Data. J. Endourol. 2011, 25, 875–880. [Google Scholar] [CrossRef]
- Shang, W.; Li, Y.; Ren, Y.; Yang, Y.; Li, H.; Dong, J. Nephrolithiasis and Risk of Hypertension: A Meta-Analysis of Observational Studies. BMC Nephrol. 2017, 18, 344. [Google Scholar] [CrossRef]
- Kittanamongkolchai, W.; Mara, K.C.; Mehta, R.A.; Vaughan, L.E.; Denic, A.; Knoedler, J.J.; Enders, F.T.; Lieske, J.C.; Rule, A.D. Risk of Hypertension among First-Time Symptomatic Kidney Stone Formers. Clin. J. Am. Soc. Nephrol. 2017, 12, 476–482. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Siani, A.; Barba, G.; Mellone, M.C.; Russo, L.; Farinaro, E.; Trevisan, M.; Mancini, M.; Strazzullo, P. A Prospective Study of Hypertension and the Incidence of Kidney Stones in Men. J. Hypertens. 1999, 17, 1017–1022. [Google Scholar] [CrossRef]
- Borghi, L.; Meschi, T.; Guerra, A.; Briganti, A.; Schianchi, T.; Allegri, F.; Novarini, A. Essential Arterial Hypertension and Stone Disease. Kidney Int. 1999, 55, 2397–2406. [Google Scholar] [CrossRef]
- Madore, F.; Stampfer, M.J.; Rimm, E.B.; Curhan, G.C. Nephrolithiasis and Risk of Hypertension. Am. J. Hypertens. 1998, 11, 46–53. [Google Scholar] [CrossRef]
- Madore, F.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Curhan, G.C. Nephrolithiasis and Risk of Hypertension in Women. Am. J. Kidney Dis. 1998, 32, 802–807. [Google Scholar] [CrossRef]
- Young, E.W.; Morris, C.D.; McCarron, D.A. Urinary Calcium Excretion in Essential Hypertension. J. Lab. Clin. Med. 1992, 120, 624–632. [Google Scholar]
- Eisner, B.H.; Porten, S.P.; Bechis, S.K.; Stoller, M.L. Hypertension Is Associated with Increased Urinary Calcium Excretion in Patients with Nephrolithiasis. J. Urol. 2010, 183, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Honey, R.J.D.A.; McLaughlin, J.M.; Bull, S.B.; Logan, A.G. High Urinary Calcium Excretion and Genetic Susceptibility to Hypertension and Kidney Stone Disease. J. Am. Soc. Nephrol. 2006, 17, 2567–2575. [Google Scholar] [CrossRef]
- Van Hooft, I.M.; Grobbee, D.E.; Frölich, M.; Pols, H.A.; Hofman, A. Alterations in Calcium Metabolism in Young People at Risk for Primary Hypertension. The Dutch Hypertension and Offspring Study. Hypertension 1993, 21, 267–272. [Google Scholar] [CrossRef]
- Hartman, C.; Friedlander, J.I.; Moreira, D.M.; Leavitt, D.A.; Hoenig, D.M.; Smith, A.D.; Okeke, Z. Does Hypertension Impact 24-Hour Urine Parameters in Patients with Nephrolithiasis? Urology 2015, 85, 539–543. [Google Scholar] [CrossRef]
- Khan, S.R. Hyperoxaluria-Induced Oxidative Stress and Antioxidants for Renal Protection. Urol. Res. 2005, 33, 349–357. [Google Scholar] [CrossRef]
- Knoll, T.; Steidler, A.; Trojan, L.; Sagi, S.; Schaaf, A.; Yard, B.; Michel, M.S.; Alken, P. The Influence of Oxalate on Renal Epithelial and Interstitial Cells. Urol. Res. 2004, 32, 304–309. [Google Scholar] [CrossRef]
- Baggio, B.; Gambaro, G.; Ossi, E.; Favaro, S.; Borsatti, A. Increased Urinary Excretion of Renal Enzymes in Idiopathic Calcium Oxalate Nephrolithiasis. J. Urol. 1983, 129, 1161–1162. [Google Scholar] [CrossRef]
- Boonla, C.; Wunsuwan, R.; Tungsanga, K.; Tosukhowong, P. Urinary 8-Hydroxydeoxyguanosine is Elevated in Patients with Nephrolithiasis. Urol. Res. 2007, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, E.C.; Lieske, J.C.; Vrtiska, T.J.; Krambeck, A.E.; Li, X.; Bergstralh, E.J.; Melton, L.J.; Rule, A.D. Clinical Characteristics of Potential Kidney Donors with Asymptomatic Kidney Stones. Nephrol. Dial. Transplant. 2011, 26, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.L.; Low, R.K.; Shami, G.S.; McCormick, V.D.; Kerschmann, R.L. High Resolution Radiography of Cadaveric Kidneys: Unraveling the Mystery of Randall’s Plaque Formation. J. Urol. 1996, 156, 1263–1266. [Google Scholar] [CrossRef]
- Rahman, I.A.; Nusaly, I.F.; Syahrir, S.; Nusaly, H.; Mansyur, M.A. Association between Metabolic Syndrome Components and the Risk of Developing Nephrolithiasis: A Systematic Review and Bayesian Meta-Analysis. F1000Research 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-D.; Chen, Y.-K.; Lin, H.-C. Increased Risk of Diabetes in Patients with Urinary Calculi: A 5-Year Followup Study. J. Urol. 2011, 186, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.H.; Wu, J.S.; Yang, Y.C.; Sun, Z.J.; Chang, C.J. The Effect of Glycemic Status on Kidney Stone Disease in Patients with Prediabetes. Diabetes Metab. J. 2016, 40, 161–166. [Google Scholar] [CrossRef][Green Version]
- Geraghty, R.; Abdi, A.; Somani, B.; Cook, P.; Roderick, P. Does Chronic Hyperglycaemia Increase the Risk of Kidney Stone Disease? Results from a Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e032094. [Google Scholar] [CrossRef] [PubMed]
- Spatola, L.; Ferraro, P.M.; Gambaro, G.; Badalamenti, S.; Dauriz, M. Metabolic Syndrome and Uric Acid Nephrolithiasis: Insulin Resistance in Focus. Metabolism 2018, 83, 225–233. [Google Scholar] [CrossRef]
- Bagnasco, S.M.; Gaydos, D.S.; Risquez, A.; Preuss, H.G. The Regulation of Renal Ammoniagenesis in the Rat by Extracellular Factors. III. Effects of Various Fuels on in Vitro Ammoniagenesis. Metabolism 1983, 32, 900–905. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Dubree, M.; Zhang, J.; McLeroy, P.; Moe, O.W. Effect of Renal Lipid Accumulation on Proximal Tubule Na+/H+ Exchange and Ammonium Secretion. Am. J. Physiol. Renal. Physiol. 2008, 294, F1315–F1322. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M. Metabolic Syndrome and Uric Acid Nephrolithiasis. Semin. Nephrol. 2008, 28, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 Diabetes Increases the Risk for Uric Acid Stones. J. Am. Soc. Nephrol. 2006, 17, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.T.; Jung, S.I.; Myung, S.C.; Kim, T.H. Correlation of Metabolic Syndrome with Urinary Stone Composition. Int. J. Urol. 2013, 20, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, S.; Murakami, T.; Uchikawa, T.; Ishikawa, S.E.; Saito, T. Effect of Glycemic Control on Calcium and Phosphorus Handling and Parathyroid Hormone Level in Patients with Non-Insulin-Dependent Diabetes Mellitus. Endocr. J. 1995, 42, 377–383. [Google Scholar] [CrossRef]
- Gotfredsen, A.; McNair, P.; Christiansen, C.; Transbøl, I. Renal Hypouricaemia in Insulin Treated Diabetes Mellitus. Clin. Chim. Acta 1982, 120, 355–361. [Google Scholar] [CrossRef]
- Eisner, B.H.; Porten, S.P.; Bechis, S.K.; Stoller, M.L. Diabetic Kidney Stone Formers Excrete More Oxalate and Have Lower Urine PH than Nondiabetic Stone Formers. J. Urol. 2010, 183, 2244–2248. [Google Scholar] [CrossRef] [PubMed]
- Semins, M.J.; Shore, A.D.; Makary, M.A.; Magnuson, T.; Johns, R.; Matlaga, B.R. The Association of Increasing Body Mass Index and Kidney Stone Disease. J. Urol. 2010, 183, 571–575. [Google Scholar] [CrossRef]
- Pigna, F.; Sakhaee, K.; Adams-Huet, B.; Maalouf, N.M. Body Fat Content and Distribution and Urinary Risk Factors for Nephrolithiasis. Clin. J. Am. Soc. Nephrol. 2014, 9, 159–165. [Google Scholar] [CrossRef]
- Choi, C.; Kim, J.K.; Han, K.; Lee, Y.G.; Han, J.H. Effect of Obesity and Metabolic Health on Urolithiasis: A Nationwide Population-Based Study. Investig. Clin. Urol. 2022, 63, 63–70. [Google Scholar] [CrossRef]
- Karelis, A.D.; Faraj, M.; Bastard, J.-P.; St-Pierre, D.H.; Brochu, M.; Prud’homme, D.; Rabasa-Lhoret, R. The Metabolically Healthy but Obese Individual Presents a Favorable Inflammation Profile. J. Clin. Endocrinol. Metab. 2005, 90, 4145–4150. [Google Scholar] [CrossRef]
- Seo, M.H.; Rhee, E.J. Metabolic and Cardiovascular Implications of a Metabolically Healthy Obesity Phenotype. Endocrinol. Metab. 2014, 29, 427–434. [Google Scholar] [CrossRef]
- Aung, K.; Lorenzo, C.; Hinojosa, M.A.; Haffner, S.M. Risk of Developing Diabetes and Cardiovascular Disease in Metabolically Unhealthy Normal-Weight and Metabolically Healthy Obese Individuals. J. Clin. Endocrinol. Metab. 2014, 99, 462–468. [Google Scholar] [CrossRef]
- Yang, H.K.; Han, K.; Kwon, H.-S.; Park, Y.-M.; Cho, J.-H.; Yoon, K.-H.; Kang, M.-I.; Cha, B.-Y.; Lee, S.-H. Obesity, Metabolic Health, and Mortality in Adults: A Nationwide Population-Based Study in Korea. Sci. Rep. 2016, 6, 30329. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-M.; Chou, Y.-H.; Li, C.-C.; Liu, C.-C.; Huang, S.-P.; Wu, W.-J.; Chen, C.-W.; Su, C.-Y.; Lee, M.-H.; Wei, Y.-C.; et al. Association of Body Mass Index and Urine PH in Patients with Urolithiasis. Urol. Res. 2009, 37, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Stoller, M.L.; Schwartz, B.F.; Kane, C.; Gentle, D.L.; Bruce, J.E.; Leslie, S.W. Impact of Body Weight on Urinary Electrolytes in Urinary Stone Formers. Urology 2000, 55, 825–830. [Google Scholar] [CrossRef]
- Lemann, J.; Pleuss, J.A.; Worcester, E.M.; Hornick, L.; Schrab, D.; Hoffmann, R.G. Urinary Oxalate Excretion Increases with Body Size and Decreases with Increasing Dietary Calcium Intake among Healthy Adults. Kidney Int. 1996, 49, 200–208. [Google Scholar] [CrossRef]
- Coe, F.L.; Strauss, A.L.; Tembe, V.; Le Dun, S. Uric Acid Saturation in Calcium Nephrolithiasis. Kidney Int. 1980, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K. Unraveling the Mechanisms of Obesity-Induced Hyperoxaluria. Kidney Int. 2018, 93, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Okada, A.; Hamamoto, S.; Iwatsuki, S.; Naiki, T.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. Proinflammatory and Metabolic Changes Facilitate Renal Crystal Deposition in an Obese Mouse Model of Metabolic Syndrome. J. Urol. 2015, 194, 1787–1796. [Google Scholar] [CrossRef]
- Amin, R.; Asplin, J.; Jung, D.; Bashir, M.; Alshaikh, A.; Ratakonda, S.; Sharma, S.; Jeon, S.; Granja, I.; Matern, D.; et al. Reduced Active Transcellular Intestinal Oxalate Secretion Contributes to the Pathogenesis of Obesity-Associated Hyperoxaluria. Kidney Int. 2018, 93, 1098–1107. [Google Scholar] [CrossRef]
- Torricelli, F.C.M.; De, S.K.; Gebreselassie, S.; Li, I.; Sarkissian, C.; Monga, M. Dyslipidemia and Kidney Stone Risk. J. Urol. 2014, 191, 667–672. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, X.; Mo, J.; Lin, H.-M.; Duan, Y.; Pu, M.; Wolbrette, D.L.; Liao, D. Metabolic Syndrome, Its Preeminent Clusters, Incident Coronary Heart Disease and All-Cause Mortality—Results of Prospective Analysis for the Atherosclerosis Risk in Communities Study. J. Intern. Med. 2007, 262, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.M.; Cook, P.; Roderick, P.; Somani, B. Risk of Metabolic Syndrome in Kidney Stone Formers: A Comparative Cohort Study with a Median Follow-Up of 19 Years. J. Clin. Med. 2021, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Kohjimoto, Y.; Sasaki, Y.; Iguchi, M.; Matsumura, N.; Inagaki, T.; Hara, I. Association of Metabolic Syndrome Traits and Severity of Kidney Stones: Results from a Nationwide Survey on Urolithiasis in Japan. Am. J. Kidney Dis. 2013, 61, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, N.M. Metabolic Syndrome and the Genesis of Uric Acid Stones. J. Ren. Nutr. 2011, 21, 128–131. [Google Scholar] [CrossRef]
- Abate, N.; Chandalia, M.; Cabo-Chan, A.V.; Moe, O.W.; Sakhaee, K. The Metabolic Syndrome and Uric Acid Nephrolithiasis: Novel Features of Renal Manifestation of Insulin Resistance. Kidney Int. 2004, 65, 386–392. [Google Scholar] [CrossRef]
- Hood, V.L.; Sternberg, K.M.; de Waal, D.; Asplin, J.R.; Mulligan, C.; Callas, P.W. Association of Urine Findings with Metabolic Syndrome Traits in a Population of Patients with Nephrolithiasis. Kidney360 2022, 3, 317–324. [Google Scholar] [CrossRef]
- Chang, C.-W.; Ke, H.-L.; Lee, J.-I.; Lee, Y.-C.; Jhan, J.-H.; Wang, H.-S.; Shen, J.-T.; Tsao, Y.-H.; Huang, S.-P.; Geng, J.-H. Metabolic Syndrome Increases the Risk of Kidney Stone Disease: A Cross-Sectional and Longitudinal Cohort Study. J. Pers. Med. 2021, 11, 1154. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Cameron, M.A.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Low Urine PH: A Novel Feature of the Metabolic Syndrome. Clin. J. Am. Soc. Nephrol. 2007, 2, 883–888. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Park, S.K.; Xu, L.H.R.; Blanco, F.; Poindexter, J.; Adams-Huet, B.; Davidson, T.L.; Sakhaee, K.; Maalouf, N.M.; Moe, O.W. Net Acid Excretion and Urinary Organic Anions in Idiopathic Uric Acid Nephrolithiasis. Clin. J. Am. Soc. Nephrol. 2019, 14, 411–420. [Google Scholar] [CrossRef]
- Stoller, M.L.; Meng, M.V.; Abrahams, H.M.; Kane, J.P. The Primary Stone Event: A New Hypothesis Involving a Vascular Etiology. J. Urol. 2004, 171, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-H.; Chang, C.-H.; Chen, S.-J.; Chen, W.-C. Randall’s Plaque, the Origin of Nephrolithiasis: Where Do We Stand Now? Urol. Sci. 2019, 30, 200. [Google Scholar] [CrossRef]
- Oh, S.-W.; Lee, J.-E.; Shin, E.; Kwon, H.; Choe, E.K.; Choi, S.-Y.; Rhee, H.; Choi, S.H. Genome-Wide Association Study of Metabolic Syndrome in Korean Populations. PLoS ONE 2020, 15, e0227357. [Google Scholar] [CrossRef]
- Howles, S.A.; Wiberg, A.; Goldsworthy, M.; Bayliss, A.L.; Gluck, A.K.; Ng, M.; Grout, E.; Tanikawa, C.; Kamatani, Y.; Terao, C.; et al. Genetic Variants of Calcium and Vitamin D Metabolism in Kidney Stone Disease. Nat. Commun. 2019, 10, 5175. [Google Scholar] [CrossRef]
- Tanikawa, C.; Kamatani, Y.; Terao, C.; Usami, M.; Takahashi, A.; Momozawa, Y.; Suzuki, K.; Ogishima, S.; Shimizu, A.; Satoh, M.; et al. Novel Risk Loci Identified in a Genome-Wide Association Study of Urolithiasis in a Japanese Population. J. Am. Soc. Nephrol. 2019, 30, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, M.; Lin, S.; Jian, R.; Li, X.; Chan, J.; Dong, G.; Fang, H.; Robinson, A.E.; GTEx Consortium. A Quantitative Proteome Map of the Human Body. Cell 2020, 183, 269–283.e19. [Google Scholar] [CrossRef] [PubMed]
- Vaxillaire, M.; Cavalcanti-Proença, C.; Dechaume, A.; Tichet, J.; Marre, M.; Balkau, B.; Froguel, P. DESIR Study Group the Common P446L Polymorphism in GCKR Inversely Modulates Fasting Glucose and Triglyceride Levels and Reduces Type 2 Diabetes Risk in the DESIR Prospective General French Population. Diabetes 2008, 57, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Marano, R.; Primiano, A.; Gervasoni, J.; Bargagli, M.; Rovere, G.; Bassi, P.F.; Gambaro, G. Stone Composition and Vascular Calcifications in Patients with Nephrolithiasis. J. Nephrol. 2019, 32, 589–594. [Google Scholar] [CrossRef]
- Prieto, D.; Contreras, C.; Sánchez, A. Endothelial Dysfunction, Obesity and Insulin Resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef]
- Yencilek, E.; Sarı, H.; Yencilek, F.; Yeşil, E.; Aydın, H. Systemic Endothelial Function Measured by Flow-Mediated Dilation Is Impaired in Patients with Urolithiasis. Urolithiasis 2017, 45, 545–552. [Google Scholar] [CrossRef]
- Sáenz-Medina, J.; Martinez, M.; Rosado, S.; Durán, M.; Prieto, D.; Carballido, J. Urolithiasis Develops Endothelial Dysfunction as a Clinical Feature. Antioxidants 2021, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Fabris, A.; Ferraro, P.M.; Comellato, G.; Caletti, C.; Fantin, F.; Zaza, G.; Zamboni, M.; Lupo, A.; Gambaro, G. The Relationship between Calcium Kidney Stones, Arterial Stiffness and Bone Density: Unraveling the Stone-Bone-Vessel Liaison. J. Nephrol. 2015, 28, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Mattace-Raso, F.U.S.; van der Cammen, T.J.M.; Hofman, A.; van Popele, N.M.; Bos, M.L.; Schalekamp, M.A.D.H.; Asmar, R.; Reneman, R.S.; Hoeks, A.P.G.; Breteler, M.M.B.; et al. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation 2006, 113, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, M.; Moochhala, S.; Robertson, W.G.; Gambaro, G.; Lombardi, G.; Unwin, R.J.; Ferraro, P.M. Urinary Metabolic Profile and Stone Composition in Kidney Stone Formers with and without Heart Disease. J. Nephrol. 2021, 35, 851–857. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Robertson, W.G.; Johri, N.; Nair, A.; Gambaro, G.; Shavit, L.; Moochhala, S.H.; Unwin, R.J. A London Experience 1995–2012: Demographic, Dietary and Biochemical Characteristics of a Large Adult Cohort of Patients with Renal Stone Disease. QJM 2015, 108, 561–568. [Google Scholar] [CrossRef]
- Patel, N.D.; Ward, R.D.; Calle, J.; Remer, E.M.; Monga, M. Vascular Disease and Kidney Stones: Abdominal Aortic Calcifications Are Associated with Low Urine PH and Hypocitraturia. J. Endourol. 2017, 31, 956–961. [Google Scholar] [CrossRef]
- Joosten, M.; Gansevoort, R.; Mukamal, K.; Navis, G.; Geleijnse, J.; Feskens, E.; Bakker, S. Urinary Magnesium Excretion and Risk of Cardiovascular Disease in the General Population. Kidney Res. Clin. Pract. 2012, 31, A40. [Google Scholar] [CrossRef][Green Version]
- Yuan, Q.; Xie, Y.; Peng, Z.; Wang, J.; Zhou, Q.; Xiao, X.; Wang, W.; Huang, L.; Tang, W.; Li, X.; et al. Urinary Magnesium Predicts Risk of Cardiovascular Disease in Chronic Kidney Disease Stage 1–4 Patients. Clin. Nutr. 2021, 40, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W.; Huang, C.-L. Hypercalciuria from Acid Load: Renal Mechanisms. J. Nephrol. 2006, 19 (Suppl. 9), S53–S61. [Google Scholar]
- Lucato, P.; Trevisan, C.; Stubbs, B.; Zanforlini, B.M.; Solmi, M.; Luchini, C.; Girotti, G.; Pizzato, S.; Manzato, E.; Sergi, G.; et al. Nephrolithiasis, Bone Mineral Density, Osteoporosis, and Fractures: A Systematic Review and Comparative Meta-Analysis. Osteoporos. Int. 2016, 27, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Nobile, M.; Sella, S.; Dalle Carbonare, L. Bone Disease in Primary Hypercalciuria. Crit. Rev. Clin. Lab. Sci. 2005, 42, 229–248. [Google Scholar] [CrossRef][Green Version]
- Persy, V.; D’Haese, P. Vascular Calcification and Bone Disease: The Calcification Paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Girfoglio, D.; Vijay, V.; Goldsmith, D.; Ferraro, P.M.; Moochhala, S.H.; Unwin, R. Vascular Calcification and Bone Mineral Density in Recurrent Kidney Stone Formers. Clin. J. Am. Soc. Nephrol. 2015, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Brueck, C.C.; Shanahan, C.M.; Schoppet, M.; Dobnig, H. Vascular Calcification and Osteoporosis—From Clinical Observation towards Molecular Understanding. Osteoporos. Int. 2007, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Carrai, P.; Camarri, S.; Pondrelli, C.R.; Gonnelli, S.; Caffarelli, C. Calcification of Cardiac Valves in Metabolic Bone Disease: An Updated Review of Clinical Studies. Clin. Interv. Aging 2020, 15, 1085–1095. [Google Scholar] [CrossRef]
- Towler, D.A. Molecular and Cellular Aspects of Calcific Aortic Valve Disease. Circ. Res. 2013, 113, 198–208. [Google Scholar] [CrossRef]
- Ortlepp, J.R.; Hoffmann, R.; Ohme, F.; Lauscher, J.; Bleckmann, F.; Hanrath, P. The Vitamin D Receptor Genotype Predisposes to the Development of Calcific Aortic Valve Stenosis. Heart 2001, 85, 635–638. [Google Scholar] [CrossRef][Green Version]
- Choi, H.S.; Rhee, Y.; Hur, N.W.; Chung, N.; Lee, E.J.; Lim, S.-K. Association between Low Bone Mass and Aortic Valve Sclerosis in Koreans. Clin. Endocrinol. 2009, 71, 792–797. [Google Scholar] [CrossRef]
- Farhat, G.N.; Cauley, J.A.; Matthews, K.A.; Newman, A.B.; Johnston, J.; Mackey, R.; Edmundowicz, D.; Sutton-Tyrrell, K. Volumetric BMD and Vascular Calcification in Middle-Aged Women: The Study of Women’s Health Across the Nation. J. Bone Miner. Res. 2006, 21, 1839–1846. [Google Scholar] [CrossRef]
- Hak, A.E.; Pols, H.A.; van Hemert, A.M.; Hofman, A.; Witteman, J.C. Progression of Aortic Calcification Is Associated with Metacarpal Bone Loss during Menopause: A Population-Based Longitudinal Study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1926–1931. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zeng, Z.; Wang, J.; Xie, L.; Li, T.; He, Y.; Qin, X.; Zhao, J. Kidney Stones and Cardiovascular Risk: A Meta-Analysis of Cohort Studies. Am. J. Kidney Dis. 2014, 64, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-S.; Shin, M.-H.; Kweon, S.-S.; Park, K.-S.; Sohn, S.-J.; Rhee, J.-A.; Choi, J.-S.; Son, M.-H. Association of Estrogen Receptor-Alpha Gene Polymorphisms with Bone Mineral Density in Postmenopausal Korean Women. J. Bone Miner. Metab. 2005, 23, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Zhang, Y.Y.; Liu, P.Y.; Liu, Y.J.; Shen, H.; Dvornyk, V.; Zhao, L.J.; Deng, H.W. Association of Estrogen Receptor Alpha and Vitamin D Receptor Gene Polymorphisms with Bone Mineral Density in Chinese Males. Calcif Tissue Int. 2004, 74, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Glader, C.A.; Dahlén, G.; Birgander, L.S.; Lorentzon, R.; Waldenström, A.; Lorentzon, M. Oestrogen Receptor Alpha Gene Polymorphism Is Related to Aortic Valve Sclerosis in Postmenopausal Women. J. Intern. Med. 2003, 254, 140–146. [Google Scholar] [CrossRef]
- Prochaska, M.; Taylor, E.N.; Curhan, G. Menopause and Risk of Kidney Stones. J. Urol. 2018, 200, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Guerra, A.; Allegri, F.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; et al. Understanding the Gut-Kidney Axis in Nephrolithiasis: An Analysis of the Gut Microbiota Composition and Functionality of Stone Formers. Gut 2018, 67, 2097–2106. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Dong, F.; Jiang, S.; Tang, C.; Wang, X.; Ren, X.; Wei, Q.; Tian, J.; Hu, W.; Guo, J.; Fu, X.; et al. Trimethylamine N-Oxide Promotes Hyperoxaluria-Induced Calcium Oxalate Deposition and Kidney Injury by Activating Autophagy. Free Radic. Biol. Med. 2022, 179, 288–300. [Google Scholar] [CrossRef]
- Ma, Y.; Ordovas, J.M. The Integration of Epigenetics and Genetics in Nutrition Research for CVD Risk Factors. Proc. Nutr. Soc. 2017, 76, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Bordoni, A.; Hrelia, S.; Bordin, L.; Biagi, P.; Semplicini, A.; Clari, G.; Manzato, E.; Baggio, B. Dietary Manipulation of Delta-6-Desaturase Modifies Phospholipid Arachidonic Acid Levels and the Urinary Excretion of Calcium and Oxalate in the Rat: Insight in Calcium Lithogenesis. J. Lab. Clin. Med. 2000, 135, 89–95. [Google Scholar] [CrossRef]
- Baggio, B.; Budakovic, A.; Nassuato, M.A.; Vezzoli, G.; Manzato, E.; Luisetto, G.; Zaninotto, M. Plasma Phospholipid Arachidonic Acid Content and Calcium Metabolism in Idiopathic Calcium Nephrolithiasis. Kidney Int. 2000, 58, 1278–1284. [Google Scholar] [CrossRef]
- Baggio, B.; Budakovic, A.; Priante, G.; Gambaro, G.; Manzato, E.; Khan, S. Dietary Fatty Acid Supplementation Modulates the Urinary Excretion of Calcium and Oxalate in the Rat. Insight into Calcium Lithogenesis. Nephron 2002, 91, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Tzelves, L.; Geraghty, R.; Lombardo, R.; Davis, N.F.; Petřík, A.; Neisius, A.; Gambaro, G.; Türk, C.; Thomas, K.; Somani, B.; et al. Duration of Follow-up and Timing of Discharge from Imaging Follow-up, in Adult Psatients with Urolithiasis After Surgical or Medical Intervention: A Systematic Review and Meta-Analysis from the European Association of Urology Guideline Panel on Urolithiasis. Eur. Urol. Focus 2022, in press. [Google Scholar] [CrossRef]
- Taylor, E.N.; Fung, T.T.; Curhan, G.C. DASH-Style Diet Associates with Reduced Risk for Kidney Stones. J. Am. Soc. Nephrol. 2009, 20, 2253–2259. [Google Scholar] [CrossRef]
- Rodriguez, A.; Curhan, G.C.; Gambaro, G.; Taylor, E.N.; Ferraro, P.M. Mediterranean Diet Adherence and Risk of Incident Kidney Stones. Am. J. Clin. Nutr. 2020, 111, 1100–1106. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambaro, A.; Lombardi, G.; Caletti, C.; Ribichini, F.L.; Ferraro, P.M.; Gambaro, G. Nephrolithiasis: A Red Flag for Cardiovascular Risk. J. Clin. Med. 2022, 11, 5512. https://doi.org/10.3390/jcm11195512
Gambaro A, Lombardi G, Caletti C, Ribichini FL, Ferraro PM, Gambaro G. Nephrolithiasis: A Red Flag for Cardiovascular Risk. Journal of Clinical Medicine. 2022; 11(19):5512. https://doi.org/10.3390/jcm11195512
Chicago/Turabian StyleGambaro, Alessia, Gianmarco Lombardi, Chiara Caletti, Flavio Luciano Ribichini, Pietro Manuel Ferraro, and Giovanni Gambaro. 2022. "Nephrolithiasis: A Red Flag for Cardiovascular Risk" Journal of Clinical Medicine 11, no. 19: 5512. https://doi.org/10.3390/jcm11195512
APA StyleGambaro, A., Lombardi, G., Caletti, C., Ribichini, F. L., Ferraro, P. M., & Gambaro, G. (2022). Nephrolithiasis: A Red Flag for Cardiovascular Risk. Journal of Clinical Medicine, 11(19), 5512. https://doi.org/10.3390/jcm11195512






