Abstract
Lateral elbow tendinopathy (LET) is a common painful musculoskeletal disorder. Several treatments have been proposed to provide pain reduction and functional recovery, including laser therapy, hyaluronic acid peritendinous injection (Hy-A), and therapeutic exercise (TE). Our study aims to assess the effectiveness of a combined approach with high-intensity laser therapy (HILT) and Hy-A injections compared to TE on pain, muscle strength, and disability in patients with painful LET. A retrospective longitudinal study was carried out by consulting the medical records of patients with a diagnosis of painful LET formulated by clinical and instrumental findings that received functional evaluations, including the Patient-Rated Tennis Elbow Evaluation (PRTEE) and muscle strength measurement at least four times: T0 (“baseline”), 1-month (T1), 3-month (T2), and 6-month follow-ups (T3). Medical records of 80 patients were analyzed. In the HILT + HyA group, the Peak-strength (p < 0.001) and mean strength (p < 0.001) significantly increased compared to the TE group between study times. For the PRTEE-total-score as for the subscales, the HILT + HyA group reported statistically significant reductions only for the comparisons of baseline versus T1 and baseline versus T2. No serious adverse events occurred. Our findings suggest that Hy-A associated with HILT might be more effective than TE for people with LET in the short–medium term.
1. Introduction
“Tennis elbow”, or lateral elbow tendinopathy (LET), is a noninflammatory condition that affects the tendon insertion or myotendinous junction of wrist muscle extensors [1], causing subacute and chronic symptoms of pain at the lateral epicondyle and disability of the elbow and sometimes of the entire upper limb. LET occurs in between 1% and 3% of the population and typically affects subjects between 30 and 60 years without gender difference [1]. Several determinants were reported to be associated with LET, such as working procedures characterized by the long-term repetitive forearm and hand movement [2], the excessive neuronal activity by nociceptors that derive from the radial nerve leading to axonal sprouting of the free nerve endings and peripheral sensitization, smoking habits, and metabolic factors, such as estrogen decline, hypercholesterolemia, and obesity [1]. LET is a degenerative overuse process of the extensor carpi radialis brevis and of the common extensor tendon [3] characterized by histological micro-rupture, vascular proliferation, and hyaline degeneration without inflammatory cells infiltrating within the tendon tissue [4]. The main clinical manifestation is hyperalgesia during active range of motion of the elbow and at the palpation in the lateral epicondyle area, which is exacerbated by prono-supination of the forearm [5]. Moreover, LET patients complain of painful handgrip with consequent functional limitation, disability in activities of daily living, time lost at work, and poor quality of life [6]. LET is usually considered a self-limiting condition, with the majority of patients recovering in 6–24 months [7], even if some clinical reports noticed symptom recurrence persisting for many years [8]. Several conservative approaches have been proposed to manage LET, including pharmacological therapy, systemic and/or local treatments (corticosteroid injections, botulinum toxin, hyaluronic acid, autologous blood, and platelet-rich plasma) [9], therapeutic exercise (TE), physical modalities, elbow braces, acupuncture, and watchful waiting [8]. Surgery is usually recommended for those patients with persistent pain and disability after a course of conservative therapy [10].
However, no consensus about the best treatment for improving pain and function in people with LET has been reached. Among rehabilitative approaches, manual therapy and TE showed positive effects on people with LET in terms of pain relief and increased tendon strength [11,12]. Among physical modalities commonly used for LET, laser therapy was previously demonstrated to improve grip strength, pain, and functional ability at midterm follow-up (5 to 26 weeks) compared to placebo [8]. The studies on laser treatment in LET have focused mainly on the efficacy of low-level laser therapy (LLLT), and few studies have investigated the efficacy of high-intensity laser therapy (HILT) [8]. However, a meta-analysis highlighted how HILT could be more effective than LLLT in terms of pain control, stiffness, and function in degenerative musculoskeletal conditions [13].
Injection therapy is widely used for the treatment of patients with LET [14]. In particular, peritendinous hyaluronic acid (Hy-A) injection seems to be an effective therapeutic option for pain control and functional improvement in these patients.
We hypothesized that a combined approach with HILT plus peritendinous Hy-A injection could improve pain control and functional recovery, considering the analgesic effect and stimulation of collagen synthesis attributable to HILT [15] and the Hy-A-related enhancement of the activity of the fibroblasts, including their adhesivity, extracellular matrix synthesis, and proliferation [16,17].
Therefore, the main objective of the study is to assess the effectiveness of the combination of HILT and Hy-A peritendinous injection on pain relief, improvement of muscle strength, functional ability, and quality of life, in a mid-long-term period, compared to TE in patients with painful LET.
2. Materials and Methods
2.1. Study Design
A retrospective cohort clinical study was conducted by the Declaration of Helsinki and the STROBE guidelines [18]. The study protocol was planned at the Department of Clinical Research, Ludes Campus, Luganoff Campus of Semmelweis University of Budapest, and written informed consent was obtained from all participants for treatment and data processing. From June 2021 to June 2022, medical records of patients with LET that were treated at the Chiparo Physical Medicine and Rehabilitation Clinic in Lecce, Italy, were consulted and selected by a specialized nurse blinded for the main outcomes of the study.
2.2. Population
We included medical records of patients between 30 and 65 years with elbow pain for at least 2 weeks and the following clinical and instrumental features: (1) no other sources of elbow pain (e.g., cervical radiculopathy); (2) minimal pain at rest (Patient-Rated Tennis Elbow Evaluation, PRTEE, pain subscale < 3); (3) positive wrist extension tests against resistance (Cozen test) [19]; (4) positive palpation test of the epicondyle; (5) ultrasound (US) evaluation showing thickening and heterogeneous echo structure of the common extensor tendon, as well as increased blood flow under Doppler. We did not consider clinical records of patients receiving regular painkillers, previous elbow injections, specific rehabilitation treatments in the previous two months, and those with US-scan-confirmed injury or rupture of any extensor tendon of the carpus or with previous elbow surgery. Additional exclusion criteria were the presence of contraindications to laser therapy and Hy-A injection, such as drug allergy, epilepsy, coagulopathies or anticoagulant therapy, neoplasms, or pregnancy. Patients with fibromyalgia, enthesitis due to seronegative arthritis, and rheumatoid arthritis were also excluded. Finally, patients who had skin infections at the injection site, systemic symptoms (e.g., fever), or were carriers of artificial cardiac pacemakers were also excluded.
2.3. Outcomes
We included medical records of patients who filled the PRTEE questionnaire [20] and who received the measurement of maximum (peak) and mean grip strength by an electronic dynamometer (Activeforce2 Sixtus Prato (PO) Italia) during contraction against the resistance of wrist extensors with the elbow flexed at 90° (Cozen test). Handgrip strength protocol provided 3 maximal contraction trials lasting 5 s each, interspersed with 15 s of rest. The PRTEE is a 15-item questionnaire assessing pain (5 items) and the degree of difficulty in performing various activities (6 specific and 4 usual activities) due to LET over the preceding week [20]. The same functional assessments were performed at different follow-ups: T1 (30 days), T2 (90 days), and T3 (180 days) after the end of treatment.
2.4. Interventions
2.4.1. HILT Plus Hyaluronic Acid (HILT + Hy-A) Group
Our HILT protocol consisted of 10 daily sessions using a LASERIX PRO device (GN med), administered via a fixed tip with a 30 mm spacer. Each HILT session had a total duration of 13 min and was divided into 3 phases. In the first phase lasting 7 min, a frequency of 18 Khz, peak power of 600 W, and 226 Joules of energy was delivered. In the second phase lasting 3 min, a frequency of 14 Khz, peak power of 900 W, and 226 Joules of energy was delivered. In the third phase lasting 3 min, a frequency of 10 Khz, peak power of 1200 W, and 226 Joules of energy was delivered. At the end of the 10 HILT sessions, and on day 7 and day 14 after the last therapeutic session, patients received US-guided (SonoSite M-Turbo ultrasound system with a 6–15 Mhz linear probe) Hy-A injections in the peritendinous area of the elbow epicondyle by the same physiatrist expert in US technique and US-guided injection (Supplementary Figures S1 and S2). The injections were conducted using a pre-filled syringe of 20 mg in 2 mL of linear Hy-A sodium salt with a molecular weight of 500–730 kDa. The needles used were 25G 25 mm. No anesthetic drugs were administered after the injections. During the therapeutic procedure with laser and Hy-A injections, patients were seated, and the affected elbow was positioned at 90° of flexion and in slight pronation. The skin was disinfected with a chlorhexidine wipe. All subjects enrolled in this group were not prescribed any therapeutic exercise and did not receive any other physical or pharmacological therapy.
2.4.2. Therapeutic Exercise Group
Our TE protocol (eccentric exercises series and static stretching exercise) consisted of three/per week clinical supervised sessions for 4 weeks for a total of 12 sessions. Eccentric exercises for LET were performed with the elbow supported on the bed in full extension, forearm in pronation, wrist in maximal extension, and hand hanging over the edge of the bed. In this position, patients were told to flex their wrist slowly until full flexion was achieved and then return to the starting position. Patients were instructed to continue with the exercise even if they experienced mild pain. However, they were instructed to stop the exercise if disabling pain occurred. Each session consisted of three sets of 10 repetitions with at least a 1 min rest interval between each set. When patients were able to perform the eccentric exercises without experiencing any pain or discomfort, the load was increased using free weights or therabands. The starting and final positions of eccentric exercises, the increase in the load, and the degree of mild or disabling pain could not be standardized but were tailored to each patient [11]. The static stretching exercises for LET were performed slowly with the elbow in extension, forearm in pronation, wrist in flexion, and ulnar deviation according to the patient’s tolerance to achieve the best stretching position result for the ECRB tendon. This position was held for 30–45 s before and after each set of eccentric exercises [11].
2.4.3. Treatments-Related Adverse Events
We reported adverse events (AEs) in both groups by consulting the clinical records of included patients [21].
2.5. Sample Size
The sample size was calculated for the comparison of the two study groups at a 6-month follow-up for the PRTEE—total score. We assumed as significant minimal change a difference of at least five points in the PRTEE at each follow-up. We also settled for standard deviations of 10. A sample size of N = 36 per study arm (72 overall) provides more than 90% power (alpha = 0.05, two-tailed) applying linear mixed models, with an intraclass-correlation between measures ρ = 0.50. To account for 10% attrition during the study period (“dropouts”), we planned to recruit 40 medical records per study group (80 overall) at baseline. Similar values were obtained in the assessment of the sample size according to peak muscle strength [22].
2.6. Statistical Analysis
Data were reported as mean ± standard error (S.E.) for continuous variables and as absolute number and percentage for dichotomous variables; differences between groups were assessed with analysis of variance and chi-square test, respectively. To assess the variation of the PRTEE subscale as the total score, peak, and mean muscle strength, linear mixed models (LMMs) were applied [23]. Intercept and time had a random component. The advantage of this approach is that it increases the precision of the estimate by using all available information concerning performance and, at the same time, allows for handling missing data with more powerful modeling of the analysis.
The LMMs were considered the two treatments: HILT + Hy-A as the reference group, the three times of the study, with baseline as the reference, and lastly, the interaction between time and treatment. Sensitivity analyses were also conducted, excluding from analysis those patients that did not reach a subjective and clinical improvement, applying the same previously described models.
Data were analyzed with SAS software (Rel. 9.4, Cary, NC, USA), and the p-value for differences was considered statistically significant for a value less than or equal to 0.05.
3. Results
In this study, the medical records of 80 patients were included (40 for each group). No statistically significant between-group differences were found for main clinical characteristics, for peak and mean strength, and for the PRTEE-total score and PRTEE-subscales (Table 1).

Table 1.
Description of the population characteristics according to type of treatment at enrollment. High-intensity laser therapy plus hyaluronic acid (Hilt + Hy-A), therapeutic exercise (TE); Patient-Rated Tennis Elbow Evaluation (PRTEE).
Hand-grip peak-strength increased during the study in both groups, and a multiplicative effect was demonstrated for the interaction between treatment and time (p < 0.001) (Figure 1).
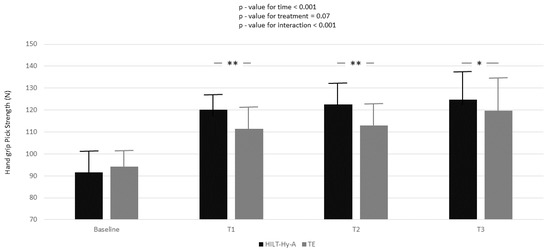
Figure 1.
Handgrip peak strength variation according to time and treatment; black box represents high-intensity laser therapy plus hyaluronic acid (HILT + Hy-A) group, whereas grey box represents therapeutic exercise (TE). Results for the linear mixed model analysis (p-values for time, treatment, and interaction of time for treatment); Bonferroni adjustment for multiple comparisons were also applied between follow-ups of study compared to baseline; *: p < 0.05; **: p < 0.001.
In the HILT + Hy-A group, the peak strength significantly increased compared to the TE group in all follow-ups (baseline-T1: 11.21 ± 1.89; p < 0.001; baseline-T2: 12.06 ± 2.94; p < 0.001; baseline-T3: 7.57 ± 3.26; p = 0.02). Nearly one-quarter (ρ = 24.5, Supplementary Table S1, Model A) of the total variation in peak strength was attributable to differences between patients. Moreover, from the unconditional means model, 84% of unexplained residual in the variation of peak strength (Model A: 210.81 ± 18.49 Nw) was associated with linear time (Model B) and 87% with the interaction between time and treatment (Model C). Model goodness increased (AIC decreased between models) with model complexity. Mean strength increased during the study period in both groups, and again a multiplicative effect was demonstrated for the interaction between treatment and time (p < 0.001) (Figure 2).
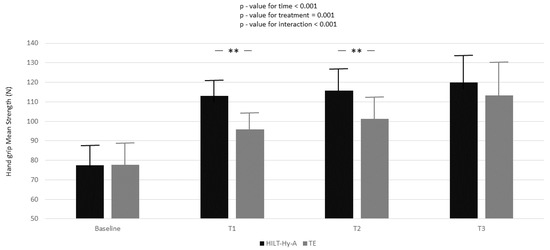
Figure 2.
Handgrip mean strength variation according to time and treatment; black box represents high-intensity laser therapy plus hyaluronic acid (HILT+Hy-A) group, whereas grey box represents therapeutic exercise (TE). Results for the linear mixed model analysis (p-values for time, treatment, and interaction for time for treatment). Bonferroni adjustment for multiple comparisons applied between follow-ups of the study compared to baseline; **: p < 0.001.
In the HILT + Hy-A group, the mean strength significantly increased compared to the TE group, only between baseline and T1 (17.24 ± 2.20; p < 0.001) and between baseline and T2 (14.49 ± 2.81; p < 0.001). Almost 15% (ρ = 14.5, Supplementary Table S2, Model A) of the total variation in peak strength was attributable to differences between patients. Again, Model B and Model C explained 84 and 89% of the unexplained residuals in the variation of peak strength (335.10 ± 29.21), respectively. Model goodness increased (AIC decreased between models) with model complexity.
Figure 3A–D report the PRTEE total score, the pain subscale score, the specific disability score, and the usual activity score. PRTEE total score and all the subscales decreased during the time points of the study. In the comparison between treatments according to different follow-ups, statistically significant differences were reported only for the comparisons between baseline and T1 and baseline and T2 for the PRTEE-score and all subscales. For the PRTEE score, almost 27% (ρ = 27.5, Supplementary Table S3, Model A) of the total variation was attributable to differences between patients. Again, Model B and Model C explained 72 and 76 percent of the unexplained residual in the variation of peak strength (83.93 ± 8.77), respectively. Model goodness increased (AIC decreased between models) with model complexity. After 6 months, 11 patients (13.8%), four in the HILT + HyA group and seven in the TE group (p-value = 0.33), did not improve.
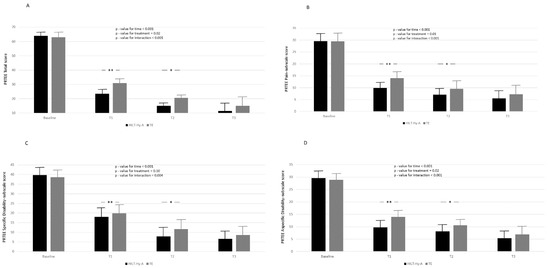
Figure 3.
Report the PRTEE total score (A), the pain subscale score (B), the specific disabil-ity score (C), and the usual activity score (D). according to time and treatment; black box represents high-intensity laser therapy plus hyaluronic acid (HILT + Hy-A) group, whereas grey box represents therapeutic exercise (TE). Results for linear mixed model analysis (p-value for time, treatment, and interaction of time for reatment). Bonferroni adjustment for multiple comparisons applied between follow-ups of the study compared to baseline; *: p < 0.05, **: p < 0.001.
4. Discussion
To our knowledge, no clinical studies investigated the effectiveness of the combined use of Hy-A and HILT on LET. Our findings suggest that combined peritendinous injections of Hy-A and HILT might be effective for improving pain, muscle strength, and disability in this population at short- and medium-term (1 to 3 month-follow-up) compared to TE.
Tendinopathy can occur because of different insults, such as certain drug treatments (e.g., fluoroquinolones), metabolic disorders (e.g., diabetes mellitus and hypercholesterolemia), and biomechanical factors [24]. In particular, overload and detraining are catabolic stimuli for tendon tissue resulting in increased synthesis of collagenase, proteinase, and pro-inflammatory cytokines [25]. To date, different studies support the use of eccentric exercise to improve pain and muscle strength in patients with LET [26]. Compared with the concentric exercise, the eccentric exercise showed a significant reduction in self-reported pain [26]. Eccentric contraction would appear to stimulate tendon cells, resulting in increased collagen cross-linking [27] and decreased neuro-vascular ingrowth that seem to modulate pain [28]. However, the relationship between exercise type and pain remains unclear in LET, and often, it is debated whether eccentric exercises should be performed with pain [29]. Other therapeutic strategies have been proposed in recent years for the treatment of pain in patients with LET, including injections of different agents, such as platelet-rich plasma (PRP), adipose-derived mesenchymal stromal cells, botulinum toxin, and Hy-A [30]. Hy-A injection seems to inhibit the pro-inflammatory response by local fibroblast [31], reduce pain, improve function, and reduce tendon rubbing in pre-insertion areas during major tendinopathies and post-surgical tendon repair [14]. Among adjunctive interventions for the management of patients with LET, physical modalities are commonly used in clinical practice [24]. In particular, LLLT seems to be useful in different musculoskeletal disorders by reducing edema and inflammation, controlling pain, and promoting tissue healing [32]. However, controversial evidence is available about the benefits of the use of LLLT in patients with LET, where only short-term pain relief was reported [33]. On the other hand, evidence about HILT is scant, with some observational studies suggesting the effectiveness of this intervention in patients with LET on pain control, functional recovery, and quality of life [34]. HILT might have analgesic and regenerative effects attributable to its ability to slow pain transmission, increase the production of morphine-mimetic substances [34], stimulate collagen production, and increase vascular permeability and blood flow within tendons by photochemical and photothermic stimulation [35]. These effects might act synergistically with the analgesic and regenerative effects of Hy-A. For what concerns safety, the combined approach proposed in our study was well tolerated, as demonstrated by the absence of AEs. Moreover, our data suggest that this approach promoted rapid clinical and functional improvement. Lastly, we cannot demonstrate a statistically significant reduction in the therapeutical failure between the two treatments, but this is not the main objective of the study, and probably the study is underpowered against this outcome.
The strengths of our study are the adequate sample size, the long-term follow-up, and no serious adverse effect occurrence in both study groups. Moreover, the character of a real-life study strengthens the results on the efficacy and safety of the combined treatment of Hy-A plus HILT in the treatment of LET. However, our study has some limitations. First, the retrospective design hampered the allocation of patients in the two groups because a randomization procedure was lacking. Moreover, a healthy worker effect could be introduced as further selection bias. In addition, our cohort might not be representative of the general population suffering from LET, such as elderly, overweight-obese people, and workers involved in repetitive and strenuous activities. The choice of a control group treated only with HILT, or alternatively, only with Hy-A, could be more informative to better define the role of each intervention in the control of LET symptomatology.
5. Conclusions
Our findings support the hypothesis that a multimodal approach might provide additional benefits without safety concerns in patients with LET. In particular, our study showed the effectiveness of the combined intervention of HILT and Hy-A injections over TE in subjects affected by LET in the short–medium term. Despite these encouraging findings, randomized controlled trials are required to prove the efficacy of the proposed approach compared to placebo or the efficacy of HILT + Hya + TE over the TE effect.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11195492/s1, Table S1: Linear mixed model, variation of handgrip peak strength during the follow-up according to treatment; Table S2: Linear mixed model, variation of handgrip mean-strength during the follow-up according to treatment; Table S3: Linear mixed model, follow-up variation of PRTEE-score according to treatments; Figure S1: Ultra-sound image of the lateral elbow, longitudinal view: (1) humerus, (2) epicondyle, (3) head of radius, (4) extensor tendons, (5) calcific lesion of the tendons; Figure S2: Ultra-sound-guided (6–15 Mhz linear probe) Hyaluronic Acid injections in the peritendinous area of the elbow epicondyle. The injections were done using a pre-filled syringe of 20 mg in 2 mL of linear Hy-A sodium salt with a molecular weight of 500-730 kDa.
Author Contributions
Conceptualization, R.P., A.D.I., A.M. and G.I.; methodology, R.P., T.P., F.B. and P.M.; formal analysis, R.P.and A.D.I.; investigation, F.B. and R.P.; resources, R.P. and A.D.I.; data curation, R.P. and A.D.I.; writing—original draft preparation, R.P., T.P., A.D.I., A.M. and G.I.; writing—review and editing, T.P., F.B., P.M., A.M. and G.I.; supervision, G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable; this is an observational retrospective study.
Informed Consent Statement
The study was developed following the Good Clinical Practice (GCP) guidelines. It was conducted within the ethical principles outlined in the Declaration of Helsinki and with the procedures defined by the ISO 9001-2015 standards for “Research and experimentation”. Written informed consent to provide information included in personal medical records was obtained from all participants.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Raffaello Pellegrino, Teresa Paolucci, Fabrizio Brindisino, Paolo Mondardini, Angelo Di Iorio, Antimo Moretti, and Giovanni Iolascon (the authors) certify that there is no conflict of interests with any financial organization regarding the material discussed in this manuscript.
References
- Cutts, S.; Gangoo, S.; Modi, N.; Pasapula, C. Tennis elbow: A clinical review article. J. Orthop. 2019, 17, 203–207. [Google Scholar] [CrossRef]
- Descatha, A.; Albo, F.; Leclerc, A.; Carton, M.; Godeau, D.; Roquelaure, Y.; Petit, A.; Aublet-Cuvelier, A. Lateral Epicondylitis and Physical Exposure at Work? A Review of Prospective Studies and Meta-Analysis. Arthritis Care Res. 2016, 68, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, F.S.; ElAttar, M.; Salem, H.F. Hand-Grip Strength and Return to Heavy Manual Work at a Mean 5-Year Follow-up After Arthroscopic Release of Recalcitrant Lateral Epicondylitis. Orthop. J. Sport. Med 2022, 10, 23259671221078586. [Google Scholar] [CrossRef] [PubMed]
- Kheiran, A.; Pandey, A.; Pandey, R. Common tendinopathies around the elbow; what does current evidence say? J. Clin. Orthop. Trauma 2021, 19, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Karabinov, V.; Georgiev, G.P. Lateral epicondylitis: New trends and challenges in treatment. World J. Orthop. 2022, 13, 354–364. [Google Scholar] [CrossRef]
- Stratford, P.W.; Levy, D.R. Assessing valid change over time in patients with lateral epicondylitis at the elbow. Clin. J. Sport Med. 1994, 4, 88–91. [Google Scholar] [CrossRef]
- Ikonen, J.; Lähdeoja, T.; Ardern, C.L.; Buchbinder, R.; Reito, A.; Karjalainen, T. Persistent Tennis Elbow Symptoms Have Little Prognostic Value: A Systematic Review and Meta-analysis. Clin. Orthop. Relat. Res 2022, 480, 647–660. [Google Scholar] [CrossRef]
- Lian, J.; Mohamadi, A.; Chan, J.J.; Hanna, P.; Hemmati, D.; Lechtig, A.; Nazarian, A. Comparative Efficacy and Safety of Nonsurgical Treatment Options for Enthesopathy of the Extensor Carpi Radialis Brevis: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials. Am. J. Sports Med. 2019, 47, 3019–3029. [Google Scholar] [CrossRef]
- de Sire, A.; Lippi, L.; Mezian, K.; Calafiore, D.; Pellegrino, R.; Mascaro, G.; Cisari, C.; Invernizzi, M. Ultrasound-guided platelet-rich-plasma injections for reducing sacroiliac joint pain: A paradigmatic case report and literature review. J. Back Musculoskelet. Rehabil. 2022, 35, 977–982. [Google Scholar] [CrossRef]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Ransom, J.E.; Smith, J.; Morrey, B.F. The epidemiology and health care burden of tennis elbow: A population-based study. Am. J. Sports Med. 2015, 43, 1066–1071. [Google Scholar] [CrossRef]
- Landesa-Piñeiro, L.; Leirós-Rodríguez, R. Physiotherapy treatment of lateral epicondylitis: A systematic review. J. Back Musculoskelet. Rehabil. 2022, 35, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Kachanathu, S.J.; Alenazi, A.M.; Hafez, A.R.; Algarni, A.D.; Alsubiheen, A.M. Comparison of the effects of short-duration wrist joint splinting combined with physical therapy and physical therapy alone on the management of patients with lateral epicondylitis. Eur. J. Phys. Rehabil. Med. 2019, 55, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Mohamad, M.S.; Yusof, A. Effects of low-level and high-intensity laser therapy as adjunctive to rehabilitation exercise on pain, stiffness and function in knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2022, 114, 85–95. [Google Scholar] [CrossRef]
- Pellegrino, R.; Brindisino, F.; Barassi, G.; Sparvieri, E.; DI Iorio, A.; de Sire, A.; Ruosi, C. Combined ultrasound guided peritendinous hyaluronic acid (500–730 Kda) injection with extracorporeal shock waves therapy vs. extracorporeal shock waves therapy-only in the treatment of shoulder pain due to rotator cuff tendinopathy. A randomized clinical. J. Sports Med. Phys. Fitness 2022, 62, 1211–1218. [Google Scholar] [CrossRef]
- Urdiales-Gálvez, F.; Martín-Sánchez, S.; Maíz-Jiménez, M.; Castellano-Miralla, A.; Lionetti-Leone, L. Concomitant Use of Hyaluronic Acid and Laserin Facial Rejuvenation. Aesthetic Plast. Surg 2019, 43, 1061. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Crimaldi, S.; Liguori, S.; Tamburrino, P.; Moretti, A.; Paoletta, M.; Toro, G.; Iolascon, G. The Role of Hyaluronic Acid in Sport-Related Tendinopathies: A Narrative Review. Medicina 2021, 57, 1088–2021. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, 31–34. [Google Scholar] [CrossRef]
- Karanasios, S.; Korakakis, V.; Moutzouri, M.; Drakonaki, E.; Koci, K.; Pantazopoulou, V.; Tsepis, E.; Gioftsos, G. Diagnostic accuracy of examination tests for lateral elbow tendinopathy (LET)—A systematic review. J. Hand Ther. 2021, in press. [Google Scholar] [CrossRef]
- Cacchio, A.; Necozione, S.; MacDermid, J.C.; Rompe, J.D.; Maffulli, N.; di Orio, F.; Santilli, V.; Paoloni, M. Cross-cultural adaptation and measurement properties of the italian version of the Patient-Rated Tennis Elbow Evaluation (PRTEE) questionnaire. Phys. Ther. 2012, 92, 1036–1045. [Google Scholar] [CrossRef]
- Pahor, M.; Chrischilles, E.A.; Guralnik, J.M.; Brown, S.L.; Wallace, R.B.; Carbonin, P. Drug data coding and analysis in epidemiologic studies. Eur. J. Epidemiol. 1994, 10, 405–411. [Google Scholar] [CrossRef]
- Stroup, W.W. Generalized Linear Mixed Models Modern Concepts, Methods and Applications; Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Singer, J.D. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. J. Educ. Behav. Stat. 1998, 23, 323–355. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, A.; Salamanna, F.; Della Bella, E.; Vittadini, F.; Gasparre, G.; Aldini, N.N.; Masiero, S.; Fini, M. The Role of Detraining in Tendon Mechanobiology. Front. Aging Neurosci. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Karanasios, S.; Korakakis, V.; Whiteley, R.; Vasilogeorgis, I.; Woodbridge, S.; Gioftsos, G. Exercise interventions in lateral elbow tendinopathy have better outcomes than passive interventions, but the effects are small: A systematic review and meta-analysis of 2123 subjects in 30 trials. Br. J. Sports Med. 2021, 55, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Baker, N.A. Effectiveness of eccentric strengthening in the treatment of lateral elbow tendinopathy: A systematic review with meta-analysis. J. Hand Ther. 2021, 34, 18–28. [Google Scholar] [CrossRef]
- Ryan, C.G.; Gray, H.G.; Newton, M.; Granat, M.H. Pain biology education and exercise classes compared to pain biology education alone for individuals with chronic low back pain: A pilot randomised controlled trial. Man. Ther. 2010, 15, 382–387. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Kim, Y.W.; Shin, I.S.; Kang, S.; Moon, H.I.; Lee, S.C. The Beneficial Effects of Eccentric Exercise in the Management of Lateral Elbow Tendinopathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3968. [Google Scholar] [CrossRef]
- Evans, J.P.; Maffulli, N.; Smith, C.; Watts, A.; Valderas, J.; Goodwin, V. Even experts cannot agree on the optimal use of platelet-rich plasma in lateral elbow tendinopathy: An international Delphi study. J. Orthop. Traumatol. 2021, 22, 47. [Google Scholar] [CrossRef]
- Mitsui, Y.; Gotoh, M.; Nakama, K.; Yamada, T.; Higuchi, F.; Nagata, K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J. Orthop. Res. 2008, 26, 1032–1037. [Google Scholar] [CrossRef]
- Cotler, H.B. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015, 2, 188–194. [Google Scholar] [CrossRef]
- Mamais, I.; Papadopoulos, K.; Lamnisos, D.; Stasinopoulos, D. Effectiveness of Low Level Laser Therapy (LLLT) in the treatment of Lateral elbow tendinopathy (LET): An umbrella review. Laser Ther. 2018, 27, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Dundar, U.; Turkmen, U.; Toktas, H.; Ulasli, A.M.; Solak, O. Effectiveness of high-intensity laser therapy and splinting in lateral epicondylitis: A prospective, randomized, controlled study. Lasers Med. Sci. 2015, 30, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Akkurt, E.; Kucuksen, S.; Yılmaz, H.; Parlak, S.; Sallı, A.; Karaca, G. Long term effects of high intensity laser therapy in lateral epicondylitis patients. Lasers Med. Sci. 2016, 31, 249–253. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).