Skin Hyperpigmentation Due to Post-Surgical Adrenal Insufficiency Regressed with the Dexamethasone Treatment
Abstract
1. Introduction
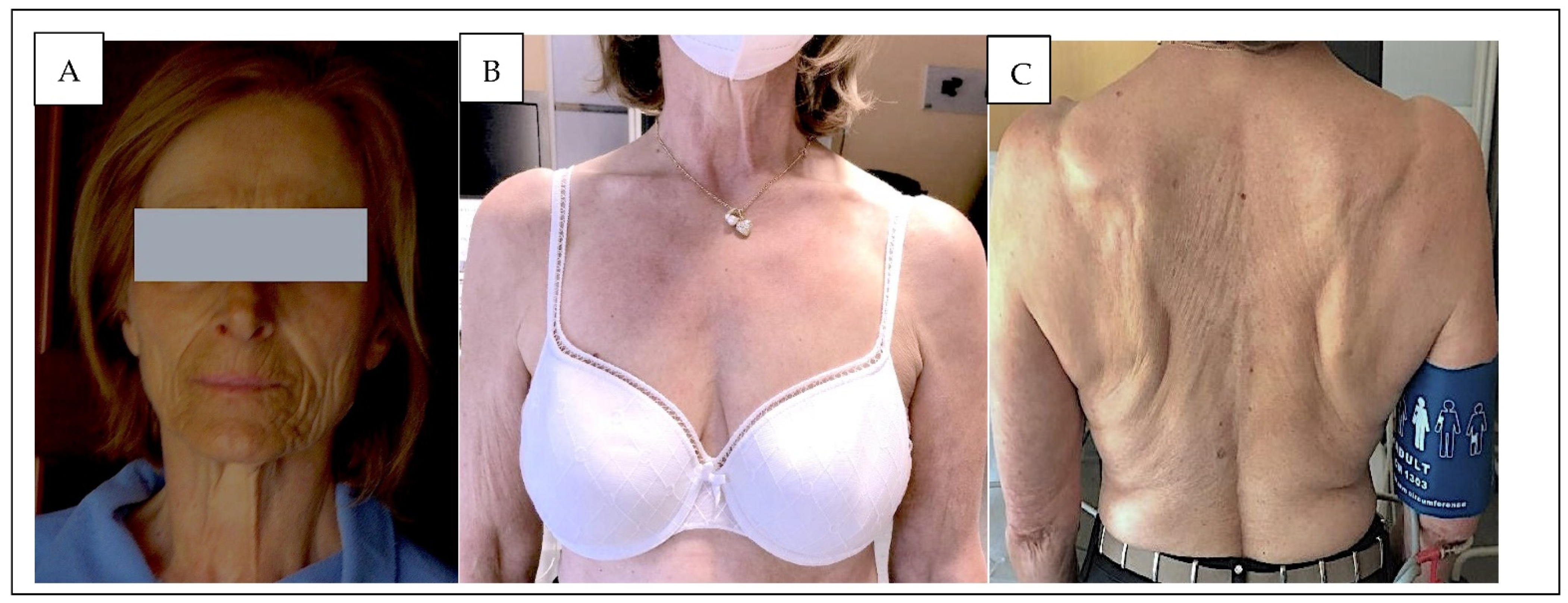
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charmandari, E.; Nicolaides, N.C.; Chrousos, G.P. Adrenal Insufficiency. Lancet 2014, 383, 2152–2167. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Rossitto, G.; Amar, L.; Azizi, M.; Riester, A.; Reincke, M.; Degenhart, C.; Widimsky, J.; Naruse, M.; Deinum, J.; Schultzekool, L.; et al. Subtyping of Primary Aldosteronism in the AVIS-2 Study: Assessment of Selectivity and Lateralization. J. Clin. Endocrinol. Metab. 2020, 105, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P. Primary Aldosteronism: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 2799–2811. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Cesari, M.; Cuspidi, C.; Maiolino, G.; Cicala, M.V.; Bisogni, V.; Mantero, F.; Pessina, A.C. Long-Term Control of Arterial Hypertension and Regression of Left Ventricular Hypertrophy with Treatment of Primary Aldosteronism. Hypertension 2013, 62, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after Adrenalectomy for Unilateral Primary Aldosteronism: An International Consensus on Outcome Measures and Analysis of Remission Rates in an International Cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar] [CrossRef]
- Citton, M.; Viel, G.; Rossi, G.P.; Mantero, F.; Nitti, D.; Iacobone, M. Outcome of Surgical Treatment of Primary Aldosteronism. Langenbeck’s Arch. Surg. 2015, 400, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, L.; Prisco, S.; Caroccia, B.; Rossi, G.P. Saga of Familial Hyperaldosteronism. Hypertension 2018, 71, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Rossitto, G.; Miotto, D.; Battistel, M.; Barbiero, G.; Maiolino, G.; Bisogni, V.; Sanga, V.; Rossi, G.P. Metoclopramide Unmasks Potentially Misleading Contralateral Suppression in Patients Undergoing Adrenal Vein Sampling for Primary Aldosteronism. J. Hypertens. 2016, 34, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Pavlaki, A.N.; Magiakou, M.A.; Chrousos, G.P. Glucocorticoid Therapy and Adrenal Suppression. [Updated 19 October 2018]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Cassarino, M.F.; Sesta, A.; Pagliardini, L.; Losa, M.; Lasio, G.; Cavagnini, F.; Pecori Giraldi, F. Proopiomelanocortin, Glucocorticoid, and CRH Receptor Expression in Human ACTH-Secreting Pituitary Adenomas. Endocrine 2017, 55, 853–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Glucocorticoid | Equivalent Dose (mg) | HPA Suppression | Plasma Half-Life (min) | Biologic Half-Life (h) |
|---|---|---|---|---|
| Cortisol | 20.0 | 1.0 | 90 | 8–12 |
| Cortisone | 25.0 | 80–118 | 8–12 | |
| Prednisone | 5.0 | 4.0 | 60 | 18–36 |
| Prednisolone | 5.0 | 115–200 | 18–36 | |
| Triamcinolone | 4.0 | 4.0 | 30 | 18–36 |
| Methylprednisolone | 4.0 | 4.0 | 180 | 18–36 |
| Dexamethasone | 0.75 | 17.0 | 200 | 36–54 |
| Betamethasone | 0.6 | 300 | 36–54 | |
| Fludrocortisone | 2.0 | 12.0 | 200 | 18–36 |
| Desoxycorticosterone acetate | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shagjaa, T.; Sanga, V.; Rossi, G.P. Skin Hyperpigmentation Due to Post-Surgical Adrenal Insufficiency Regressed with the Dexamethasone Treatment. J. Clin. Med. 2022, 11, 5379. https://doi.org/10.3390/jcm11185379
Shagjaa T, Sanga V, Rossi GP. Skin Hyperpigmentation Due to Post-Surgical Adrenal Insufficiency Regressed with the Dexamethasone Treatment. Journal of Clinical Medicine. 2022; 11(18):5379. https://doi.org/10.3390/jcm11185379
Chicago/Turabian StyleShagjaa, Tungalagtamir, Viola Sanga, and Gian Paolo Rossi. 2022. "Skin Hyperpigmentation Due to Post-Surgical Adrenal Insufficiency Regressed with the Dexamethasone Treatment" Journal of Clinical Medicine 11, no. 18: 5379. https://doi.org/10.3390/jcm11185379
APA StyleShagjaa, T., Sanga, V., & Rossi, G. P. (2022). Skin Hyperpigmentation Due to Post-Surgical Adrenal Insufficiency Regressed with the Dexamethasone Treatment. Journal of Clinical Medicine, 11(18), 5379. https://doi.org/10.3390/jcm11185379






